Abstract
Objective:
Compare long-term costs and outcomes of lurasidone to aripiprazole among adults with schizophrenia in the US who previously failed ≥1 atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) based on an indirect comparison of outcomes data from clinical trials.
Methods:
A 5-year Markov cohort model was developed to compare long-term effectiveness of lurasidone to aripiprazole, including total discontinuations, relapse rates, and hospitalization rates. Cost inputs included pharmacy, mental health, and medical costs associated with cardiometabolic risks (diabetes and cardiovascular [CV] events). Effectiveness inputs were derived from an indirect comparison of aripiprazole and lurasidone using common comparators from CATIE. Cardiometabolic risks were derived from claims data analysis for diabetes, weight change and CV events, and Framingham body mass index (BMI) risk equation. Cost inputs were derived from published sources and Red Book. Costs and outcomes were discounted at 3% and tested with sensitivity analyses.
Results:
Over 5 years, total discounted costs for lurasidone and aripiprazole patients were $86,480 and $90,500, respectively. During this period, the number of relapses per patient, hospitalizations per patient, diabetes rates, and CV events per 1000 patients, respectively, were estimated to be lower for lurasidone (0.442, 0.245, 7.29%, and 37.3) than aripiprazole (0.478, 0.369, 7.36%, and 37.8). Results were sensitive to lurasidone and aripiprazole hospitalization rates. At a willingness-to-pay threshold of $50,000 per hospitalization avoided, lurasidone had a 100% probability of being more cost-effective than aripiprazole.
Limitations:
The model was based on results from various comparative clinical trials. Differences in patient population and study methods may change estimates from the model. The model does not account for patient heterogeneity.
Conclusions:
Based on this model, when switching from another atypical antipsychotic, lurasidone had fewer relapses and hospitalizations with a lower incidence of diabetes and CV events than aripiprazole. Additionally, lurasidone may be less costly than aripiprazole among adults with schizophrenia.
Introduction
Schizophrenia is a chronic and disabling disorder with a prevalence of 0.51% in the USCitation1. Schizophrenia imposes a significant burden on patients, caregivers, and society, resulting in an estimated total annual excess cost of $62.7 billion (2002) in the USCitation2,Citation3. The indirect costs comprised $32.4 billion (of which $21.6 billion was due to unemployment), direct non-healthcare costs comprised $7.6 billion (including living costs offset), and direct healthcare costs comprised $22.7 billion ($6.9 billion outpatient, $5.0 billion drugs, $2.8 billion inpatient, $8.0 million long-term care) of the total $62.7 billionCitation3.
Schizophrenia remains one of the most challenging diseases to treat due to high morbidity and mortality, its variable nature, the heterogeneity of clinical response, and the side-effects of treatmentCitation4,Citation5. Atypical antipsychotics are standard of care for schizophrenia and are generally used as initial therapy because typical antipsychotic agents (first-generation antipsychotics) are associated with a high incidence of adverse events (AEs), including sedation, weight gain, sexual dysfunction, movement disorders, and tardive dyskinesiaCitation4,Citation6–8. However, some atypical antipsychotics are also associated with metabolic effects, such as weight gain, hyperglycemia, insulin resistance, and lipid abnormalities. The American Diabetes Association (ADA) Consensus on Antipsychotic Drugs and Obesity and Diabetes recognizes that certain atypical antipsychotic agents are also associated with increased risk of developing metabolic syndrome, new onset diabetes, and cardiovascular (CV) diseaseCitation9. It has been reported that the prevalence of metabolic syndrome and diabetes in patients taking atypical antipsychotics is ∼2-times that of the general populationCitation10,Citation11. In addition, patients on atypical antipsychotics have been found to be 9% more likely to develop diabetes than those taking conventional antipsychoticsCitation12,Citation13. Metabolic side-effects of atypical antipsychotics, especially weight gain, may contribute to premature treatment discontinuation and poor adherenceCitation14,Citation15, which can lead to symptom worsening, relapse, and greater healthcare resource utilizationCitation16,Citation17. The cardiometabolic consequences of treatment with atypical antipsychotics may increase mortality in individuals with schizophreniaCitation18.
Lurasidone is an atypical antipsychotic FDA approved for adults with schizophrenia in October 2010Citation1Citation9. The efficacy of lurasidone was established in five 6-week, multi-center, double-blind, randomized, fixed-dose, placebo- and active-controlled studiesCitation19–24. Furthermore, a large, 1-year, double-blind, randomized, non-inferiority study of lurasidone vs quetiapine XR demonstrated that lurasidone was associated with fewer relapses and significantly fewer relapse-related hospitalizationsCitation25. The safety of lurasidone has been previously demonstrated in multiple short-term and longer-term clinical studiesCitation19–24. Treatment with lurasidone has been associated with minimal mean changes in weight, body mass index, and metabolic outcomes (lipids and glycemic control)Citation19–24.
There is a need among decision-makers for comprehensive clinical and economic evaluations of available drugs for schizophrenia since the costs of treatment, complications, and relapses can be significant. Antipsychotics are one of Medicaid’s most commonly prescribed drug classes, and management strategies are being used by Medicaid programs more frequently in recent years to control the use of atypical antipsychoticsCitation26.
The potential for lowering the risk for CV diseases and reducing relapses and hospitalizations provides an important basis to compare lurasidone to aripiprazole. The objective of this model was to assess the cost-effectiveness of lurasidone compared to aripiprazole, following a switch from one of the generic atypical antipsychotics, in the treatment of patients with schizophrenia from a US payer perspective. The cost-effectiveness model compared the cardiometabolic profile, relapses, and hospitalizations for lurasidone to aripiprazole.
Patients and methods
Model design
An economic model was developed in Microsoft Excel to assess the cost-effectiveness of lurasidone and aripiprazole for treating adult patients with schizophrenia. The cost-effectiveness analysis was conducted over a 5-year time horizon from a third-party payer perspective in the US. Costs included in the analysis were pharmacy, mental health, diabetes management, and CV event-related costs, inflated to 2012 US dollars using the Medical Care Component of the Bureau of Labor Statistics’ Consumer Price IndexCitation27. A standard discount rate of 3% was used for both costs and outcomes.
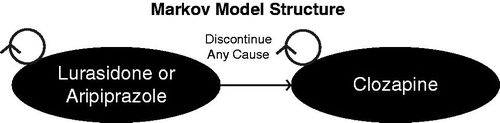
Costs and outcomes associated with lurasidone and aripiprazole were modeled using a Markov cohort analysis, which allows for a percentage of a hypothetical cohort to transition between a mutually exclusive set of states. Patients in the modeled cohort initiated treatment on the model comparator (Drug 1; lurasidone or aripiprazole) after failing a generic atypical antipsychotic. Patients failing a generic antipsychotic were defined as patients treated with generic atypical antipsychotics (olanzapine, risperidone, quetiapine, or ziprasidone) that were discontinued due to lack of efficacy or adverse event. Upon discontinuing lurasidone or aripiprazole for any cause (e.g., AE or lack of efficacy), patients were switched to clozapine and remained on clozapine for the duration of the analysis ().
The model outcomes included relapses and hospitalizations. For each outcome, the per-patient mean value was estimated over the 5-year time horizon. The incremental cost-effectiveness ratios (ICERs) were calculated as the difference in cost divided by the difference in outcome between the comparator agents. One-way and probabilistic sensitivity analyses were also conducted to test model robustness.
Model inputs
Patient characteristics
The population for the model included adult patients diagnosed with schizophrenia. Patient characteristics for the base case scenario were specified to reflect the average schizophrenia patient enrolled in the lurasidone clinical trialsCitation29: male (73% of patients were male), 38 years old, weight of 77.3 kg, BMI of 26.3, with a mean of 192 mg/dL total cholesterol, 48 mg/dL high-density lipoprotein (HDL), and 120 mmHg systolic blood pressure (SBP). In addition, based on the trial data, 5.5% of patients in the model were assumed to have diabetes and 67% to be smokers. In the model, patient sex, HDL, SBP, and smoking were static and did not change over time. Patient age, total cholesterol, and diabetes were adjusted based on time (for age), therapy (for diabetes), and time on therapy (for total cholesterol, weight, and BMI). Patient characteristics were used to estimate the risk of CV events in the model based on Framingham risk equationsCitation30.
Effectiveness parameters
Annual transition probabilities for total discontinuation, discontinuation due to lack of efficacy, and hospitalization were based on rates from published studies (). Since lurasidone and aripiprazole have not been compared in a direct head-to-head clinical trial, an adjusted indirect treatment comparison using a common comparator was used to obtain model inputsCitation31,Citation32. Available long-term head-to-head schizophrenia studies for lurasidone include a 52-week comparison with quetiapine XRCitation25 and a 12-month comparison with risperidoneCitation33. Available long-term head-to-head schizophrenia studies for aripiprazole include a 52-week maintenance study vs oral haloperidolCitation34 and a 52-week comparison with olanzapineCitation35. As required studies to obtain an indirect comparison in a single step (A-B from A-C and C-B) were not available, we conducted a multi-step indirect comparison (A-C, C-D, D-B) using an additional study. Both quetiapine and olanzapine were included in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE); hence, we used the CATIE phase 1 study as the intermediaryCitation14.
Table 1. Discontinuation and hospital rates.
The 12-month safety and maintenance of efficacy for flexibly dosed lurasidone vs quetiapine XR in chronic schizophrenia was evaluated in a randomized, double-blind, parallel-group study with 151 patients randomized to lurasidone and 85 patients randomized to quetiapine XRCitation25. Discontinuation was defined as dismissal from the study as a result of non-compliance to treatment regimen or evaluations, pregnancy during study, insufficient clinical response, intolerable AEs, protocol violation, withdrawal of consent, or patient was lost to follow-up. Hospitalization was defined in the study as a hospital admission that was not pre-planned for other therapeutic purposes. Hospitalization and discontinuation data for lurasidone were obtained directly from this study. The hazard ratio for lurasidone vs quetiapine XR from this study was used to estimate the annual discontinuation rates of lurasidone vs other atypical antipsychotics studied in CATIE.
Chrzanowski et al.Citation35 conducted a 52-week, open-label extension to a 26-week comparison of aripiprazole with olanzapine in 214 patients with acute relapsing and chronic schizophrenia. This study was conducted to determine the long-term efficacy of aripiprazole and olanzapine in patients who either completed the original 26-week study or met the definition of relapse after >2 weeks of treatment. In the Chrzanowski et al. study, discontinuation was defined as withdrawal from the study due to intolerable AEs, lack of efficacy, withdrawn consent from patient, death, or other known cause such as patient lost to follow-up. Hospitalization was not measured in this study. Discontinuation data for aripiprazole were obtained directly from this study. As no hospitalization rate was available for aripiprazole, the estimation of this rate was based on the relative risk of lack of efficacy for aripiprazole in Chrzanowski et al. and the hospitalization rate for olanzapine from the CATIE trial. All-cause discontinuations and discontinuations due to lack of efficacy for aripiprazole vs olanzapine were used to estimate the annual discontinuation rates of aripiprazole vs other CATIE atypical antipsychotics.
CATIE was a randomized, double-blind study of four atypical antipsychotics (olanzapine, quetiapine, risperidone, and ziprasidone) and one first-generation antipsychotic (perphenazine). Patients with schizophrenia were randomized to one of the five treatments and followed for 18 months or until discontinuation of therapy. The study measured AEs and discontinuation rates among the different antipsychotics treatmentsCitation14. In the CATIE study, discontinuation was defined as all-cause discontinuation of study medications. Discontinuations were also categorized into discontinuations due to inadequate therapeutic effect, unacceptable side-effects, patient inability or refusal to take the assigned antipsychotic, and administrative reasonsCitation36. Hospitalization was defined as hospital admission for an exacerbation of schizophreniaCitation14.
The relative risk for all-cause discontinuation for aripiprazole vs olanzapine was 1.349, and the relative risk of discontinuation due to lack of efficacy was 1.851Citation3Citation5. Then the rates of total discontinuation and discontinuation due to lack of efficacy of olanzapine from CATIECitation14 were multiplied by these relative risks to obtain estimates of the discontinuation rates for aripiprazole. As no hospitalization rates were included in Chrzanowski et al.Citation35, the hospitalization rate for aripiprazole was estimated based on the relative risk of lack of efficacy for aripiprazole and the hospitalization rate for olanzapine from the CATIE trial. Similarly, in the 12-month double-blind, parallel-group study evaluating lurasidone vs quetiapine XR, lurasidone was found to have a relative risk of discontinuation for any cause of 0.787, a relative risk of discontinuation due to lack of efficacy of 0.728, and a relative risk of hospitalization of 0.404 vs quetiapine XR. The respective rates for lurasidone were, therefore, based on the rates for quetiapine from the CATIE trial and these relative risks. Standard errors for the transition probabilities were calculated based on the proportions and sample sizes from the published studies.
Cardiometabolic parameters
The model structure also incorporated the costs and outcomes of cardiometabolic consequences of treatment with lurasidone and aripiprazole associated with weight gain, an increase in lipid levels, and a higher risk of diabetesCitation37. As such, cardiometabolic parameters in the model included annual weight change (kg/year), annual cholesterol change (mg/dL/year), and diabetes relative risk ().
Table 2. Cardiometabolic parameters.
The model incorporated these risk factors by utilizing data from comparative clinical trialsCitation25,Citation35 of the rate of weight gain and lipid increase of the atypical antipsychotics and data from a large retrospective analysis of the risk of diabetesCitation38. In order to track the time on therapy to estimate the amount of weight gain and lipid increase, time-dependent sub-states based on the time a segment of the cohort initiates a new therapy were incorporated into the cohort analysisCitation39. For each annual cycle in the model, the patient cohort age increases, baseline weight is adjusted by time on atypical antipsychotic therapy, total cholesterol is adjusted by time on atypical antipsychotic therapy, and diabetes is adjusted by the relative risk based on the atypical antipsychotic. These adjusted risk factors were then applied to the Framingham 10-year CV risk profile using the BMI Framingham risk equationCitation30, and the 10-year risks were adjusted to 1-year risks to calculate the expected number of annual CV disease events based on the cohort risk factors. According to the BMI equation, CV risk is a function of age, BMI, untreated SBP, treated SBP, smoking, and diabetes.
Mortality rates
Published age- and sex-specific mortality tables were used to determine patient mortality over the 5-year time horizonCitation40. The mortality risk due to CV events and suicide was estimated separately in the model; therefore, the population mortality rates were adjusted to exclude the increased mortality risks associated with suicide and CV disease among patients with schizophrenia. Patient suicides were calculated based on a rate of 579 per 100,000 patient years from a published systematic review of suicide ratesCitation41. CV disease mortality was estimated by multiplying the number of patients experiencing a CV event by a fatal CV event rate of 9.5%Citation42.
Drug costs and resource utilization
Annual drug costs were estimated based on the wholesale acquisition cost (WAC) of lurasidone, aripiprazole, and clozapine as reported in Red Book as of October 23, 2012Citation43. Annual costs for lurasidone were based on patient utilization from a 12-month, multi-center, double-blind, parallel-group study of flexibly dosed lurasidone (40–160 mg/day)Citation25, in which 15% of patients received a dose of 40 or 80 mg and 85% of patients received a dose of 120 or 160 mg for a mean daily dose of 124 mg. The reported mean daily dose of aripiprazole (22 mg) and clozapine (332 mg) was used to estimate the annual drug costs ().
Table 3. Annual treatment costs and resource utilization.
Resource utilization costs were obtained from the published literature (). The annual psychiatric care costs were obtained from a published prospective, observational, non-interventional study of schizophrenia in the US, comparing patients with and without a relapseCitation44. This study included the following cost components: costs of medications (antipsychotics, other psychotropics, such as mood stabilizers, anticholinergics, antidepressants, anti-anxiety, and sleep agents), psychiatric hospitalizations, day treatment, emergency services, psychosocial group therapy, medication management, individual therapy, and assertive community treatment (ACT)/case management. Costs for patients with a relapse and a psychiatric hospitalization vs those with a relapse and no psychiatric hospitalization were differentiated by subtracting the hospitalization costs from the former group.
The annual attributable costs of diabetes management were obtained from a published studyCitation45 that included costs associated with hospital inpatient care, outpatient and physician office visits, emergency visits, nursing facility stays, home health visits, visits with other health professionals, and prescription drug and medical supply use. The rate of diabetes was multiplied by the annual diabetes cost to estimate the total costs of diabetes.
The annual costs of a CV event were estimated based on the 1-month attributable costs from a large administrative claims analysis in the USCitation42 that included costs for myocardial infarction, cardiac arrest, congestive heart failure, angina pectoris, transient ischemic attack, hemorrhagic stroke, ischemic stroke, peripheral vascular disease, coronary artery bypass graft surgery, and coronary angioplasty. The number of CV events was multiplied by the cost per event to estimate the total cost of CV disease events for the cohort.
Sensitivity analyses
The robustness of the model results was tested using a one-way deterministic sensitivity analysis and a probabilistic sensitivity analysis. The one-way deterministic sensitivity analysis was conducted to quantify the impact of uncertainty around the mean value of individual model parameters. For the low and high values, the one-way sensitivity analysis used the 95% confidence interval (CI) based on the mean and standard error for all model parameters.
In addition, several scenarios were conducted to evaluate the potential impact on the cost-effectiveness results, including:
Using the lipid Framingham risk equation in place of the BMI Framingham risk equation;
Changing the discount rate from 3% to a range of 0–5%; and
Running the analysis with (a) pharmacy costs only and (b) removing cardiometabolic costs.
A probabilistic sensitivity analysis was conducted to simultaneously quantify the impact of uncertainty of all model parameters by sampling from each parameter’s defined distribution and 95% CI. The results of the probabilistic sensitivity analysis were presented in a cost-effectiveness acceptability curve.
Results
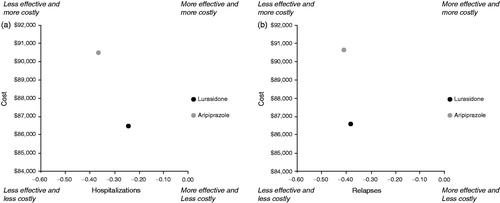
Over the 5-year time horizon, lurasidone had lower total discounted costs compared to aripiprazole, with a predicted cost of $86,480 for lurasidone and $90,500 for aripiprazole (). Lurasidone had lower mental health, diabetes, and CV disease costs, while aripiprazole had a lower pharmacy cost. When compared to aripiprazole, lurasidone was associated with fewer relapses per patient (0.442 vs 0.478) and hospitalizations per patient (0.245 vs 0.369). Additionally, lurasidone had a lower rate of diabetes and number of CV disease events per 1000 patients compared to aripiprazole (7.29% vs 7.36% and 37.3 vs 37.8, respectively). Lurasidone cost $4019 less and resulted in 0.04 fewer relapses per patient and 0.12 fewer hospitalizations per patient. The comparative analysis of cost and effectiveness was in favor of lurasidone vs aripiprazole for both outcome measures ().
Table 4. Discounted clinical outcomes and costs for atypical antipsychotics.
Sensitivity analyses
Results of the various scenarios indicated that the model results were insensitive to the CV risk equation, cardiometabolic costs (CV and diabetes costs), and the discount rate. Lurasidone dominated aripiprazole when the lipid CV risk equation is used, cardiometabolic costs are excluded, and discount rates are varied from 0–5%. When mental health costs are excluded, lurasidone no longer dominates aripiprazole and results in an ICER of $12,180 per hospitalization avoided and $42,447 per relapse avoided.
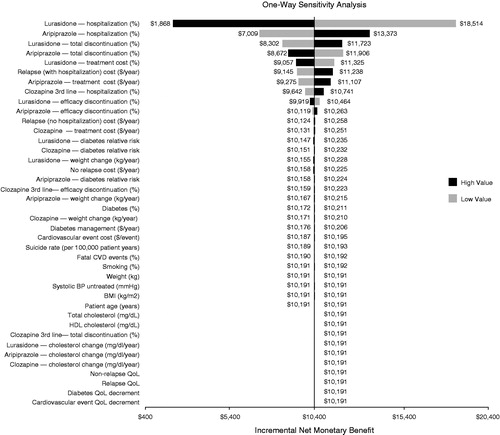
shows the results of the one-way sensitivity analysis comparing lurasidone and aripiprazole, assuming a willingness-to-pay threshold of $50,000 per hospitalization avoided. At this threshold, lurasidone is the preferred strategy with an incremental net monetary benefit of $7377 compared to aripiprazole. As shown in the tornado diagram, at the tested willingness-to-pay threshold, the model results are robust. The model results were insensitive to model parameters over the tested ranges, including lurasidone hospitalization rate ($1868, $18,514), aripiprazole hospitalization rate ($7009, $13,373), lurasidone total discontinuation rate ($8302, $11,723), aripiprazole total discontinuation rate ($8672, $11,906), lurasidone treatment cost ($9057, $11,325), relapse with hospitalization cost ($9145, $11,238), aripiprazole treatment cost ($9275, $11,107), clozapine hospitalization rate ($9642, $10,741), and lurasidone efficacy discontinuation rate ($9919, $10,464).
The probabilistic results showed a mean cost of $86,529 (95% CI = $81,423–$91,719) for lurasidone and $90,550 (95% CI = $85,190–$96,054) for aripiprazole (). At a willingness-to-pay threshold of $50,000 per hospitalization avoided, lurasidone had a 100% probability of being more cost-effective than aripiprazole and at a willingness-to-pay threshold of $50,000 per relapse avoided, lurasidone had a 99.3% probability of being more cost-effective than aripiprazole ().
Figure 4. Cost-effectiveness acceptability curve: hospitalization avoided (a) and relapse avoided (b).
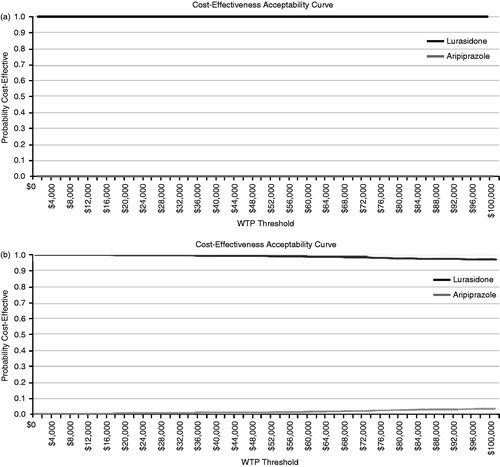
Table 5. Probabilistic sensitivity analysis results.
Discussion
Payers generally require clinical and economic evaluations of drugs to make formulary decisions. The clinical development program for lurasidone included other atypical antipsychotics such as risperidoneCitation33, olanzapineCitation22, and quetiapine XRCitation25 as active comparators in different short-term and long-term clinical trials. These clinical trials provided comparable data on efficacy and cardiometabolic AEs for lurasidone vs the atypical antipsychotics used as active comparators. Lurasidone showed comparable efficacy to risperidone, olanzapine, and quetiapine XR in clinical trials with a better cardiometabolic AE profile measured by blood glucose, lipid parameters, and BMI. This model was developed to mimic real-world treatment patterns to provide clinical and economic comparisons of lurasidone vs aripiprazole. As schizophrenia patients are often required to step through a generic atypical antipsychotic prior to receiving a branded medication, this model evaluated patients who relapsed and switched from their previous generic antipsychotic to either of the two branded atypical antipsychotics lurasidone or aripiprazole.
This model compared relapses, hospitalizations, and cardiometabolic profiles of lurasidone vs aripiprazole using an indirect treatment comparison. Indirect treatment comparisons are increasingly being used to compare treatments where direct head-to-head studies are not availableCitation46. In a review article published in 2006, among the agents that were available in the market, aripiprazole was shown to have the lowest relative tendency to cause weight gainCitation47. In the Comparison of Antipsychotics for Metabolic Problems (CAMP) study, switching patients from olanzapine, quetiapine, or risperidone to aripiprazole was shown to be effective in helping improve lipid profiles (lower non-HDL cholesterol and triglycerides levels) and increase weight lossCitation48. More recently, in a review of labels for all atypical antipsychotics, including lurasidone, the relative tendency to cause weight gain was reportedly lower for lurasidone (number need to harm [NNH] = 20) compared to aripiprazole (NNH = 63)Citation49. In the absence of head-to-head comparative data between lurasidone and aripiprazole, a decision analysis such as this provides insight into a comparison of the efficacy and costs associated with each of the therapies. This insight may be used to guide healthcare decision-makers with regard to evaluating the benefits of one therapy vs the other.
This model demonstrated that lurasidone was less costly and more effective than aripiprazole in terms of hospitalizations avoided and relapses avoided. At a willingness-to-pay threshold of $50,000 per hospitalization avoided, lurasidone was the preferred therapeutic option with a potential net monetary benefit of $7377 compared to aripiprazole over a 5-year time period. Sensitivity analyses showed that the ‘dominance’ of lurasidone (less costly and more effective) was unaffected by variance in model inputs.
Limitations
There are a number of limitations inherent in cost-effectiveness modeling that apply to this model. First, the cost-effectiveness model does not account for patient heterogeneity, a limitation of all Markov cohort models. In the base case, patients were assumed to reflect the average schizophrenia patient enrolled in the lurasidone clinical trialsCitation29. To the extent that a specific patient population differs from these characteristics, the model results may change, and the baseline characteristics of the patient population would need to be modified to better represent a specific health plan’s patient population.
In addition, the model is based on the results of various comparative clinical trials, including: Study 234Citation25, a study comparing aripiprazole and olanzapineCitation35; and CATIE Phase 1Citation14. In order to include lurasidone and aripiprazole in the model, these drugs were compared indirectly with a multi-step analysis using CATIE, which may introduce more uncertainty than a simple indirect comparison. While healthcare decision-makers increasingly recognize indirect treatment comparisons as acceptable in the absence of direct head-to-head comparisons, the comparability of the studies included in the indirect treatment comparison may introduce additional uncertainty.
Differences in the patient population and study methods may change the estimates from this model. CATIE was a real-world comparative effectiveness study, while the lurasidone and aripiprazole studies were controlled clinical trials. The lurasidone study included patients who had a prior response, while the aripiprazole study included both patients with a response and those who previously relapsed. Disease severity, measured by CGI-Severity, was 4.9 in acute patients at 6-week trial baseline and 3.0 at 12-month study baseline for lurasidone compared to 4.4 in acute patients and 3.0 in stable patients at baseline for ariprirazoleCitation25,Citation35. Differences in patient characteristics such as gender, race, age, and duration of illness may impact the estimates from this model, as aripiprazole patients were more likely to be female, white, older, and with a longer duration of illness.
Drug costs in the model were estimated based on the mean drug utilization and published costs as of October 23, 2012. Resource costs were estimated based on the published literature. While the authors have addressed potential variations in drug costs in the sensitivity analysis, the model results may be impacted by variations in costs.
The model was developed from the payer perspective and only reflects the impact of direct costs (pharmacy, mental health, and cardiometabolic outcomes). It does not include indirect costs associated with lost productivity.
Lastly, the only adverse events included in the analysis were those associated with cardiometabolic outcomes. Other potential adverse events such as tardive dyskinesia and akathisia were not included. Cardiometabolic risks were assumed to depend on patient characteristics and weight and lipid changes caused by the atypical antipsychotic agents. Weight and lipid changes were assumed to occur in a linear manner based on the amount of time on therapy. These changes were modeled at the cohort level for the average patient. Outcomes and CV risks for individual patients may vary. As this analysis only incorporates some risk factors, it may under-estimate the CV risks and costs associated with atypical antipsychotics. The longer-term studies of lurasidone include two 12-month active-comparator controlled trialsCitation25,Citation33 and three open-label trialsCitation50–53. These investigations evaluated lurasidone for up to 22 months. However, given its relatively recent FDA approval, cardiometabolic data on lurasidone beyond the timeline of these studies are not currently available, and long-term studies will be necessary to demonstrate these cardiometabolic advantages.
Conclusions
The results of this indirect economic analysis indicate lurasidone may be a less costly and more effective treatment option compared to aripiprazole for adult patients with schizophrenia over a 5-year period. This may be driven by lurasidone’s clinical benefits including preventing hospitalizations and relapses in patients with schizophrenia, and its favorable cardiometabolic AE profile. Further investigation of lurasidone’s cost-effectiveness in real-world settings, through prospective comparative effectiveness studies or retrospective claims database analyses, may be warranted.
Transparency
Declaration of funding
Supported by funding from Sunovion Pharmaceuticals Inc. The funding organization provided feedback on the design of the model and the inputs/sources used in the model. The funding organization also reviewed the manuscript.
Declaration of financial/other relationships
KR, MH, and FG are employees of Sunovion Pharmaceuticals Inc. KO’D and KM are employees of Xcenda, a consulting company that provides services to several pharmaceutical companies, including Sunovion Pharmaceuticals Inc.
Acknowledgments
Laurie Kozbelt from Xcenda provided editorial assistance in the preparation of this manuscript, funded by Sunovion Pharmaceuticals Inc.
References
- Wu EQ, Shi L, Birnbaum H, et al. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med 2006;36:1535-40
- Awad AG, Voruganti LNP. The burden of schizophrenia on caregivers. Pharmacoeconomics 2008;26:149-62
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. 2nd edn. Am J Psychiatry 2004;161(2 Suppl):1-56
- Tandon R, Belmaker RH, Gattaz WF, et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 2008;100:20-38
- Moore TA. Schizophrenia treatment guidelines in the United States. Clin Schizophr Relat Psychoses 2011;5:40-9
- International Psychopharmacology Algorithm Project. Schizophrenia Algorithm: Side Effects of Antipsychotics. http://www.ipap.org/schiz/index.php?screen=side_effects, Accessed April 12, 2012
- McEvoy JP, Scheifler PL, Frances A. Expert consensus guideline series: treatment of schizophrenia 1999. J Clin Psychiatry 1999;60(11 Suppl):1-80
- American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus Development Conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601
- McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32
- Bresee LC, Majumdar SR, Patten SB, et al. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based study. Schizophr Res 2010;117:75-82
- Mathews M, Muzina DJ. Atypical antipsychotics: new drugs, new challenges. Cleve Clin J Med 2007;74:597-606
- Sernyak MJ, Leslie DL, Alarcon RD, et al. Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002;159:561-6
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Eng J Med 2005;353:1209-23
- Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophr Res 2004;66:51-7
- Liu-Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med 2005;3:21 . http://www.biomedcentral.com/1741-7015/3/21
- Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull 2006;32:724-42
- Kelly DL, McMahon RP, Liu F, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry 2010;71:304-11
- Latuda (lurasidone HCl) tablets [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc. May 2012
- Ogasa M, Kimura T, Nakamura M, et al. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacol (Berl) 2013;225:519-30
- Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 2009;70:829-36
- Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry 2011;168:957-67
- Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res 2013;47:670-7
- Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 2013;145:101-9
- Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind non-inferiority study. Schizophr Res 2013;147:95-102
- Abouzaid S, Jutkowitz E, Foley KA, et al. Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag 2010;13:247-54
- United States Department of Labor. Bureau of Labor Statistics. Consumer Price Index, All Urban Consumers. http://data.bls.gov/cgi-bin/surveymost?cu, Accessed October 23, 2012
- McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not repond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163:600-10
- Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention: a double-blind, placebo-controlled study (PEARL 3). Poster presented at: American Psychiatric Association; May 5-9, 2012; Philadelphia, PA
- D’Agostino RB, Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices–Part 2. Value Health 2011;14:429-37
- Song F, Altman DG, Glenny A, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326:472
- Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharm 2012a;27:165-76
- Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 2003;6:325-37
- Chrzanowski WK, Marcus RN, Torbeyns A, et al. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology 2006;189:259-66
- Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 2003;29:15-31
- McDermott S, Moran R, Platt T, et al. Heart disease, schizophrenia, and affective psychoses: epidemiology of risk in primary care. Community Ment Health J 2005;41:747-55
- Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry 2004;161:1709-11
- Rajagopalan K, O’Day K, Meyer K, et al. Time-on-Therapy for atypical antipsychotics in a Markov Cohort Analysis. Poster presented at: ISPOR Annual International Meeting; June 2-6, 2012; Washington DC
- Arias E. United States life tables, 2007. National vital statistics reports; vol 59 no 9. Hyattsville, MD: National Center for Health Statistics, 2011
- Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol 2010;24:81-90
- O’Sullivan AK, Rubin J, Nyambose J, et al. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics 2011;29:693-704
- Red Book Online. Micromedex 2.0. Thomson Reuters. http://www.micromedexsolutions.com Accessed October 23, 2012
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry 2010;10:2
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596-615
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14:417-28
- Llorente MD, Urrutia V. Diabetes, psychiatric disorders, and the metabolic effects of antipsychotic medications. Clin Diabetes 2006;24:18-24
- Stroup TS, McEvoy JP, Ring KD, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: Comparison of Antipsychotics for Metabolic Problems (CAMP). Am J Psychiatry 2011;168:947-56
- Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother 2012b;13:1599-613
- Data on File [lurasidone HCl, NDA 200603, Clinical Study Report D1050229E]. Fort Lee, NJ: Sunovion Pharmaceuticals Inc. 2011-2012
- Data on File [lurasidone HCl, NDA 200603, Clinical Study Report D1050231E]. Fort Lee, NJ: Sunovion Pharmaceuticals Inc. 2011-2012
- Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with luradisone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry http://dx.doi/org:10.4088/JCP.12m08084. 2013;74:507-15 [Epub ahead of print]
- Data on File [lurasidone HCl, NDA 200603, Clinical Study Report D1001048]. Fort Lee, NJ: Sunovion Pharmaceuticals Inc. 2011-2012