Abstract
Objective:
To report the design and preliminary results of a mirror-image study comparing total psychiatric hospitalisation rates pre- and post-switch to aripiprazole once-monthly, an extended release injectable solution.
Methods:
A multi-center, open-label mirror-image study of patients (18–65 years) with schizophrenia to compare total psychiatric hospitalisation rates between retrospective treatment with oral standard-of-care (SOC) anti-psychotics and prospective treatment with aripiprazole once-monthly in a naturalistic community setting in North America. Total psychiatric hospitalisation rates were assessed between retrospective (Months −4 to −1) and prospective treatment periods (Months 4–6) for patients who completed ≥3 months aripiprazole once-monthly.
Results:
One hundred and eighty-three patients entered the prospective phase. After switching to aripiprazole once-monthly, total psychiatric hospitalisation rates for the 3-month prospective period were significantly lower (p < 0.0001, Exact McNemar’s test) compared with the retrospective 3-month period when the same patients received SOC anti-psychotics (6.6% [n = 8/121] vs 28.1% [n = 34/121], respectively; rate ratio = 0.24). Similarly, total psychiatric hospitalisation rates for all patients who entered the prospective treatment phase were significantly lower (p < 0.0001, Exact McNemar’s test) for the prospective 6 months following switch to aripiprazole once-monthly, compared with the retrospective 6-month SOC period (14.2% [n = 26/183] vs 41.5% [n = 76/183], respectively; rate ratio = 0.34). Common treatment-emergent adverse events (occurring in ≥5% of patients) were psychotic disorder (7.7%), akathisia (7.2%), and insomnia (7.2%). Discontinuation (all causes) during the prospective phase was 44.8% (n = 82/183).
Limitations:
Mirror-image studies do not include a parallel active control; as each patient serves as their own control, it cannot be determined whether other treatments may have similar effects. Treatment and trial effects may be difficult to separate. Independent factors such as admission patterns, insurance coverage, availability of hospital beds, and community support may influence rates of hospitalisation.
Conclusions:
Switching to aripiprazole once-monthly substantially reduced total psychiatric hospitalisation rates compared with retrospective rates in the same patients taking oral SOC.
Introduction
The World Health Organisation (WHO) estimates that schizophrenia, depression, epilepsy, dementia, alcohol dependence, and other mental, neurological, and substance-use (MNS) disorders constitute 13% of the global burden of disease, surpassing both cardiovascular disease and cancerCitation1. The social and economic consequences of mental ill health are considerable, with the costs of treatment as high as, or potentially higher than, that for heart diseases, cancer, and diabetes combinedCitation2. Worldwide, schizophrenia affects ∼24 million people worldwideCitation3. The social and economic burden of schizophrenia is particularly high due to its chronicity, with frequent relapses and patients generally requiring lifelong treatment. Direct costs include those associated with inpatient (hospitalisation), outpatient, and long-term care, medication costs, as well as criminal justice costsCitation4. Indirect costs arise from the productivity loss suffered by individuals with schizophrenia, family members, and caregivers. As such, the total costs of treating schizophrenia are high. Indeed, studies in the US have estimated the overall (direct and indirect) costs attributed to schizophrenia to total $62.7 billion in 2002, with direct healthcare costs accounting for $22.7 billion of this sumCitation5. More recently, it has been reported that the estimated total cost of psychotic disorders in Europe was €93.9 billion in 2010Citation2.
Non-adherence to anti-psychotic medication is a known risk factor for relapse and hospitalisationCitation6, which contributes to the cost burden. Indeed, it has been estimated that ∼60% of patients are non-adherent to anti-psychotic medication early in treatment and early non-adherence predicts poor adherence laterCitation4. Early non-adherence is also associated with a greater number of hospitalisations, longer duration of hospitalisations, and increased cost of careCitation4. Gaps in medication adherence as brief as 1–10 days have been shown to be associated with a 2-fold increase in hospitalisation riskCitation7. Furthermore, it has been estimated that up to 50% of the direct medical costs of psychiatric hospitalisation can be attributed to non-adherence to anti-psychotic medicationCitation8. Strategies to reduce hospitalisation rates through improving medication adherence are a key focus for improving outcomes and reducing costs in patients with schizophreniaCitation9,Citation10. Long-acting injectable anti-psychotic medications have been reported to reduce relapse relative to oral agents, possibly by improving adherenceCitation11, and reduced re-hospitalisation rates in early-episode patients compared with use of oral formulations of the same compoundsCitation12.
A number of second-generation, long-acting injectable anti-psychotics are currently available for the treatment of schizophreniaCitation13. In addition, a long-acting intramuscular injectable formulation of the atypical anti-psychotic aripiprazole (aripiprazole once-monthly) has recently been approved for treatment of schizophrenia by the Food and Drug Administration in the USCitation14 and has been submitted for regulatory approval in the European Union. Aripiprazole once-monthly represents the first dopamine partial agonist available in the long-acting formulationCitation15. In a recent study of patients with schizophrenia requiring chronic treatment, aripiprazole once-monthly significantly delayed time to relapse compared with placeboCitation15. For patients initiating aripiprazole once-monthly, the US label recommends that patients continue treatment with oral aripiprazole or another oral anti-psychotic for 14 consecutive days to maintain therapeutic anti-psychotic concentrations during initiation of therapyCitation16. In the current study, total psychiatric hospitalisation rates were assessed in patients diagnosed with schizophrenia previously treated with oral standard-of-care (SOC) anti-psychotics, before and after prospective treatment with long-acting injectable aripiprazole, using a mirror-image study design (6 months pre- and post-initiation with aripiprazole once-monthly). Here, we report details of the study design as well as findings from a preliminary analysis.
Methods
Patients
Eligible patients were aged 18–65 years of age with a current diagnosis of schizophrenia as defined by the Diagnostic and Statistical Manual of Mental Health Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria, a history of illness of more than 1 year, and 7 months of hospitalisation data. Patients needed to have at least one inpatient psychiatric hospitalisation within 4 years (48 months) prior to screening, but have been managed as outpatients for the 4 weeks prior to signing the Informed Consent Form (ICF) and for the full duration of the screening period. Patients also needed to have been prescribed oral anti-psychotic treatment in the 7 months prior to screening. Eligible patients must also have been deemed, in the investigator’s judgment, to require a change in treatment for any reason (e.g., lack of efficacy, poor compliance, or side-effects) and have the potential to benefit from extended treatment with a long-acting injectable formulation. Subjects with a current DSM-IV-TR diagnosis other than schizophrenia, including schizoaffective disorder, major depressive disorder, bipolar disorder, delirium, dementia, and amnestic or other cognitive disorder, were excluded.
Study design
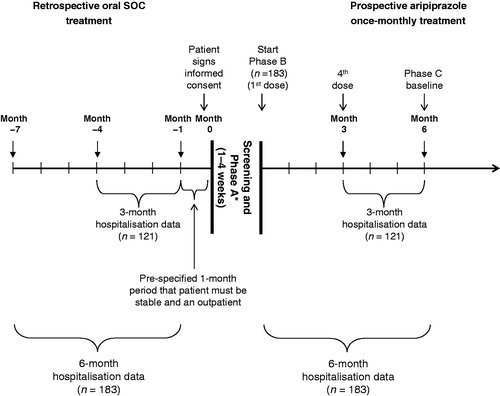
This phase IIIb, multi-center, open-label study used a mirror-image study design to assess hospitalisation rates in patients with schizophrenia treated retrospectively with oral SOC anti-psychotics (6 months) followed by prospective treatment with aripiprazole once-monthly (6 months) in a naturalistic community setting in North America. In accordance with the Declaration of Helsinki, the ethics committee at each site approved the protocol. The study is registered with ClinicalTrials.gov (Identifier: NCT01432444). The study commenced on October 13, 2011.
The study consisted of a prospective screening phase and three subsequent treatment phases. Eligibility was determined during the initial screening phase (2–28 days). Retrospective hospitalisation data were collected for the 7-month period prior to screening, allowing for a 4-week outpatient treatment period as well as 6 months of retrospective hospitalisation and intervention data (including psychiatric and non-psychiatric data). Patients meeting the inclusion/exclusion criteria, with no previous history of oral aripiprazole or those who had a previous history of aripiprazole but were receiving a different oral anti-psychotic at screening and the investigator assessed as prudent to cross-titrate, entered the first phase of the prospective treatment arm: the oral conversion phase (Phase A). In Phase A, patients not already receiving aripiprazole were cross-titrated to oral aripiprazole monotherapy (10–30 mg) over a period of 1–4 weeks based on investigator’s judgment. Any patients unable to tolerate oral aripiprazole or requiring inpatient psychiatric hospitalisation based on the investigator’s judgment were withdrawn from the study. Patients already receiving oral aripiprazole treatment or who had been treated with oral aripiprazole in Phase A or those with a history of aripiprazole whom the investigator considered not to require cross-titration entered a 6-month, open-label treatment phase (Phase B) during which they received 400 mg aripiprazole once-monthly, by intramuscular injection in the gluteal muscle, with an option to decrease to 300 mg for tolerability. Patients received concomitant oral aripiprazole (10–20 mg) for the first 14 days following the initial 400 mg dose of aripiprazole once-monthly. Patient visits were conducted at baseline (Week 0) and Weeks 1, 2, 4, and every 4 weeks thereafter up to 24 weeks during Phase B.
Patients who completed Phase B and whom the investigator believed would receive benefit from continued treatment with aripiprazole once-monthly were then eligible to enter Phase C. In this phase, patients continued to receive aripiprazole once-monthly (400 mg or 300 mg) every 28 (−2/+10) days.
End-points
Total psychiatric hospitalisation rates (proportion of patients with ≥1 psychiatric hospitalisation) were assessed between the retrospective oral anti-psychotic treatment period (i.e., Months −4 to −1), while on oral SOC anti-psychotic treatment and the prospective aripiprazole once-monthly treatment period (last 3 months [i.e., Months 4–6 in Phase B after initiation treatment with 400 mg aripiprazole once-monthly]) in patients treated with aripiprazole once-monthly for at least 3 months (see ).
Figure 1. Study design. * Patients who were already receiving oral aripiprazole treatment entered the open-label treatment phase (Phase B) without entering the oral conversion phase (Phase A). SOC, standard-of-care.
Other end-point comparisons between the retrospective and prospective period included the cumulative duration of inpatient psychiatric hospitalisations, mean duration of inpatient psychiatric hospitalisations, number, and mean duration of all other (non-inpatient) psychiatric treatment visits (including partial hospitalisations, intensive outpatient programs, assertive community treatment programs, emergency-room visits, and hospitalisations for psychosocial reasons), number of non-psychiatric hospitalisations per subject, cumulative duration of non-psychiatric hospitalisations, mean duration of non-psychiatric hospitalisations, and the number and mean duration of all other non-psychiatric treatment visits including, but not limited to, emergency-room visits.
Additional end-points assessed in Phase B included mean change from baseline to Week 24 in Positive and Negative Syndrome Scale (PANSS) total score and positive and negative sub-scale scores and Clinical Global Impressions–Severity (CGI-S) score; mean change in CGI–Improvement (CGI-I) score, all-cause discontinuation rate, time to discontinuation, and the proportion of responders (i.e., defined as ≥30% decrease from baseline in PANSS total score or a score of 1 [very much improved] or 2 [much improved] on the CGI-I scale). Subject-rated perception of treatment was evaluated and assessed using the Drug Attitude Inventory (DAI), Subjective Well-being under Neuroleptic Treatment scale–short version (SWN-S), and the Quality of Life Scale (QLS). Safety and tolerability were also systematically assessed during Phase B for patients receiving aripiprazole once-monthly. Safety variables included adverse events (AEs), clinical laboratory tests, vital signs, electrocardiograms, physical-examination findings, weight, height, body mass index, extrapyramidal symptoms, suicidality using the Columbia Suicide Severity Rating ScaleCitation17, investigator rating of injection-site reaction, and subject-reported visual analog scale score of injection-site pain.
Data analytic procedures and statistical analysis
The current analysis reports the total psychiatric hospitalisation rates at the cut-off date of the preliminary analysis (November 16, 2012). The analysis for the full Data set will be reported separately.
Hospitalisation rates were assessed for patients who received aripiprazole once-monthly for ≥3 months during Phase B. Differences between the proportions of total psychiatric hospitalisations in the retrospective oral anti-psychotic treatment period (Months −4 to −1) and the prospective aripiprazole once-monthly treatment period (Months 4–6 in Phase B) were assessed using a statistical test of significance at alpha level 0.0148 for the preliminary analysis. The alpha level was re-calculated using Lan-Demets alpha spending function with the property similar to the Pocock test, based on the fact that this analysis is conducted with ∼20% of the sample size originally planned at final analysis. Total psychiatric hospitalisations were considered to fall in the last 3 months of the retrospective or prospective period (see ) if hospitalised during this time or if hospitalisation was ongoing and overlapped with the start of the hospitalisation period.
Comparisons of total psychiatric hospitalisation rates were also made between the 6-month retrospective period (Months −7 to −1) with oral anti-psychotic treatment and all patients entering the 6-month prospective Phase B period (Months 1–6) following the switch to aripiprazole once-monthly.
A sensitivity analysis was conducted for the preliminary data set to assess the impact of potential discontinuation on the comparison of total psychiatric hospitalisation rates. This will also be conducted for the full data set. The analysis compared imputed total psychiatric hospitalisation rates between the 6-month retrospective and prospective Phase B periods for all patients who received treatment with aripiprazole once-monthly. In the sensitivity analysis, patients who discontinued from Phase B due to psychiatric AEs (with the exclusion of discontinuation due to insomnia) or lack of efficacy were considered as having one imputed total psychiatric hospitalisation during the 6-month prospective period.
Exact McNemar’s test was performed for the analysis of hospitalisation rates pre- and post-initiation of aripiprazole once-monthly for the preliminary results (reported here) and for the final results. In addition, a Chi-squared test will be performed on the final analysis. The rate ratio was determined by the ratio of rates for pre- and post-switch to aripiprazole once-monthly.
Results
Patients
At the first data cut-off point, 183 patients had entered Phase B and either completed or discontinued treatment. Of these, 104 patients entered Phase B from Phase A having received treatment in the oral conversion phase. The remaining 79 patients entered directly into Phase B. A total of 181 patients initiated treatment with aripiprazole once-monthly; two patients entered Phase B but did not receive treatment. Neither of these patients was hospitalised. Demographics and baseline characteristics for the 181 patients that received treatment with aripiprazole once-monthly are shown in . Patient disposition and reasons for discontinuation for all patients entering Phase B are shown in . Disease severity at baseline was moderate (CGI-S scale 3.9 ± 0.8 for the patients receiving treatment with aripiprazole once-monthly in Phase B). Eighty-nine per cent of patients (n = 161/181) started and continued on 400 mg aripiprazole once-monthly. Of the remaining patients, 7.2% (n = 13) had their dose decreased to 300 mg, 3.3% (n = 6) decreased and then increased the dose, and 0.5% (n = 1, protocol violation) started on 300 mg aripiprazole once-monthly.
Table 1. Demographics and baseline characteristics for all patients that received treatment in Phase B (n = 181).
Table 2. Patient disposition and reasons for discontinuation.
In total, results of the 183 patients who had either completed or discontinued in Phase B are reported here. There were another 115 patients still ongoing in Phase B at the data cut-off point and their data were not included in this report; these data will be reported in the full data set analysis.
Total psychiatric hospitalisation rates
Hospitalisation rates were calculated for 121 patients who had completed ≥3 months’ treatment in Phase B. The rates for total psychiatric hospitalisation were significantly lower (p < 0.0001, Exact McNemar’s test) following the switch to aripiprazole once-monthly in Phase B (Months 4–6) compared with the 3-month retrospective treatment period (Months −4 to −1) when the same patients were treated with oral anti-psychotics (6.6% [n = 8/121] vs 28.1% [n = 34/121]; respectively; rate ratio = 0.24) (). The discordant rates of total psychiatric hospitalisation, upon which the McNemar test is conducted, are presented in .
Figure 2. Total psychiatric hospitalisation rates following the switch to aripiprazole once-monthly (prospective) compared with the same patients treated with oral anti-psychotics (retrospective). p-value derived from Exact McNemar test.
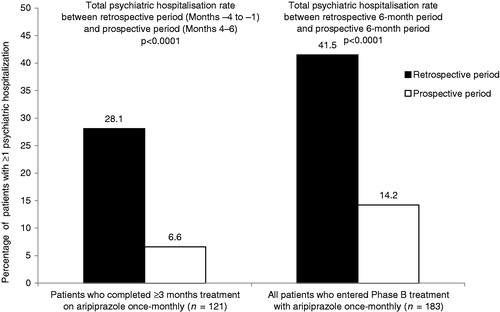
Table 3. Analysis of total psychiatric hospitalisation rates following the switch to aripiprazole once-monthly (prospective) compared with the same patients treated with oral anti-psychotics (retrospective) between retrospective period Months −4 to −1 and prospective period Months 4–6 and between retrospective period Months −7 to −1 and prospective period Months 1–6.
Similarly, rates of total psychiatric hospitalisation for all patients who entered Phase B (n = 183) were significantly lower (p < 0.0001, Exact McNemar’s test) for Months 1–6 following the switch to aripiprazole once-monthly compared with the retrospective 6-month period (Months −7 to −1) when the same patients were treated with oral anti-psychotics (14.2% [n = 26/183] vs 41.5% [n = 76/183], respectively; rate ratio = 0.34) (). Discordant rates of total psychiatric hospitalisation for the 6 months in the prospective period only and retrospective period only also are presented in . Data for total psychiatric hospitalisation rates were imputed for 23 patients in the sensitivity analysis. Results of sensitivity analysis (using the imputed total psychiatric hospitalisation rates) for Months 1–6 following the switch to aripiprazole once-monthly compared with the retrospective 6-month period (Months −7 to −1) showed significant reductions (p < 0.0001) in total psychiatric hospitalisations following the switch to aripiprazole once-monthly (19.7% [n = 36/183] vs 41.5% [n = 76/183], respectively, rate ratio = 0.47). The discordant rate of total psychiatric hospitalisations using the same sensitivity analysis also showed significant reductions prospectively (8.7%, n = 16/183) compared with the retrospective rate (30.6%, n = 56/183 retrospective; p < 0.0001, rate ratio = 0.29).
Safety
Of the 183 patients who either completed or discontinued the study (Phase B) at data cut-off time-point, 181 patients received at least one dose of aripiprazole once-monthly and were evaluable for safety. During oral conversion (Phase A), 8.1% (n = 12/148) of patients discontinued due to AEs. Of these, nine patients were categorised as experiencing psychiatric disorders (including worsening of psychoses, increased paranoia, agitation, anxiety, decreased self-care, insomnia, accidental overdose, and a suicide attempt) and three patients had nervous-system disorders (akathisia). All-cause discontinuations during the prospective Phase B were 44.8% (n = 82/183). In Phase B, 26 patients discontinued due to AEs. The most common AEs (by system organ class) leading to discontinuation included: psychiatric disorders (11.0%; n = 20) and nervous-system disorders (1.1%; n = 2); four other AEs leading to discontinuation occurred in four different system organ classes and were not considered causally related to study treatment. Reasons for withdrawal of consent were not associated with AEs.
The incidence of treatment-emergent AEs (TEAEs) during the 6-month treatment period with aripiprazole once-monthly is reported in . For all patients receiving at least one dose of aripiprazole once-monthly (n = 181), the most common TEAEs (occurring in ≥5% of patients) were psychotic disorder (7.7%), akathisia (7.2%), insomnia (7.2%), paranoid schizophrenia (5.5%), back pain (5%), and schizophrenia (5%). Rates of AE reporting were highest (38.1%) during the first month of treatment and decreased in subsequent months, with 18.3% reported in Month 2, and 6.5% in Month 6. The majority (93%) of AEs reported were mild or moderate in intensity. Overall, 36 patients (19.9%) receiving aripiprazole once-monthly experienced serious TEAEs during treatment; all AEs that were experienced by more than one patient were categorised as psychiatric disorders.
Table 4. Incidence of treatment-emergent adverse events occurring in ≥2% of all patients treated in Phase B (n = 181).
Discussion
Results of the preliminary analysis of the present study show the benefits of aripiprazole once-monthly in terms of reducing total psychiatric hospitalisations compared with the retrospective phase where patients were treated with oral SOC anti-psychotics. Also notable, the AEs were in line with historical data on oral aripiprazole. A reduction in hospitalisation is likely to translate to a reduction in healthcare resource utilisation and may, therefore, impact on the overall cost-effectiveness of aripiprazole once-monthly in the maintenance treatment of patients with schizophrenia. The potential for cost savings is supported by a recent study comparing healthcare resource usage and costs before and after initiating long-acting injectable anti-psychotics among Medicaid-insured patients with schizophreniaCitation18. Reductions in the mean number of hospitalisations and hospital lengths of stay were associated with the initiation of long-acting anti-psychotic therapy; and, as a result, annualised hospital payments were much lower (all cause: $16,249 ± $36,404 vs $7380 ± $21,087, p < 0.001; schizophrenia-related: $13,388 ± $31,614 vs $5645 ± $15,767, p < 0.001)Citation18. Similar analyses have confirmed that patients initiating long-acting injectable anti-psychotics incur less healthcare costs in comparison to patients initiating oral anti-psychoticsCitation19. Therefore, the greater initial acquisition costs of long-acting injectable anti-psychotics compared with their oral formulations is likely to be offset by reduced health-resource utilisationCitation19–22.
Although preliminary, the results presented here support findings from other studies demonstrating that switching from oral medication to a long-acting injectable anti-psychotic leads to reduced hospital admissions and inpatient resource useCitation23–29. Indeed, results from a recent systematic review and meta-analysis of mirror-image studies following patients eligible for long-acting injectable anti-psychotics for at least 12 months (at least 6 months on each oral anti-psychotic and long-acting injectable) showed superiority of the long-acting injectable treatments compared with oral anti-psychotics in delaying time to hospitalisation (rate ratio = 0.4) as well as decreasing the number of hospitalisationsCitation29.
This mirror-image study was designed with a prospective treatment phase, which enabled the analysis of the incidence of AEs following the switch to aripiprazole once-monthly. Discontinuations due to AEs and TEAEs were different in this study than in previously reported, randomised controlled trials of aripiprazole once-monthlyCitation15,Citation30, perhaps due to the open-label, naturalistic design with only monthly treatment visits. Treatment-emergent psychiatric AEs and discontinuations due to psychiatric AEs perhaps reflect the inherent risk of emerging psychiatric symptoms when switching to any other anti-psychotic medication, as documented in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studyCitation31. Moreover, the sensitivity analysis accounting for psychiatric AEs leading to hospitalisation did not alter the study findings and found significantly lower rate ratios similar to those of the main efficacy analysis.
The preliminary findings of this study need to be considered in the context of several limitations. The use of a mirror-image study design does not include a parallel active control group; instead, each patient serves as their own control. As a result, it cannot be determined whether other treatments may have had similar effects. An additional limitation of a mirror design is that it is difficult to separate a drug treatment effect from a trial effect. Results from mirror-image studies may also be influenced by independent factors such as admission patterns, insurance coverage, availability of hospital beds, and availability of community support. Since the study is not blinded, it is unknown what influence the study design had on the clinical decision to hospitalise or not to hospitalise any given patient. Finally, the study was only designed to switch patients from oral to long-acting injectable anti-psychotic medication and data on switching from long-acting injectable anti-psychotic to oral medication is not available for comparison.
The final analysis of the complete data set will add to the preliminary findings reported here, providing data from a larger patient group, with a greater variety of prior anti-psychotic experience, and additional end-points including both psychiatric and non-psychiatric hospitalisation rates, duration of hospitalisation, effects on psychopathology based on PANSS, and CGI-S. The larger sample will also allow a better understanding of the safety and tolerability profile in a naturalistic environment.
Conclusion
In the current preliminary analysis, a switch from SOC oral anti-psychotics to aripiprazole once-monthly reduced total psychiatric hospitalisation rates. Substantially reduced rates of total psychiatric hospitalisation suggest aripiprazole once-monthly may offer significant cost savings to the healthcare system.
Transparency
Declaration of funding
This study was supported by Otsuka Pharmaceutical Development & Commercialization, Inc. and H. Lundbeck A/S.
Declaration of financial/other relationships
JMK has received honoraria for lectures and/or consulting from Alkermes, Amgen, Bristol-Myers Squibb, Cephalon, Eisai, Boehringer Ingelheim, Eli Lilly, Genentech, Intracellular Therapeutics, Janssen, Johnson and Johnson, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Pierre Fabre, Proteus, Roche, Sunovion and Targacept. He is a shareholder of MedAvante. RAB, ARD, BRJ, RMQ TP-S, RS, and JZ are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, NJ). AE is an employee of H. Lundbeck A/S (Deerfield, IL).
Acknowledgments
Editorial support for the preparation of this manuscript was provided by Dr Marion James and Dr Suzanne Patel at Ogilvy Healthworld Medical Education; funding was provided by Otsuka Pharmaceutical Development & Commercialization, Inc. and H. Lundbeck A/S.
Clinical trial registration
NCT01432444. Registry: ClinicalTrials.gov
Previous presentations
This data has been presentation at the Annual Meeting of the American Psychiatric Association 2013 and the New Research Approaches for Mental Health Interventions Annual Meeting 2013.
References
- Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature 2011;475:27-30
- Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:718-79
- World Health Organization. WHO. http://www.who.int/mental_health/management/schizophrenia/en. Accessed 2012
- Offord S, Lin J, Mirski D, et al. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther 2013;30:286-97
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Novick D, Haro JM, Suarez D, et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010;176:109-13
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatric Serv 2004;55:886-91
- Weiden P, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull 1995;21:419-29
- Marcus S, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull 2008;34:173-80
- Keith S. Advances in psychotropic formulations. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:996-1008
- Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia—A critical systematic review and meta-analysis of randomised long-term trials. Schizophrenia Res 2011;127:83-92
- Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011;168:603-9
- De Berardis D, Marini S, Carano A, et al. Efficacy and safety of long acting injectable atypical antipsychotics: a review. Current Clin Pharmacol 2013:[Epub ahead of print]
- FDA. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
- Kane J, Sanchez R, Perry P, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012;73:617-24
- Otsuka America Pharmaceuticals Inc. Abilify Maintena Prescribing Information. 2013. http://www.otsuka-us.com/Products/Documents/Abilify.M.PI.pdf
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266-77
- Bera R, Offord S, Zubek D, et al. Impact on healthcare resource usage and costs among Medicaid-insured schizophrenia patients after initiation of treatment with long-acting injectable antipsychotics. J Med Econ 2013;16:522-8
- Lin J, Wong B, Offord S, et al. Healthcare cost reductions associated with the use of lai formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Rers 2013. [Epub ahead of print]
- De Graeve D, Smet A, Mehnert A, et al. Long-acting risperidone compared with oral olanzapine and haloperidol depot in schizophrenia: a Belgian cost-effectiveness analysis. Pharmacoeconomics 2005;23(1 Suppl):35-47
- Edwards NC, Locklear JC, Rupnow MF, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics 2005;23(1 Suppl):75-89
- Olivares JM, Rodriguez-Martinez A, Buron JA, et al. Cost-effectiveness analysis of switching antipsychotic medication to long-acting injectable risperidone in patients with schizophrenia: a 12- and 24-month follow-up from the e-STAR database in Spain. Appl Health Econ Health Policy 2008;6:41-53
- Haddad P, Taylor M, Niaz OS. First-generation antipsychotic long-acting injections v. oral antipsychotics in schizophrenia: systematic review of randomised controlled trials and observational studies. Br J Psychiatry 2009;52:S20-8
- Asseburg C, Willis M, Lothgren M, et al. Hospitalisation utilisation and costs in schizophrenia patients in finland before and after initiation of risperidone long-acting injection. Schizophr Res Treat 2012;2012:791468 . doi: 10.1155/2012/791468
- Carswell C, Wheeler A, Vanderpyl J, et al. Comparative effectiveness of long-acting risperidone in New Zealand: a report of resource utilization and costs in a 12-month mirror-image analysis. Clin Drug Investig 2010;30:777-87
- Spill B, Konoppa S, Kissling W, et al. Long-term observation of patients successfully switched to risperidone long-acting injectable: a retrospective, naturalistic 18-month mirror-image study of hospitalization rates and therapy costs. Int J Psychiatry Clin Pract 2010;14:53-62
- Fuller M, Shermock K, Russo P, et al. Hospitalisation and resource utilisation in patients with schizophrenia following initiation of risperidone long-acting therapy in the Veterans Affairs Healthcare System. J Med Econ 2009;12:317-24
- Peng X, Ascher-Svanum H, Faries D, et al. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res 2011;3:9-14
- Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull [Epub ahead of print]
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for the treatment of schizophrenia: a double-blind, randomized, non-inferiority study vs. oral aripiprazole. 51st Annual Meeting of the American College of Neuropsychopharmacology (ACNP); 2-6 December 2012; Hollywood, FL
- Essock S, Covell N, Davis S, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5