Abstract
Objective:
The study to Evaluate Patient OutComes, Safety, and Tolerability of Fingolimod (EPOC; NCT01216072) aimed to test the hypothesis that therapy change to oral Gilenya (Novartis AG, Stein, Switzerland) (fingolimod) improves patient-reported outcomes compared with standard-of-care disease-modifying therapy (DMT) in patients with relapsing multiple sclerosis; safety and tolerability were also assessed. This communication describes the study rationale and design.
Methods:
EPOC is a phase 4, open-label, multi-center study conducted in the US and Canada of patients with relapsing forms of multiple sclerosis who are candidates for therapy change. Therapy change eligibility was determined by the treating physician (US patients) or required an inadequate response to or poor tolerance for at least 1 MS therapy (Canadian patients). Patients were randomly assigned in a 3:1 ratio to 6 months of treatment with once-daily oral fingolimod 0.5 mg or standard-of-care DMTs. The primary study end-point was the change from baseline in treatment satisfaction as determined by the global satisfaction sub-scale of the Treatment Satisfaction Questionnaire for Medication. Secondary end-points included changes from baseline in perceived effectiveness and side-effects, and measures of activities of daily living, fatigue, depression, and quality-of-life. A 3-month open-label fingolimod extension was available for patients randomly assigned to the DMT group who successfully completed all study visits.
Results:
Enrollment has been completed with 1053 patients; the patient population is generally older and has a longer duration of disease compared with populations from phase 3 studies of fingolimod.
Limitations:
Inclusion criteria selected for patients with a sub-optimal experience with a previous DMT, limiting the collection of data on therapy change in patients who were satisfied with their previous DMT.
Conclusions:
Results of the EPOC study are anticipated in early 2013 and will inform treatment selection by providing patient-centered data on therapy switch to fingolimod or standard-of-care DMTs.
Trial Registration:
ClinicalTrials.gov NCT01216072.
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) in which symptoms of inflammation and neurodegeneration often begin in early adulthood, when patients have completed their education and started their careers and familiesCitation1. The timing of disease onset frequently has a substantial socioeconomic impact on the patient, including potential loss of employment and the ability to live independentlyCitation2,Citation3. Thus, in addition to radiographic and clinical measures of disease, it is important to include the patient perspective on how disease-modifying therapies (DMTs) may affect quality-of-life and daily functioning.
Patient-reported outcome (PRO) instruments are increasingly used in clinical research to obtain the patient perspective on disease symptoms, treatment efficacy, and outcomeCitation4,Citation5. Even with identical standard disease measures, the differences in the physical and psychological disease aspects among patients may be dramaticCitation6 and under-appreciated without the addition of PROs. PROs are particularly useful in chronic diseases for which the goal of treatment is to slow disease progression, manage symptoms, and preserve quality-of-lifeCitation5.
Although there are numerous PRO instruments available for use in patients with MS, they have not been widely used in pivotal trials of DMTsCitation5,Citation7,Citation8. One of the primary reasons to consider PROs in such trials is that patient preferences and quality-of-life are important factors for selecting treatment and assessing tolerability. However, PROs have been largely secondary outcomes in these studies.
Gilenya (Novartis AG, Stein, Switzerland) (fingolimod [FTY720]), a sphingosine 1-phosphate receptor modulator, is the first once-daily oral therapy approved for relapsing MS; several large studies have demonstrated its efficacy on relapse activity, disability progression, and magnetic resonance imaging (MRI) measures. In the 12-month Trial Assessing Injectable Interferon Versus Fingolimod Oral in Relapsing–Remitting Multiple Sclerosis (TRANSFORMS), which studied 1292 patients with relapsing MS, oral fingolimod 0.5 mg reduced the annualized relapse rate by 52% compared with intramuscular (IM) interferon (IFN) β–1a (0.16 vs 0.33; p < 0.001); this effect was observed in both treatment-naive (0.15 vs 0.31) and previously treated patients (0.26 vs 0.53)Citation9. Fingolimod 0.5 mg also more effectively reduced MRI lesion activity (1.7 vs 2.6 new or enlarged T2 lesions; p < 0.004) and preserved brain volume (−0.31% vs −0.45%; p < 0.001) than IFNβ-1a IMCitation9. In the 24-month Fingolimod Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) study of 1272 patients with relapsing MS, fingolimod 0.5 mg reduced the annualized relapse rate by 54% compared with placebo (0.18 vs 0.40; p < 0.001); a greater effect of fingolimod was observed in treatment-naive patients (0.17 vs 0.46 with placebo) than in patients with a history of prior DMT (0.28 vs 0.53)Citation10,Citation11. Furthermore, fingolimod 0.5 mg significantly reduced the risk of 3-month confirmed disability progression compared with placebo (hazard ratio, 0.70 [95% CI = 0.52–0.96]; p = 0.02), significantly slowed MRI disease activity (2.5 vs 9.8 new or enlarged T2 lesions; p < 0.001), and preserved brain volume at 24 months (−0.84% vs −1.31%; p < 0.001)Citation11.
Few data are available for the PROs of treatment satisfaction, adverse events, or health-related quality-of-life (HRQoL) associated with fingolimod treatment to date. An exploratory analysis of a fingolimod phase 2 study demonstrated that patients treated with fingolimod had improved HRQoL and reduced symptoms of depression compared with those receiving placebo after 6 months of treatmentCitation12. Post-hoc analysis of the TRANSFORMS study data showed that, after 1 year of fingolimod treatment, patients experienced significantly less deterioration of their ability to perform daily activities compared with patients treated with IFNβ-1a IM, as measured by the activities scale of the Patient-Reported Indices for Multiple Sclerosis (PRIMUS-Activities)Citation13. However, there are currently no published data on PROs associated with switching therapy to fingolimod from other DMTs, and no data on patient satisfaction with fingolimod.
The study to Evaluate Patient Outcomes, Safety and Tolerability of Fingolimod (EPOC; NCT01216072) was the first phase 4 clinical trial conducted in the US after approval of fingolimod. EPOC aims to investigate PROs associated with switching therapy to fingolimod vs standard-of-care DMT in patients with relapsing MS who were previously treated with a DMT, testing the hypothesis that switching therapy to fingolimod improves PROs vs switching to standard-of-care DMT. Further objectives of the EPOC study are to evaluate the safety and tolerability of fingolimod after an immediate change from a previous DMT with no associated washout period. The EPOC study rationale and design, as well as the demographic and baseline disease characteristics of the enrolled study population, are presented here and compared with baseline data from phase 3 trials of fingolimod.
Methods
Study design
This is a 6-month, open-label, parallel-group, randomized multi-center study conducted at neurology centers in the US and Canada. The study was initiated as a phase 3b trial, then amended to be phase 4 after fingolimod approval by the Food and Drug Administration. Standard-of-care DMTs used in this study are IFNβ-1b 0.25 mg subcutaneously (SC) every other day, IFNβ-1a 30 µg IM once weekly, IFNβ-1a 22 or 44 µg SC 3-times weekly, or glatiramer acetate (GA) 20 mg SC once daily.
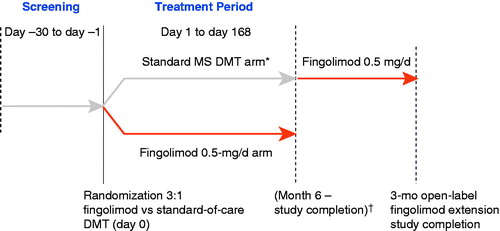
A 28-day screening period was followed by randomization (3:1) to fingolimod 0.5 mg once daily or standard-of-care DMT for 6 months (); randomization utilized an interactive voice response system. Patients randomly assigned to treatment with fingolimod changed from their pre-randomization DMT with no washout period. Patients randomized to the DMT group either remained on their pre-randomization DMT or changed to another DMT based on the investigator’s judgment. A 3-month extension was available for patients randomized to the DMT group who successfully completed all study visits; during the extension, patients switched to fingolimod 0.5 mg with no washout period.
Figure 1. Study design. * IFNβ-1b 0.25 mg SC every other day, IFNβ-1a 30 µg IM once weekly, IFNβ-1a 22 or 44 µg SC 3 times weekly, or glatiramer acetate 20 mg SC once daily. † Patients in the MS DMT arm who completed the study were eligible for a 3-month open-label fingolimod extension study. DMT, disease-modifying therapy; IFN, interferon; IM, intramuscular; MS, multiple sclerosis; SC, subcutaneous.
Study sites were selected based on historical participation in other Novartis MS trials and in conjunction with a feasibility questionnaire assessing the staff experience with MS research. Site visits were made to ensure that the staff was experienced with the MS indication and with Good Clinical Practice/International Conference on Harmonization guidelines. All sites received protocol training. Designated, trained staff at each site entered the data required by the protocol into the Electronic Case Report Forms. The site investigator certified the completeness and accuracy of the data. A contract research organization reviewed the data for completeness and accuracy, and was responsible for the statistical analysis of the data from the combined sites.
In accordance with the 2008 Declaration of Helsinki, the protocol and informed consent form was reviewed and approved by a properly constituted institutional review board/independent ethics committee/research ethics board before study start; informed consent was obtained from each patient at enrollment.
Patients
Eligible participants were men and women aged 18–65 years with relapsing forms of MS, as defined by 2005 revised McDonald criteriaCitation14, and an Expanded Disability Status Scale (EDSS) score of 0–5.5. Patients must have received a single, continuous MS DMT, excluding natalizumab, for ≥6 months before study initiation and be candidates for a change in therapy (therapy change from natalizumab to fingolimod will be assessed in a separate study). Candidacy for therapy change was determined by the treating physician. For Canadian patients, only those with the relapsing-remitting form of MS and who had an inadequate response to, or were unable to tolerate, one or more therapies for MS were eligible. Patients must have been naive to fingolimod treatment.
Patients were excluded if they had a history of any of the following: chronic immune system disease other than MS; immunodeficiency; malignancy other than localized basal cell carcinoma within the past 5 years; cardiac arrest, myocardial infarction, ischemic heart disease, or coronary spasm within 6 months; Mobitz type II second-degree heart block, third-degree atrioventricular block, or an increased QTc interval (>470 ms); bone marrow transplant; or alcohol abuse within the past 5 years. Further exclusion criteria at the time of screening were macular edema; active systemic infection; a negative test for varicella zoster immunoglobulin G antibodies; positive tests for hepatitis B, hepatitis C, or HIV; tuberculosis; uncontrolled diabetes; uncontrolled or poorly controlled hypertension or asthma; cardiac failure; severe respiratory disease or pulmonary fibrosis; and chronic liver or biliary disease. Patients were also excluded if they had been treated with the following medications: immunosuppressants, immunoglobulins, or monoclonal antibodies within 6 months before screening; any live or live attenuated vaccines within 1 month before screening; cladribine, cyclophosphamide, or mitoxantrone at any time; and class Ia or class III antiarrhythmic drugs at time of screening.
Assessments
Patient-reported outcomes
The primary study end-point is the change from baseline in patient-reported treatment satisfaction at 6 months in patients treated with fingolimod vs DMT as determined by the global satisfaction sub-scale of the Treatment Satisfaction Questionnaire for Medication (TSQM). Secondary PRO-specific end-points include the change from baseline in effectiveness, side-effects, and convenience sub-scales of TSQM, activities of daily living using PRIMUS-Activities, fatigue using the Fatigue Severity Scale (FSS), depression using the Beck Depression Inventory (BDI-II), and HRQoL using the Short Form Health Survey v2 standard (SF-36 v2 standard). An additional secondary end-point is the physician-reported Clinical Global Impression of Improvement (CGI-I). Key primary and secondary assessments are to be made according to the schedule in ; characteristics of each PRO instrument are listed in . PROs are assessed during the core treatment period only, not during the extension phase.
Table 1. Schedule of PRO and CGI-I assessments.
Table 2. PRO instrument characteristics.
Safety
Other secondary study end-points include the safety and tolerability of fingolimod immediately after changing from prior DMT and during the 6 months of ensuing treatment, monitored via adverse event (AE) reporting. Hematology and blood chemistry were assessed at baseline and at the 6-month or final study visit; serology was assessed at baseline. A standard 12-lead electrocardiogram was performed on all patients at screening; patients receiving fingolimod were monitored for 6 h after the first dose for signs and symptoms of bradycardia. MS relapse activity were assessed by neurologic examination and EDSS score, and relapse severity will be judged by the investigator. The lack of prior DMT washout in the study design necessitates the use of pooled historical fingolimod phase 3 clinical data to determine whether AEs were potentially fingolimod related or are carried over from prior DMT treatment. Patients participating in the extension phase were monitored for AEs, including disease relapses.
Statistical analysis
For the primary end-point, ∼1000 patients (fingolimod ∼750 patients; standard-of-care DMT ∼250 patients) will have 90% power to detect a significant difference between treatment groups in the change from baseline, assuming an effect size of 0.25, a significance level of 5%, and 10% rate of unevaluable patients. The primary variable will be analyzed by an analysis of covariance model with baseline TSQM global satisfaction sub-scale as a covariate and treatment group as a main effect. The least squares mean, least squares mean difference of the treatment groups, and 95% CI for the difference in the two treatment groups based on the fitted linear model will be reported. Missing data will be imputed using the last-observation-carried-forward method.
Results
Study enrollment for EPOC was completed October 27, 2011, with a total of 1053 patients in the US (n = 1032) and Canada (n = 21). Patient demographics and baseline disease characteristics are shown in . Enrolled patients were predominantly women (76.8%) and white (81.0%), with a mean age of 45.8 (SD 9.8) years. Patients had a mean duration of MS symptoms of 12.0 (SD = 8.5) years, mean 1.4 (SD = 2.0) relapses over the previous 2 years, and a mean baseline EDSS score of 2.4 (SD = 1.3) at enrollment. At screening, patients were treated with GA (33.7%), IFNβ-1a IM (25.4%), IFNβ-1a SC (24.7%), or IFNβ-1b (16.1%).
Table 3. Baseline demographics, disease characteristics, and MS treatment history of patients enrolled in the EPOC study.
Comparison with phase 3 study populations
The patient population enrolled in the EPOC study was generally older and had a longer duration of disease compared with populations from phase 3 studies of fingolimod (). Although baseline EDSS scores are similar between the EPOC patient population and those patients participating in the phase 3 development program, the EPOC population experienced fewer relapses in the previous 1–2 years. To gain insight on whether these differences were solely attributable to the protocol inclusion requirement for prior DMT treatment, the EPOC study population was compared with TRANSFORMS participants with a history of prior DMT treatment at any time. EPOC participants were still older, with longer disease duration and fewer relapses than DMT-experienced TRANSFORMS participants.
Table 4. Comparison of EPOC study population characteristics with phase 3 studies of fingolimod.
Discussion
EPOC is a phase 4, randomized, active-comparator, open-label, multi-center study designed to measure PROs associated with fingolimod treatment and the safety of switching from a standard-of-care DMT to fingolimod with no washout period. The EPOC study is unique in that it is powered to measure PRO end-points, with a primary end-point focused on treatment satisfaction, providing a patient’s perspective on therapy. Previous studies of the major currently available DMTs usually assessed PROs as secondary end-points and were, therefore, not powered to necessarily reveal the impact of DMT on such measuresCitation7. The primary variable is the global satisfaction scale of the TSQM which asks the question, ‘Taking all things into account, how satisfied or dissatisfied are you with this medication?’, and is rated on a 7-point response scale ranging from ‘extremely satisfied’ to ‘extremely dissatisfied’Citation15. Patient satisfaction affects patients’ health-related decisions and treatment-related behaviors, which may substantially affect treatment outcomesCitation15. Therefore, it is important to understand the level of patient satisfaction with a medication—as well as its clinical efficacy and safety—when assessing its overall effectiveness in practice.
Patients who enrolled in the EPOC study were not required to have had a recent relapse, allowing for inclusion of patients with less severe disease or those for whom prior DMT provided some clinical benefit. This aspect of the study design aimed to enroll a patient population that better reflected the ‘real world’ clinical population. EPOC participants were generally older and had a longer disease duration than in phase 3 studies. This is not solely attributable to the requirement for prior DMT treatment, as EPOC participants were also older and had longer disease duration than the sub-group of patients with prior DMT treatment from the TRANSFORMS study. Instead, the EPOC study population is likely to consist mainly of patients that did experience some clinical benefit from their prior DMT, demonstrated by the fact that they experienced fewer relapses in the 2 years preceding enrollment compared with the TRANSFORMS sub-group. Baseline EDSS scores for EPOC participants were also comparable to other study populationsCitation16,Citation17. The differences in patient age, disease duration, and recent relapse history between EPOC and other studies indicate that the EPOC population represents a segment of the MS population that is not typically captured in clinical trials.
A unique aspect of the EPOC study design is the focus on DMT switching. Few data have been published regarding the patient’s perspective on the switch from one DMT to another. Although reported rates of therapy switching vary widelyCitation18–24, studies agree that adverse effects (38–50%) and a perceived lack of efficacy (30–53%) are the most frequently cited reasons for therapy switching or discontinuationCitation18,Citation22,Citation23. These two complaints—adverse effects and lack of efficacy—are unique to each patient and drive adjustments in therapy and patient education. However, the increasing availability of new oral MS treatments will likely drive patients to consider therapy change for other reasons as well. For US patients, the EPOC inclusion requirement that a patient be a candidate for therapy change was given room for interpretation by the treating physician. This allowed for the recruitment of patients who were candidates for therapy change for a wide variety of reasons beyond lack of efficacy or poor tolerability. Thus, PRO data will help to guide treatment selection across a broad sample of patients with the aim of ultimately improving treatment adherence and, consequently, clinical outcomes. However, it should be noted that this inclusion criterion precludes the measurement of any perceived benefit of fingolimod in patients who were satisfied with their previous DMT.
In the TRANSFORMS study, patients in the fingolimod treatment groups achieved better results than those in the IFNβ1-a IM group with respect to clinical and MRI-related end-points. The EPOC study will provide additional comparative data on switch tolerability in more than 1000 patients and will extend beyond AE reporting to include aspects important to patient satisfaction and overall quality-of-life, including physical and emotional functioning in daily life. Additionally, this study will assess the safety of switching therapies without a washout period. Results of the EPOC study are anticipated in early 2013.
Related ongoing phase 4 trials of fingolimod in patients with MS include a study of disease control and safety in patients with relapsing remitting MS switching from natalizumab (NCT01499667) and an open-label, real-world health outcomes and safety study of fingolimod in patients with relapsing MS. An additional study will assess treatment retention for fingolimod vs other approved first-line DMTs.
Conclusions
The EPOC study will provide data to fill the gaps in our knowledge regarding patient experience, allowing evidence-based treatment selection that considers the patient perspective.
Transparency
Declaration of funding
Novartis Pharmaceuticals Corporation funded the study and editorial support for the preparation of this manuscript. All authors (MC, DW, LB, LP, LS, KM) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; were involved in drafting the manuscript and revising it critically for important intellectual content; and had final approval of the version to be published. Additionally, MC and DW were principal investigators who enrolled patients and followed them through study completion. LB was the internal medical director responsible for all medical activities in the study. LP and LS were the clinical personnel responsible for the execution of the day-to-day clinical activities. These activities included interaction with clinical site personnel (e.g., study investigators and co-ordinators) and ensuring data integrity was maintained. KM was the study statistician responsible for all statistical output for the study. The study sponsor conceived the study and participated in data collection, data analysis, and interpretation of the data; as well as writing of the manuscript. However, the decision to submit the manuscript to the Journal of Medical Economics and final approval of the manuscript was made solely by the authors.
Declaration of financial/other relationships
MC declares receiving research support, speakers’ fees, and/or consulting fees from Acorda Therapeutics, Bayer HealthCare Pharmaceuticals, Biogen Idec, EMD Serono, Genzyme, Novartis, Pfizer, Sanofi-Aventis, and Teva. DW declares receiving research support and/or consulting fees from Abbott Laboratories, Acorda Therapeutics, Allergan, Avanir Pharmaceuticals, Biogen Idec, Cephalon, Elan, Eli Lilly, EMD Serono, Facet Biotech, Genentech, Genzyme, GlaxoSmithKline, Hoffman LaRoche, National Institutes of Health, National MS Society, Novartis, Ono Pharmaceutical, Opexa Therapeutics, Pfizer, Questcor, Sanofi-Aventis, Teva, UCB, and XenoPort. LB, LS, LP, and KM declare receiving salary from Novartis.
Acknowledgments
Novartis Pharmaceuticals Corporation funded the study and editorial support for the preparation of this manuscript, which was provided by Valerie P. Zediak, PhD, and Erica S. Wehner, RPh, CMPP, from Complete Healthcare Communications, Inc.
References
- Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000;343:938–52
- Pike J, Jones E, Rajagopalan K, et al. Social and economic burden of walking and mobility problems in multiple sclerosis. BMC Neurol 2012;12:94
- Salter AR, Cutter GR, Tyry T, et al. Impact of loss of mobility on instrumental activities of daily living and socioeconomic status in patients with MS. Curr Med Res Opin 2010;26:493-500
- Dinan MA, Compton KL, Dhillon JK, et al. Use of patient-reported outcomes in randomized, double-blind, placebo-controlled clinical trials. Med Care 2011;49:415-9
- Doward LC, McKenna SP, Meads DM, et al. The development of patient-reported outcome indices for multiple sclerosis (PRIMUS). Mult Scler 2009;15:1092-102
- Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118:622-9
- Miller D, Rudick RA, Hutchinson M. Patient-centered outcomes: translating clinical efficacy into benefits on health-related quality of life. Neurology 2010;74(3 Suppl):S24-35
- Rudick RA, Miller DM. Health-related quality of life in multiple sclerosis: current evidence, measurement and effects of disease severity and treatment. CNS Drugs 2008;22:827-39
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Devonshire V, Havrdova E, Radue EW, et al. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 2012;11:420-8
- Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- Montalban X, Comi G, O'Connor P, et al. Oral fingolimod (FTY720) in relapsing multiple sclerosis: impact on health-related quality of life in a phase II study. Mult Scler 2011;17:1341-50
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod (FTY720) treatment improves the performance of daily activities compared with intramuscular interferon β-1a: Patient-Reported Indices for Multiple Sclerosis (PRIMUS)-Activities results from a phase III study (TRANSFORMS). Presented at: American Academy of Neurology 62nd Annual Meeting; April 10-17, 2010; Toronto, Canada
- Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005;58:840-6
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12
- Klawiter EC, Cross AH, Naismith RT. The present efficacy of multiple sclerosis therapeutics: is the new 66% just the old 33%? Neurology 2009;73:984-90
- Uitdehaag BM, Barkhof F, Coyle PK, et al. The changing face of multiple sclerosis clinical trial populations. Curr Med Res Opin 2011;27:1529-37
- Cunningham A, Gottberg K, von Koch L, et al. Non-adherence to interferon-beta therapy in Swedish patients with multiple sclerosis. Acta Neurol Scand 2010;121:154-60
- Milanese C, Beghi E, Giordano L, et al. A post-marketing study on immunomodulating treatments for relapsing-remitting multiple sclerosis in Lombardia: preliminary results. Neurol Sci 2005;26(4 Suppl):S171-3
- Portaccio E, Zipoli V, Siracusa G, et al. Switching to second-line therapies in interferon-beta-treated relapsing-remitting multiple sclerosis patients. Eur Neurol 2009;61:177-82
- Reynolds MW, Stephen R, Seaman C, et al. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin 2010;26:663-74
- Rio J, Porcel J, Tellez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler 2005;11:306-9
- Ruggieri RM, Settipani N, Viviano L, et al. Long-term interferon-beta treatment for multiple sclerosis. Neurol Sci 2003;24:361-4
- Wong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci 2011;38:429-33
- Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-3
- Moran PJ, Mohr DC. The validity of Beck Depression Inventory and Hamilton Rating Scale for Depression items in the assessment of depression among patients with multiple sclerosis. J Behav Med 2005;28:35-41
- Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130-9
- Jeffery D, Calabresi P, Goodin D, et al. Oral fingolimod (FTY720) vs placebo in relapsing-remitting multiple sclerosis (RRMS): baseline data from a 2-year phase III trial (FREEDOMS II). Presented at: The Consortium of Multiple Sclerosis Centers Annual Meeting; June 2-5, 2010; San Antonio, TX
