Abstract
Purpose:
To model the economic impact of annual relapses/relapse-related hospitalizations among adults with schizophrenia treated with lurasidone or quetiapine extended-release (XR).
Methods:
A probabilistic model estimating per-patient-per-year (PPPY) direct mental healthcare (MH) cost differences due to relapses/relapse-related hospitalizations was developed using relapse and relapse-related hospitalization rates from a 12-month, double-blind, parallel-group, global comparison study of lurasidone vs quetiapine XR (all patients previously treated with lurasidone or quetiapine XR for 6 weeks). Analyses were conducted for both all subjects and clinical responders. Direct costs associated with inpatient and outpatient mental healthcare-related services were obtained from a large, prospective, observational study of schizophrenia treatment in usual-care settings for relapsing and non-relapsing patients, including psychiatric hospitalizations, emergency services, medication management, and outpatient individual therapy. Model robustness was tested using univariate and probabilistic sensitivity analyses.
Results:
Model-estimated PPPY MH cost savings associated with relapse-related hospitalization rates in all subjects were $3276 for lurasidone vs quetiapine XR. Lurasidone resulted in PPPY MH cost savings of $2702 vs quetiapine XR in all subjects, using relapse rates. Sensitivity analyses indicated lurasidone had lower 1-year MH costs than quetiapine XR in 100% and 99.7% of simulations, using relapse-related hospitalization rates and relapse rates, respectively, in all subjects. Similar results were seen in clinical responders.
Limitations:
The model represents a simplification of treatment patterns and response to treatment. Cost of treatment with lurasidone and quetiapine XR was not included in the model. Estimates of cost savings are likely conservative, as the model did not assess the impact of long-term cardiometabolic consequences. Indirect costs associated with relapses and non-mental health-related costs were also excluded from the model.
Conclusion:
Adults treated for schizophrenia with lurasidone are predicted to have lower 12-month MH costs compared to those treated with quetiapine XR due to fewer relapses and relapse-related hospitalizations.
Introduction
Schizophrenia is a mental disorder characterized by disruption of cognition, affect, and behavior. In the US, the population prevalence is ∼1%, or an estimated 2.4 million adultsCitation1, with a diagnosed prevalence of 0.51%Citation2. While the course of the disease is variable, patients often experience acute relapses followed by periods of stabilityCitation3.
Schizophrenia imposes a significant toll on patients, caregivers, and society in terms of direct and indirect costsCitation4. Compared with matched controls, the total annual excess cost of schizophrenia in the US was estimated at $62.7 billion in 2002. Of this economic burden, 52% was due to indirect costs ($32.4 billion, including unemployment, reduced workplace productivity, premature mortality from suicide, and family caregiving), 36% was due to direct healthcare costs ($22.7 billion, including outpatient, inpatient, long-term care, and medications), and the remaining 12% was due to direct non-healthcare costs ($7.6 billion)Citation5. Although schizophrenia accounts for a lower number of hospital stays compared with other mental illnesses, data from 2008 from the Healthcare Cost and Utilization Project show that patients admitted for schizophrenia have the second-longest average length of stay (11.1 days), highest average total cost per stay ($7500), and highest aggregate cost of hospitalization ($2.7 billion) compared with other mental health and substance abuse disordersCitation6.
A primary driver of medical costs in patients with schizophrenia is relapse and associated care (including hospitalizations). One study suggests that patients who experienced a relapse of psychotic symptoms within the previous 6 months incurred 4-times higher costs than schizophrenia patients without a recent relapse (p < 0.01)Citation7. Similarly, in a recently published, prospective, observational study (1997–2003); the 12-month direct mental healthcare costs for patients with a prior relapse (within the previous 6 months) were 3-times higher than costs for patients without a relapse ($33,187 vs $11,771; p < 0.01). These costs were driven by significantly higher psychiatric hospitalization costs for patients with a prior relapse vs patients without a relapse ($19,298 vs $1055; p < 0.01)Citation8. These results underscore the economic importance of relapse prevention.
Atypical anti-psychotic agents in addition to lurasidone and quetiapine extended-release (XR) include oral asenapine, aripiprazole, clozapine, iloperidone, olanzapine, quetiapine, risperidone and ziprasidone, as well as a number of long-acting injectable formulationsCitation9. More recently, paliperidone and its long-acting injectable formulation, paliperidone palmitate, have also become available. Despite these therapeutic advances, schizophrenia remains one of the most challenging diseases to treat due to the heterogeneity of clinical response, the adverse events associated with treatment, and the disease’s association with high morbidity and mortalityCitation10,Citation11. Given these substantial challenges, atypical anti-psychotic agents may confer substantial economic benefits if they can prevent or delay relapses among patients with schizophrenia.
Quetiapine XR is an established, widely used, well-tolerated agent approved by the Food and Drug Administration (FDA) in 2007 and is also approved in Europe for the maintenance treatment of schizophrenia based, in part, on demonstration of relapse prevention efficacy in a double-blind, randomized withdrawal studyCitation12. LurasidoneCitation13 received FDA approval in October 2010 and recently received approval in Canada for the treatment of patients with schizophrenia. Evidence from open-label continuation trialsCitation14,Citation15 and a randomized, double-blind, long-term trialCitation16 suggests that efficacy of lurasidone is maintained over a 12-month treatment period with a low potential for adverse effects on weight and metabolic parameters, thus making it a promising candidate for maintenance treatment. Typically, schizophrenia patients are initiated on a generically available atypical anti-psychotic, such as risperidone. However, they may be switched to another atypical anti-psychotic agent, including more costly branded products, after discontinuation due to lack of efficacy or adverse effects. Hence, there is a need for comparative effectiveness research that demonstrates the impact of agents, such as quetiapine XR and lurasidone, on relapses and associated costs to assess the potential long-term efficacy of atypical anti-psychotics and their real-world economic impact from a payer perspective. Accordingly, the objective of this study was to model the potential economic impact of annual relapses and relapse-related hospitalizations among adults with schizophrenia treated with lurasidone or quetiapine XR tablets based on available head-to-head data.
Patients and methods
Model design
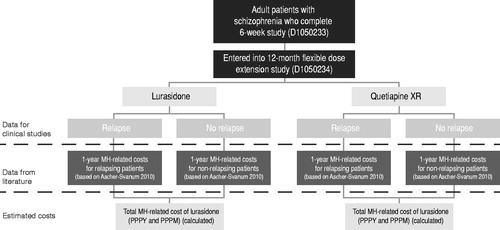
An economic model was developed in Microsoft Excel® to estimate the potential economic impact of annual relapses and relapse-related hospitalizations among adults treated for chronic schizophrenia with lurasidone or quetiapine XR. The model compares the direct mental healthcare costs from the perspective of a third-party payer over a 1-year time horizon and includes a probabilistic simulation to estimate 95% confidence intervals (CIs) for the 1-year costs. As shown in the model structure in , the primary model outcome was the total mental healthcare-related costs for a patient treated with either lurasidone or quetiapine XR over a 1-year period (cost per patient per year [PPPY] and cost per patient per month [PPPM]). Inputs used in the model include rate of clinical relapse and relapse-related hospitalization and direct mental healthcare-related costs.
Model inputs
Relapse rates and relapse-related hospitalization rates for lurasidone and quetiapine XR were obtained from Study D1050233 and study D1050234. Study D1050233 was a 6-week, double-blind, randomized placebo-controlled trial involving fixed doses of lurasidone (80 mg/day or 160 mg/day) and quetiapine XR (600 mg/day) (active control) monotherapy in patients with chronic schizophreniaCitation17. Quetiapine XR was chosen as the comparator in this study since it was a widely utilized anti-psychotic with a well-established knowledge base about its efficacy, safety, and tolerability in schizophrenia.
Eligibility criteria for the study include: 18–75 years of age; DSM-IV primary diagnosis of schizophrenia with acute exacerbation of psychotic symptoms <2 months; minimum duration of illness of at least 1 year; PANSS total score ≥80 at baseline; CGI-S score ≥4 at baseline. Patients who completed this study were eligible to participate in Study D1050234, a 12-month, double-blind, parallel-group study using a non-inferiority design, to compare flexibly dosed lurasidone 40–160 mg/day (n = 151) and quetiapine XRCitation18 200–800 mg/day (n = 85) in adult patients with schizophreniaCitation19.
In Study D1050234, the primary relapse prevention analysis sub-group was defined, a priori, as all subjects who were randomized to either once-daily lurasidone or quetiapine XR in the initial 6-week treatment study and who met clinical response criteria at the end of week 6 (defined as ≥20% reduction in Positive and Negative Symptoms Scale (PANSS) total score from acute study baseline and a CGI-S ≤ 4 at Day 42). Response in the 6-week, double-blind study was defined as ≥20% improvement in PANSS total score and Clinical Global Impression Scale–Severity of Illness (CGI-S) score ≤4 at completion of the study.
The primary end-point for Study D1050234 was time to relapse for lurasidone compared with quetiapine XR in a non-inferiority analysis utilizing a Cox proportional hazards model. Relapse was defined as: either a worsening of ≥30% PANSS total score from Study D1050233 at 6 weeks and CGI-S ≥ 3; or re-hospitalization for worsening of psychosis; or emergence of suicidal ideation, homicidal ideation, and/or risk of harm to self or others. Patients who were randomized to placebo in the 6-week, double-blind study D1050233 and entered into the non-randomized, 12-month, double-blind study D1050234 were excluded from the analysis of the primary end-point. The observed relapse-related hospitalization rates and relapse rates for lurasidone and quetiapine XR in the overall population and responder population are shown in Citation19. The population included in the model was adults with schizophrenia, as defined in the 6-week, double-blind, placebo-controlled study (D1050233)Citation17. For purposes of the model, the analysis was conducted on both the full study population (base case) and the responder population (secondary analysis) after excluding the placebo group. Analyses were also conducted using the Kaplan-Meier probabilities of relapse and relapse-related hospitalization, respectively, for lurasidone (23.7% and 10.0%) and quetiapine XR (33.6% and 25.2%) instead of the absolute ratesCitation20.
Table 1. Observed relapse-related hospitalization rates and relapse ratesCitation15.
Mental healthcare-related costs
Direct costs associated with inpatient and outpatient mental healthcare-related services for patients with and without relapses were obtained from published literature. Ascher-Svanum et al.Citation8 conducted an analysis of adults with schizophrenia in the US in order to assess the direct costs of relapse and to identify predictors of relapse during treatment. The data source for the analysis was a large (n = 2327) prospective, observational study of schizophrenia treatment in usual-care settings, conducted between 1997–2003. Ascher-Svanum et al.Citation8 identified patients with and without a relapse in the 6-month pre-period and calculated their subsequent total 1-year direct mental healthcare costs. Relapse was defined as having any of the following recorded in a patient’s medical record: psychiatric hospitalization, use of emergency services, use of a crisis bed, or a suicide attempt. Resource costs were obtained from published average wholesale prices (medications), daily per-diem costs at study sites (hospitalizations), and relative value units of other resource utilization. Costs were aggregated into the following components: medications (anti-psychotics and other psychotropics, such as mood stabilizers, anti-cholinergics, antidepressants, anti-anxiety, and sleep agents), psychiatric hospitalizations, day treatment, emergency services, psychosocial group therapy, medication management, outpatient individual therapy, and assertive community treatment (ACT)/case management. Ascher-Svanum et al.Citation8 evaluated the total 1-year direct mental healthcare costs for patients who had a relapse in the prior 6-month period (‘relapsing patients’) and those who did not (‘non-relapsing patients’). lists the mental healthcare-related costs for relapsing and non-relapsing patients used in the model. As the model evaluated mental healthcare-related costs associated with a 1-year period following treatment with lurasidone or quetiapine XR during which a relapse may have occurred; drug costs for treatment with lurasidone and quetiapine XR were not included. As the costs from Ascher-Svanum et al.Citation8 were in 2000 US dollars (USD), all cost estimates were inflated to 2011 USD, using the Medical Care Component of the Bureau of Labor Statistics Consumer Price IndexCitation21.
Table 2. Cost inputs in 2011 USDCitation8,Citation17.
Model outcomes
The model outcome of interest was the total mental healthcare-related costs PPPY and PPPM for patients treated with lurasidone or quetiapine XR. The model estimated the PPPY costs for each treatment as the sum of the product of the relapse rates () and each mental healthcare cost component () for relapsing patients and the product of the proportion of non-relapsing patients and each mental healthcare cost component for non-relapsing patients.
To calculate the PPPY costs associated with relapse-related hospitalizations, the above calculations were modified by multiplying the psychiatric hospitalization cost component by the relapse-related hospitalization rate instead of the clinical relapse rate. PPPM costs were calculated by dividing PPPY costs by 12.
The model outcomes were calculated using a probabilistic analysis consisting of 1000 Monte Carlo simulations based on the mean value, the standard error, and the distribution for each model parameter (frequency of relapses and mental healthcare-related costs). The relapse and relapse-related hospitalization rates for lurasidone or quetiapine XR were modeled as a beta distribution using the mean observed relapse rate and standard errors from the 12-month, double-blind, parallel-group study (Study D1050234)Citation19 (). The 1-year costs were modeled as a normal distribution using the mean costs and standard errors from the Ascher-Svanum et al.Citation8 retrospective analysis (). The mean and 95% CI were estimated for the total mental healthcare-related (and all resource) costs PPPY for lurasidone and quetiapine XR.
Outcomes were estimated for all subjects (base case) and for the responder population (secondary analysis). Costs were modeled using the overall relapse rates and limiting the re-hospitalization costs to patients who had an observed re-hospitalization for worsening of psychosis during the 12-month, double-blind, parallel-group study (Study D1050234)Citation19.
Sensitivity analysis
The probabilistic analysis provided a mean and 95% CI for all costs, as well as the probability that one drug was less costly than another based on the proportion of simulations in which the costs were lower.
In order to determine the impact of specific model parameters on the model output, univariate analyses were also performed. Each model input was singly varied around the base case value using the 95% CI range, while keeping other inputs constant, to determine the effect on the model results.
Given that lurasidone patients had a longer time to relapse and relapse-related hospitalization compared with quetiapine patients, a sensitivity analysis was also conducted to assess the impact of using the Kaplan-Meier probabilities from Study D1050234 instead of the absolute relapse and relapse-related hospitalization rates.
Results
Base case
All subjects/relapse-related hospitalization rates
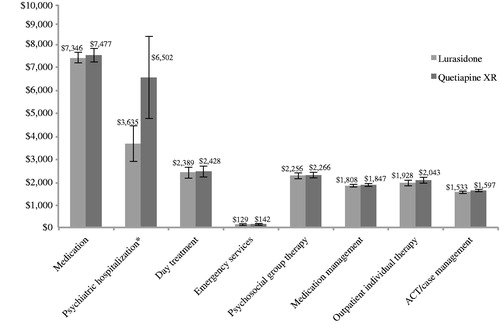
The model results estimated a 100% likelihood that the 1-year mental healthcare-related direct costs would be less for lurasidone than quetiapine XR in all subjects using relapse-related hospitalization rates. The mental healthcare-related estimated direct costs PPPY for all subjects using relapse-related hospitalization rates are contained in . Over a 1-year period, the total mental healthcare-related costs for lurasidone were estimated to be $21,025 PPPY (95% CI = $20,059, $21,979) compared with $24,301 PPPY (95% CI = $22,479, $26,295) for quetiapine XR, representing a $3276 cost savings. The total PPPM cost for lurasidone was estimated to be $1752 compared with $2025 PPPM for quetiapine XR, representing a potential $273 cost saving. Medications and psychiatric hospitalizations were the largest cost components, together comprising over 50% of the total costs.
Figure 2. Mental healthcare-related estimated direct costs per patient per year (all subjects/relapse-related hospitalization rates). * Using relapse-related hospitalization rates to estimate costs of psychiatric hospitalizations. ACT, assertive community treatment; error bars represent the 95% confidence interval for each value.
All subjects/relapse rates
Using relapse rates to estimate the costs of hospitalization for all subjects, the model estimated a 99.7% likelihood that the mental healthcare-related direct costs would be less for lurasidone than for quetiapine XR. Lurasidone resulted in a total estimated PPPY cost of $24,567 compared with $27,269 for quetiapine XR, amounting to a potential annual cost savings of $2702 per patient.
Secondary analysis
Clinical responders/relapse-related hospitalization rates
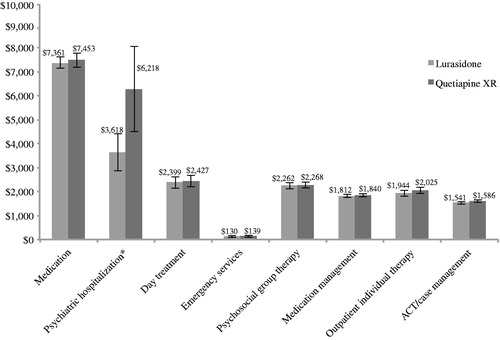
When the model patient population was restricted to patients who were responders in the 6-week, double-blind D1050233 study, the model estimated a 100% likelihood that mental healthcare-related direct costs would be lower for lurasidone than quetiapine XR using relapse-related hospitalizations rates. The mental healthcare-related estimated direct costs PPPY for responders using relapse-related hospitalization rates are contained in . Over a 1-year period, the total mental healthcare-related costs were $21,069 (95% CI = $20,051, $22,040) for lurasidone compared with $23,956 (95% CI = $22,181, $25,799) for quetiapine XR, representing a cost savings of $2888 PPPY.
Figure 3. Mental healthcare-related estimated direct costs per patient per year (clinical responders/relapse-related hospitalization rates). * Using relapse-related hospitalization rates to estimate costs of psychiatric hospitalizations. ACT, assertive community treatment; error bars represent the 95% confidence interval for each value.
Clinical responders/relapse rates
Using relapse rates to estimate the costs of hospitalization for the clinical responder population, the model estimated a 96.5% likelihood that the mental healthcare-related direct costs would be less for lurasidone than for quetiapine XR. Lurasidone resulted in a total estimated PPPY cost of $24,920 compared with $26,835 for quetiapine XR, amounting to an annual potential cost savings of $1914 per patient.
Sensitivity analyses
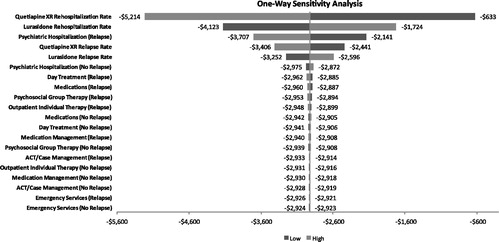
Results of the univariate sensitivity analysis for the base case and secondary analyses, using relapse-related hospitalization rates to estimate hospitalization costs, indicated that the model results were most sensitive to the relapse-related hospitalization rates for quetiapine XR and lurasidone. In the all subjects analysis, even at the highest relapse-related hospitalization rate tested for lurasidone (11.4%), lurasidone was still associated with a lower cost, resulting in a net cost savings of $2129 (). Variability of the cost parameters, including costs of medication management, psychosocial group therapy, ACT/case management, outpatient individual therapy, and emergency services, had a negligible impact on the outcome. In the all subjects analysis using the relapse rates, results were sensitive to the quetiapine XR relapse rate, where at the lowest tested relapse rate of 18.6% quetiapine XR resulted in a PPPY cost saving of $427. Results for the responder population using relapse-related hospitalization rates were insensitive to all tested parameter values (). Results for the responder population using relapse rates were sensitive to quetiapine XR relapse rate and lurasidone relapse rate.
Figure 4. Univariate sensitivity analysis (all subjects/relapse-related hospitalization rates) using relapse-related hospitalization rates to estimate costs of psychiatric hospitalizations. Note, the output of the one-way sensitivity analysis is depicted as a tornado diagram, in which the width of the arms represents the influence of that parameter on the model results, while holding all other parameters constant.
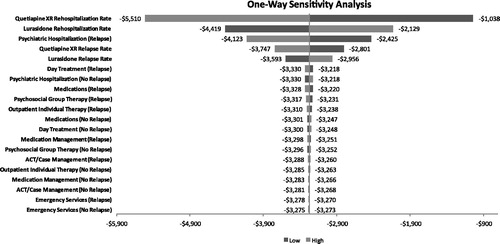
Figure 5. Univariate sensitivity analysis (clinical responders/relapse-related hospitalization rates) using relapse-related hospitalization rates to estimate costs of psychiatric hospitalizations. Note, the output of the one-way sensitivity analysis is depicted as a tornado diagram, in which the width of the arms represents the influence of that parameter on the model results, while holding all other parameters constant.
Model results estimated using risk-related probability estimates were similar to those using the absolute rates. In the responder population, using relapse-related hospitalization probabilities the PPPY costs were $22,010 vs $26,744 and using relapse probabilities the PPPY costs were $25,801 vs $28,951, respectively, for lurasidone and quetiapine XRCitation20.
Discussion
Over a 1-year period, relapsing schizophrenia patients have been shown to incur 3-times higher costs than patients without a relapseCitation8. Therefore, the present economic analysis based on observed relapse rates from this 12-month double-blind study D1050234Citation19 provides valuable insight into the potential long-term effectiveness of lurasidone and quetiapine XR in schizophrenia.
The results of the economic model in this analysis found that adults treated for schizophrenia with lurasidone have lower 1-year direct mental healthcare-related costs than those treated with quetiapine XR. This observation is likely due to a lower number of relapses and relapse-related hospitalizations observed in the clinical study. In the two study cohorts of interest, the estimated PPPY cost was substantially lower for lurasidone vs quetiapine XR (−$3276 for all patients and −$2888 for clinical responders) using relapse-related hospitalization rates. Cost savings were also found for lurasidone vs quetiapine XR when the relapse rates from the study were used to calculate the hospitalization costs (−$2702 PPPY for all patients and −$1914 PPPY for the clinical responder population). Results were similar when Kaplan-Meier probabilities were used instead of relapse rates. In the univariate sensitivity analyses, the rates of relapse or relapse-related hospitalization for lurasidone and quetiapine XR were the factors which most impacted model results. In addition to its demonstrated efficacy in managing symptoms and preventing relapses in the longer term study, lurasidone may have the potential to reduce mental healthcare-related costs in the treatment of adults with schizophrenia.
As with all economic models, this model has a number of strengths and limitations. This model’s unique strength is that the clinical data inputs for relapses and relapse-related hospitalizations were drawn from a direct comparison of lurasidone and quetiapine XR in a 12-month, comparative, parallel-group, double-blind study. Additionally, cost comparisons between the two drug cohorts were homogeneous, given that cost inputs were drawn from a large-scale representative study of relapsing and non-relapsing patients from a real-world analysis in usual-care settings.
It is important to note that this model represents a simplification of treatment patterns and response to treatment from what is seen in actual practice. In the real world, patients with schizophrenia are likely to be treated with a variety of treatment modalities, including psychological and rehabilitative interventions in addition to or instead of pharmacotherapy. The scope of our analysis is, therefore, limited in that it focuses only on the direct comparison of two pharmacologic therapies. Cost inputs for the model were derived from real-world data based on the 3-year US schizophrenia care and assessment projectCitation8. However, since the Ascher-Svanum et al.Citation8 study was non-randomized, the possibility of selection bias exists. To counteract this, Ascher-Svanum et al. conducted propensity score matching to control for selection bias and supplemental analyses. Propensity score matching allowed assessment of the robustness of results while excluding high-cost patients who were continuously hospitalized. Additionally, the Ascher-Svanum et al. analysis was not able to assess the reason for a psychiatric hospitalization; thus, it is possible that some hospitalizations may have been attributable to another mental health condition. However, it is expected that the costs are likely reflective of actual schizophrenia-related costs, given that the total mental healthcare-related costs from two other data sourcesCitation5,Citation22 indicate that the costs are in a similar range. Finally, the definition of relapse used in the study comparing lurasidone and quetiapine XR was slightly different than that used in the Ascher-Svanum et al. analysis. Both include psychiatric hospitalization and suicidal ideation/attempt, but the clinical trial also included patient outcomes based on the PANSS and CGI-S. This could potentially increase the relapse rate in the trial compared to what would be observed in the real world and may, consequently, over-estimate the costs.
Secondly, cohort-level annual average pharmacy costs for relapsing and non-relapsing patients were included in the model, as it focuses on the impact of costs for relapsing and non-relapsing patients after therapy with lurasidone or quetiapine XR. As such, it is quite likely that the model under-estimates the economic advantage of lurasidone, as the current pharmacy costs, using the weighted average cost based on the doses utilized in Study D1050234, are lower for lurasidone than for quetiapine XR (wholesale acquisition costs of $9116 and $9853, respectively, based on average daily doses of 124 mg and 630 mg, respectively)Citation23.
Thirdly, drug-specific characteristics that may impact patient response to treatment, including adverse events, medication adherence, and medication persistence, were not evaluated in the model. However, it could be expected that the real-world difference in persistence between the two agents would favor lurasidone, given the lower all-cause discontinuations and discontinuations due to insufficient clinical effect observed in the clinical studiesCitation17,Citation19.
Furthermore, the model did not include other relevant clinical outcomes such as cardiometabolic conditions and other comorbidities common in schizophrenia patients. While atypical anti-psychotics have been associated with an increased risk of cardiovascular disease-related factors, data from this study also showed that lurasidone has a favorable cardiometabolic profile compared to quetiapine XRCitation17,Citation19,Citation24. Therefore, it is expected that this model may under-estimate the total cost savings, as it did not include the impact of these long-term cardiometabolic consequences.
Finally, indirect costs associated with relapses such as lost productivity and caregiver time, as well non-mental healthcare-related costs, were also excluded from the model. The only outcomes evaluated were relapse and relapse-related hospitalization; other relevant clinical outcomes such as cardiometabolic conditions and other comorbidities common in schizophrenia patients were not included. As such, it is likely that the true annual costs for adults with schizophrenia are higher than identified in this analysis. Thus, the inclusion of only mental healthcare-related costs may have provided a more conservative estimate of the cost savings for lurasidone, as patients with relapse are likely to have higher all-cause medical costs.
Conclusions
Overall findings from this model and associated sensitivity analyses demonstrate that lurasidone is a cost-saving therapeutic option compared with quetiapine XR. The results of this model are unique in that the clinical benefits of fewer relapses and relapse-related hospitalizations associated with the use of lurasidone vs quetiapine XR are drawn from a single, 12-month, parallel group, double-blind study. In this model, adults treated for schizophrenia with lurasidone had lower 1-year direct mental healthcare costs than those treated with quetiapine XR due to fewer relapses and relapse-related hospitalizations. The estimated PPPY cost was also substantially lower for lurasidone vs quetiapine XR in all subjects, irrespective of the outcome measure considered (relapse or relapse-related hospitalization). These results may provide valuable insights about the potential effectiveness of lurasidone, in terms of relapses and relapse-related hospitalizations to physicians, pharmacists, patients, and payers.
Transparency
Declaration of funding
Supported by funding from Sunovion Pharmaceuticals Inc. The funding organization provided feedback on the design of the model and the inputs/sources used in the model. The funding organization also reviewed the manuscript.
Declaration of financial/other relationships
KR, AP, and AL are employees of Sunovion Pharmaceuticals Inc. KO’D and KM are employees of Xcenda, a consulting company that provides services to several pharmaceutical companies, including Sunovion Pharmaceuticals Inc., and received funding to complete this analysis.
Acknowledgments
Laurie Kozbelt provided editorial support in the preparation of this manuscript, funded by Sunovion Pharmaceuticals Inc.
References
- Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23
- Wu EQ, Shi L, Birnbaum H, et al. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med 2006;36:1535-40
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: American Psychiatric Publishing; 2000
- Awad AG, Voruganti LNP. The burden of schizophrenia on caregivers. Pharmacoeconomics 2008;26:149-62
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Wier LM, Levit K, Stranges E, et al. HCUP facts and figures: statistics on hospital-based care in the United States, 2008. Rockville, MD: Agency for Healthcare Research and Quality, 2010. http://www.hcup-us.ahrq.gov/reports.jsp. Accessed April 20, 2012
- Almond S, Knapp M, Francois C, et al. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry 2004;184:346-51
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry 2010;10:2
- National Institute of Mental Health; Bethesda, MD. Schizophrenia. NIH Publication No. 06-3517. Mental Health Medications 2006;1–25
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 2004;161(Suppl):1–56
- Tandon R, Belmaker RH, Gattaz WF, et al. World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 2008;100:20-38
- Peuskens J, Trivedi J, Malyarov S, et al. Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patients. Psychiatry 2007;4:34-50
- Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc, 2011
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract 2011;65:189-210
- Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507-15
- Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 2012;27:165-76
- Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res 2013;145:101-9
- Seroquel [package insert]. Wilmington, DE: AstraZeneca, 2011
- Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12-month double-blind noninferiority study. Schizophr Res 2013;147:95-102
- Rajagopalan K, O’Day K, Meyer K, et al. Costs of relapses and relapse-related hospitalizations among adults treated with atypical antipsychotics for schizophrenia. Poster presented at: American Society of Health-System Pharmacists 2012 Summer Meeting & Exhibition; June 9–13, 2012; Baltimore, MD
- Bureau of Labor Statistics; Washington, DC. Consumer Price Index, All Urban Consumers. USA: US Bureau of Labor Statistics. http://data.bls.gov/cgi-bin/surveymost?cu. Accessed October 3, 2011
- Singh G, Mannalithara A, Rajagopalan K, et al. Incremental treatment costs in hospitalized schizophrenia patients with comorbid type 2 diabetes mellitus. Poster presented at: American Society of Health-System Pharmacists 2012 Summer Meeting & Exhibition; June 9–13, 2012; Baltimore, MD
- RED BOOK Online. Micromedex 2.0. Thomson Reuters. http://www.micromedexsolutions.com Accessed October 9, 2012
- Citrome L. Lurasidone in schizophrenia: new information about dosage and place in therapy. Adv Ther 2012;29:815-25