Abstract
Objectives:
To conduct a retrospective analysis of the association between drug tolerability and potential economic impact measured by medical resource utilization (MRU) for prophylaxis of invasive antifungal infections (IFI) after allogeneic hematopoietic stem cell transplantation (alloHCT).
Methods:
An open-label, multi-center study (IMPROVIT) included patients (≥12-years old) who were randomized to receive oral voriconazole (VOR) or oral itraconazole (ITR) from the alloHCT day for at least 100 days and up to 180 days. Trial data on discontinuation and MRU for the first 100 days were analyzed.
Results:
Two hundred and twenty-four patients were in VOR and 241 in ITR, with similar demographic distributions (average age of 43 years, 58% male, 92% Caucasian). All-cause and study drug intolerance discontinuations were less frequent with VOR than ITR (50% vs 63%, p = 0.0137; 7% vs 22%, p < 0.0001). VOR patients had longer study drug exposure (median = 96 vs 68 days, p < 0.0001; mean = 68 vs 60 days, p = 0.0044). ITR patients were 2-times more likely (p = 0.0110) to use other antifungals vs VOR patients. Controlling for treatment and key baseline variables, longer IFI prophylaxis was associated with fewer hospital days (p < 0.0001) and less other antifungal use (p < 0.0001). Patients who discontinued prophylaxis during the first 100 days incurred 10 more hospital days (p < 0.0001) and 17 more other antifungal days (p < 0.0001) compared to their counterparts. Eight more prophylaxis days were associated with ∼1 less hospital day and 3.6 less other antifungal days (p < 0.0001).
Key limitation:
MRU data collection was limited to the first 100 days post-transplant, which may not fully capture the real-world utilization and outcomes.
Conclusions:
Patients’ ability to tolerate and continue their antifungal prophylaxis after alloHCT is associated with less use of MRU such as other antifungals and hospital days. In the current resource-constrained healthcare environment, it is important to consider the potential economic impact of the tolerability of antifungal prophylaxis.
Introduction
Invasive fungal infections (IFI) after allogeneic hematopoietic stem cell transplantation (alloHCT) are major causes of morbidity, mortality, and cost. Mortality from Aspergillus infections following alloHCT is high, with reported rates of 50–90%Citation1. The adoption of antifungal (AF) prophylaxis is an important strategy to reduce IFI incidence and improve patient outcomes. Currently, there are several antifungal agents recommended by the National Comprehensive Cancer NetworkCitation2 for the prophylaxis of IFIs in alloHCT patients, including fluconazole, itraconazole, voriconazole, posaconazole, and various amphotericin B formulations. However, the optimal antifungal prophylaxis, in terms of efficacy and tolerability, is unclear.
The use of fluconazole prophylaxis to prevent invasive Candida infection has effectively decreased the number of infections during the pre-engraftment period. However, fluconazole’s lack of activity against mold infections limits its value as prophylaxis during the post-engraftment period of riskCitation3–5. Itraconazole, a broad spectrum antifungal with activity against molds, has proven efficacy in the treatment of both invasive aspergillosis and invasive candidiasis. Nevertheless, the variable bioavailability and poor tolerability of itraconazole limit the drug’s usefulness as prophylaxis over a prolonged periodCitation6–8. It has been widely recognized that a critical issue with itraconazole is treatment adherence, as there are significantly more adverse effects (especially gastrointestinal complaints), laboratory abnormalities, and withdrawals due to adverse effects with itraconazole than with fluconazoleCitation3,Citation6,Citation9–11.
Voriconazole is another broad-spectrum triazole antifungal with activity against a wide range of yeasts and filamentous fungi, including Candida, Aspergillus, Fusarium, and Scedosporium species. Voriconazole has demonstrated safety and efficacy as first-line treatment for invasive aspergillosis and as first-line treatment of serious Candida infectionsCitation3,Citation12–14. The IMPROVIT trial (Voriconazole vs Itraconazole in Primary Prophylaxis of Invasive Fungal Infection in Subjects with Allogeneic Hematopoietic Stem Cell Transplants) was the first large-scale, prospective, randomized, open-label study to compare the efficacy, safety, and tolerability of voriconazole to itraconazole as antifungal prophylaxis in alloHCT recipientsCitation15. The IMPROVIT trial revealed that voriconazole was better-tolerated than itraconazole, with a longer treatment duration and less need for other systemic antifungal therapyCitation15. We postulated that overall medical resource utilization (MRU) and unscheduled treatment costs may be less for the patients who can better tolerate and stay longer on their antifungal prophylaxis treatment.
In the current environment of tighter healthcare budgets and stricter management guidelines, it is important to evaluate the economic impact of new treatments in addition to efficacy and safety end-points. Therefore, the objective of this study was to conduct a retrospective medical resource use analysis based on the IMPROVIT trial (prophylaxis with voriconazole and itraconazole up to 100 days) and to evaluate the association between drug tolerability and potential economic impact. The rationale for the 100-day data analysis was because the primary composite end-point of IMPROVIT included the ability to tolerate study drug for at least 100 days, and a major secondary end-point was the success of prophylaxis at Day 100.
Patients and methods
Clinical trial
IMPROVIT is a prospective, open label, multi-center study for primary IFI prophylaxis after alloHCT. Patients ≥12 years old were randomized (stratified by conditioning regimen intensity and donor type) to receive oral voriconazole or oral itraconazole prophylaxis from the day of HCT (Day 0) for at least 100 and up to 180 days. All subjects were followed for 180 days, irrespective of prior discontinuation for any reason. The two trial treatments were (1) Voriconazole IV 6 mg/kg BID for the first 24 h and then 200 mg BID or 100 mg PO BID; and (2) Itraconazole IV 200 mg BID for the first 2 days and then 200 mg PO BID. More details of the trial including the recommended use of both study drugs have been reported elsewhereCitation15.
Ethical conduct of the trial
The IMPROVIT trial was performed in accordance with the protocol, International Conference on Harmonization Good Clinical Practice guidelines, and applicable local regulatory requirements and laws for institutional review board (IRB) and ethics committee approvals. Tolerability and MRU end-points discussed in this paper were part of the original study design reviewed by IRB and ethics committees.
Study population and end-point
The study population consists of patients with alloHCT who received at least one dose of randomized study drug (i.e., modified intent to treat [MITT] population of the IMPROVIT). Patients from one study site were excluded due to a suspected Good Clinical Practice breach (10 received voriconazole, 14 received itraconazole)Citation15.
The primary composite end-point of IMPROVIT is the success of prophylaxis at Day 180, i.e., survival to Day 180 with no breakthrough IFI and no discontinuation of study drug for >14 days in total during the 100-day prophylaxis. For this analysis, secondary clinical end-points of safety and tolerability (adverse events, treatment duration, and discontinuation), as well as economic end-points of medical resource use during the first 100 days of prophylaxis were assessed.
Resource utilization
MRU related to IFI/prophylaxis recorded during the first 100 days of the trial was summarized for each patient and treatment group.
The specific MRU measures included: (1) Inpatient bed days (the number of [re]hospitalizations, total hospital days, general ward days, and other special care days such as intensive care unit [ICU], hematological/oncology unit, and transplant unit days); (2) Emergency room/day hospital (outpatient days); (3) Treatment procedures and physician services received in hospital related to prophylaxis; (4) Outpatient services related to IFI/prophylaxis (number of unscheduled outpatient visits, number of days in homecare); and (5) Use of other systemic antifungal medications besides study drug (identified by members of trial steering group). These MRU data collected (via trial case report forms) during the first 100 days of the trial were summarized for each patient and treatment group. Hospital length of stay was calculated by subtracting the admission date from the discharge date plus one. Lengths of stay for multiple admissions were summed as the total hospital days for analyses.
Statistical analysis
Statistical analyses were performed using SAS® software (SAS Institute, Cary, NC) version 9.2. All confidence intervals (CIs), statistical tests, and resulting p-values were 2-sided. All hypothesis testing was performed at the 5% significance level.
In univariate analysis, continuous MRU variables were tested for baseline treatment group differences by the non-parametric Wilcoxon-Mann-Whitney test due to the non-normality of these variables. Categorical variables and patient population frequency were tested by Pearson chi-square (or Fisher’s exact test for frequency table with small sample size).
In multivariable analysis, logistic regression analysis was used to assess the impact of treatment and treatment duration on the use of other concomitant antifungals. Furthermore, generalized linear models (GLM) were used to evaluate the impact of treatment, treatment duration, and treatment discontinuation on continuous MRU end-points (i.e., total hospital days and other concomitant AF treatment duration) while controlling for baseline covariates and factors. Data were reviewed and tested to identify appropriate distributional family and link. Gamma distributions were selected by the modified Park test from the exponential family for total hospital days. Finally, given that no distribution in the exponential family was found to fit the skewed response variables perfectly, bootstrap validations were performed to confirm univariate and multivariate sensitivity on the analysis results. In the bootstrap procedure, the original dataset of size n (i.e., 465) becomes a parent population from which samples of size n are randomly drawn with replacementCitation16. Two thousand bootstrap samples were created and, for each of the samples, the mean difference between treatment groups was estimated, and the final multivariate model was refit with new regression parameters. The sample mean and standard error of the 2000 treatment comparisons on hospital days as well as regression parameters were computed and used to formulate 95% confidence intervals. These estimates were compared with those obtained in the GLM regression models.
Results
Patients
Among the total of 465 subjects analyzed, 224 patients were in the voriconazole and 241 patients in the itraconazole groups. Similar age, sex, race distributions, and clinical characteristics were identified (). The conditioning regimen was myeloablative for the majority of subjects: 125 (55.8%) in the voriconazole group and 143 (59.3%) in the itraconazole group (p = 0.44). Approximately 25% of subjects had both mismatched/unrelated donors and myeloablative conditioning regimen in each treatment group: 59 (26%) in the voriconazole group and 58 (24%) in the itraconazole group. The most commonly reported primary diagnosis was acute myeloid leukemia: 98 (44%) subjects in the voriconazole group and 109 (45%) subjects in the itraconazole group ().
Table 1. Patient baseline characteristicsa.
Treatment tolerability
Treatment discontinuation/duration up to the first 100 days of the trial study is presented in . On average, voriconazole patients stayed on the study drug 8 days longer than itraconazole (mean: 68 vs 60 days, p = 0.0044). Median study treatment exposure was 96 days and 68 days for voriconazole and itraconazole, respectively. More itraconazole patients discontinued study treatment before day 100 compared to voriconazole (63% vs 50%, p = 0.0137). The reasons for treatment discontinuation are also summarized in . The most frequently reported reason for discontinuation was adverse events. More details on adverse events are described in Marks et al.Citation15. The number of patients who discontinued due to intolerance of study medication was significantly higher for itraconazole than voriconazole patients (22% vs 7%, p < 0.0001).
Table 2. Treatment discontinuation/duration (MITT overall).
Medical resource utilization
Resource utilization on total hospital days, ER/day hospital visits, outpatient visits, concomitant treatment procedures, and other AF use during the first 100 days is presented in . No significant difference in the mean total days of hospitalization during the study period was observed between voriconazole and itraconazole (37 vs 38 days, respectively). As expected, almost all patients had either hematological/oncology or transplant unit stay with an average length of 35 days. Only 16 (8%) voriconazole and 14 (7%) itraconazole subjects were reported as having at least one ICU stay, with an average duration of 15 days for both treatment groups. There was no statistically significant difference in outpatient services use or concomitant treatment procedures between the treatment groups.
Table 3. Non-drug medical resource utilization.
Use of other concomitant antifungal drugs
The concomitant use of systemic antifungal medications other than the study drugs (other AF) is presented in . Fewer voriconazole patients had other AF use compared to itraconazole patients (25% vs 39%, p = 0.0014), with significantly shorter duration of other AF exposure (29 days vs 42 days, p = 0.0075). The most commonly used other AFs were caspofungin (i.v.), liposomal amphotericin (AmBisome) (i.v.), and fluconazole (oral). There was a significant difference in the use of caspofungin, fluconazole, and voriconazole between the treatment groups: fewer voriconazole patients were administered caspofungin and fluconazole compared to itraconazole patients (10% vs 18%, p = 0.0142 and 5% vs 13%, p = 0.0032, respectively). It is worth mentioning that more than 12% of itraconazole patients were found to have cross-over to voriconazole prophylaxis, significantly higher than the voriconazole–itraconazole switch (less than 2%) (p < 0.0001).
Table 4. Other concomitant antifungal usea.
Impact of treatment on resource use
Multiple logistic regression analysis was conducted to assess the impact of treatment on the use of other AF while controlling for baseline covariates and factors (). This showed that itraconazole patients were 2-times more likely (p = 0.0110) to use concomitant antifungals than voriconazole patients. Logistic regression analyses were also conducted to evaluate the impact of treatment on the use of inpatient care (re-hospitalization and the use of ICU) and outpatient services. Consistent with the findings of the univariate tests, no significant treatment impact was identified on these resource utilization components.
Table 5. Impact of study drug prophylaxis and prophylaxis duration on other antifungal use (n = 460).
Association between treatment tolerability and resource use
Controlling for treatment and key baseline variables, prophylaxis duration was found to be associated with the key resource utilization components (): longer IFI prophylaxis was associated with fewer total hospital days (p < 0.0001) and less other concomitant AF use (p < 0.0001). Figure 1. further demonstrates the association between prophylaxis exposure and total hospital days as well as other AF use based on the coefficient obtained from the GLM regression analysis. The red dots in indicate that the mean difference of 8 days of prophylaxis between voriconazole and itraconazole group was associated with ∼1 fewer hospital day and 3.6 fewer other AF days.
Figure 1. Association between Prophylaxis Treatment Duration and Resource Utilization (Total Hospital Days and Other Antifungal Days).
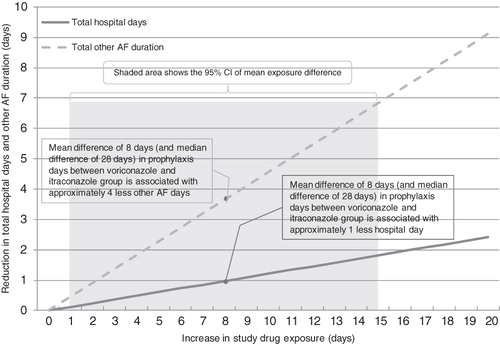
Table 6. Impact of study drug treatment duration on total hospital days and other antifungal duration, controlling for key baseline variables.
Similarly, treatment discontinuation was significantly associated with greater resource use. A series of logistic regression analyses showed that, while controlling for treatment and baseline variables, discontinued patients were 2-times more likely to be re-hospitalized (p = 0.0123), 3-times more likely to require ICU admission (p = 0.0546), and almost 6-times more likely to use other AF (p < 0.0001), compared to the patients without treatment discontinuation. Further GLM analyses showed that patients who discontinued prophylaxis during the first 100 days after alloHCT incurred 10 more hospital days (p < 0.0001) and 17 more other AF days (p < 0.0001), compared with their counterparts after controlling for treatment and key baseline variables.
Bootstrapping validation
A bootstrap validation was conducted on the mean difference of per patient total hospital days (itraconazole minus voriconazole). No significant difference in total hospital days was identified between the two treatment groups (mean difference = 0.9036 day, 95% CI = 0.8058, 1.0014). Among 2000 iterations, 1328 (66%) mean differences were reported as positive, indicating higher per-patient hospital days of itraconazole compared to voriconazole. The multivariate bootstrap analyses revealed almost identical coefficients compared to the GLM regressions for total hospital days.
Discussion
To our knowledge, this study is the first to assess the medical resource utilization consequences of drug tolerability for mold active azoles as primary prophylaxis in alloHCT based on a randomized, open-label clinical trial study. By dissecting the components of the composite measure of the original clinical trial (i.e., tolerability for at least 100 days and the success of prophylaxis at Day 100), this study puts the findings of the trial in a context that may be more clear to decision-makers. The difference in tolerability observed between voriconazole and itraconazole and associated resource utilization consequences may affect clinicians’ decisions regarding treatment selection in the real-world.
A desired outcome given the treatment goal of patients on prophylaxis drug is the ability to achieve longer treatment time. Voriconazole has demonstrated better tolerability with a longer treatment duration compared to itraconazole for the primary prophylaxis of post-alloHCT subjects at risk of fungal infectionCitation11. Consistent with the clinical trial findings, our analysis showed that all-cause and study drug intolerance discontinuations were significantly less frequent with voriconazole than itraconazole during the first 100 days after alloHCT. Voriconazole patients stayed significantly longer on the study drug (28 days longer in median and 8 days longer in mean) compared to itraconazole patients. Furthermore, voriconazole patients tended to have less other AF use and shorter other AF treatment duration compared to itraconazole patients. The 8-day mean difference in prophylaxis treatment resulted in ∼1 fewer hospital day and 3.6 fewer other AF days, which may be attributable to the higher intolerance rate seen in itraconazole patients. Voriconazole’s tolerability advantage did not translate into a statistically significant difference compared to itraconazole in total hospital days and the use of other non-drug medical resources (ICU days, outpatient services, and concomitant treatment procedures), which may be due to a common challenge of using clinical trial data for economic analysis. In that case, direct comparison between treatment groups is likely under-powered to evaluate differences in continuous outcomes that are usually more heterogeneous with large standard deviations.
Regardless of treatment, longer prophylaxis duration was associated with fewer hospital days and less other AF use. Patients who discontinued prophylaxis before Day 100 had longer hospital stays and more other AF use, compared to the patients who were able to remain on their prophylaxis for the first 100 days. These findings indicate that a patient who can stay longer on his or her mold-active azole prophylaxis treatment will incur less medical resource use.
Our study confirmed that, on economic grounds, drug tolerability should be a key determinant in the selection of an antifungal agent for prophylaxisCitation3,Citation17. The decision to use a mold-active agent should take into consideration the patients’ risk of drug-related adverse effects. Clinicians’ awareness about adverse events and vulnerable population may influence the choice of a drug and patients’ adherence to treatment, and eventually treatment outcomes. The major adverse effects of itraconazole were related to gastrointestinal intolerance, including nausea, vomiting, and diarrhea. Previous studies have shown that significantly more adverse effects, especially gastrointestinal complaints, and withdrawals due to adverse effects were associated with itraconazole compared to fluconazole or voriconazoleCitation9–11,Citation15.
Drug tolerability also greatly influences patient satisfaction, treatment adherence, and treatment outcomes. It was shown that the better tolerability of voriconazole compared with itraconazole was reflected in patient satisfaction scores measured by the Treatment Satisfaction Questionnaire for Medication (TSQM): voriconazole patients reported higher convenience and global satisfaction scores at 2 weeks after the start of study treatmentCitation15. It has been recognized that tolerability limits the use and effect of antifungal treatment and should be considered in the clinical decisions in order to increase treatment adherenceCitation3,Citation18,Citation19.
A previous literature review study revealed that there is a pressing need to incorporate the collection of economic outcome measures in clinical trials for systemic antifungal therapiesCitation20. The current study demonstrated that treatment tolerability has a substantial impact on resource utilization in terms of total hospital days and other concomitant systemic antifungal drug use. Future economic evaluations of antifungal prophylaxis agents should take into account tolerability issues and the economic consequences of that tolerability.
There are several limitations of our study. First, this economic analysis only focused on the first 100 days of prophylaxis because complete data collection on MRU was limited to the first 100 days per protocol. If the actual duration of exposure to study drugs is used (i.e., up to Day 180), the mean difference in treatment duration between voriconazole and itraconazole is more than doubled (increased from 8 days to 17 days). Therefore, the benefit of voriconazole from prolonged treatment exposure may have been under-estimated in this truncated economic analysis or over-estimated if the findings were different from Day 100 to 180. There may also be a bias against voriconazole in this study because the duration on the study drug was generally longer compared to itraconazole and only the patients that remained on study drug had complete MRU data collection per study protocol. Once the study drug was stopped, the sites may no longer collect resource utilization information such as outpatient visits and treatment procedures per protocol. Therefore, the period of observation for these events was longer for voriconazole patients compared to itraconazole patients. Furthermore, the results generated from a clinical trial setting may not reflect real-world use and outcomes.
In addition, no further high-risk sub-group analyses were conducted (e.g., for a high risk sub-group defined by several combined risk factors such as mismatched/unrelated donors with myeloablative conditioning regimen as well as primary diagnosis and comorbidity). The current findings may not be generalizable to different risk sub-groups. However, we conducted some sub-group analysis based on donor type and conditioning regimen to compare treatment difference in total hospital days and did not find any different trends compared to the main analysis.
Finally, this study only considered MRU consequences and did not attempt to conduct a cost analysis or cost-effectiveness analysis. The MRU data collected in the trial were from multiple countries. Cost analysis based on one country unit price may not be generalizable to other countries. For example, the cost of a hospital day in the US can be much higher than that in the UK. Thus, the cost impact of tolerability of antifungal prophylaxis may be different from country to country. Furthermore, given that there was no difference in 180-day survival between VOR and ITR in the IMPROVIT trial, no efficacy/effectiveness end-point and trade-offs were analyzed in this study. A comprehensive cost-effectiveness evaluation considering other efficacy end-points and economic consequences may be warranted.
Conclusion
Voriconazole was better tolerated than itraconazole, and better tolerability was associated with less use of medical resources such as other antifungals and hospital days. This difference may actually be under-estimated in this truncated economic analysis, with the period of observation for MRU events being significantly longer for voriconazole patients. The potential economic impact of the tolerability of antifungal prophylaxis needs to be considered in the current resource-constrained healthcare environment.
Transparency
Declaration of funding
The IMPROVIT study was sponsored by Pfizer Inc. Research funding for this work and preparation of this manuscript was funded by Pfizer Inc.
Declaration of financial/other relationships
Xin Gao, Xiang Ji, and Jennifer Stephens were employed by Pharmerit International, who were paid consultants to Pfizer in connection with this analysis and the development of content for this manuscript. Miriam Tarallo and Haran Schlamm were employed by Pfizer Inc, the manufacturer of antifungal agents. The work was performed at Pharmerit International, Bethesda, MD. David Marks has received honoraria for advisory board work for Pfizer, Merck, Gilead and Schering Plough. JME Peer Reviewers have no relevant financial or other relationships to disclose.
Acknowledgments
Previous Presentations: Schlamm H, Gao X, Ji X, Stephens J, Tarallo M. ‘Economic evaluation of voriconazole versus itraconazole for primary prophylaxis of invasive fungal infection in allogeneic hematopoietic stem cell transplant’; 21st ECCMID 2011, Milan Italy; May 7–10, 2011. Gao X, Ji X, Stephens J, Schlamm H, Tarallo M. ‘Association between Drug Tolerability and Medical Resource Use in Prophylaxis of Invasive Fungal Infections after Allogeneic Hematopoietic Stem Cell Transplant’; ISPOR 16th Annual International Meeting; May 21–25, 2011; Baltimore, MD.
References
- Denning DS, Stevens DA. Antifungal and surgical treatment of invasive aspergillosis: review of 2121 published cases. Rev Infect Dis 1990;12:147-201
- Clinical practice guidelines in oncology. Prevention and treatment of cancer-related infections. National Comprehensive Cancer Network (NCCN); 2009
- McCoy D, Depestel DD, Carver PL. Primary antifungal prophylaxis in adult hematopoietic stem cell transplant recipients: current therapeutic concepts. Pharmacotherapy 2009;29:1306-25
- Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation — a prospective, randomized, double-blind study. J Infect Dis 1995;171:1545-52
- Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992;326:845-51
- Vardakas KZ, Michalopoulos A, Falagas ME. Fluconazole versus itraconazole for antifungal prophylaxis in neutropenic patients with haematological malignancies: a meta-analysis of randomised-controlled trials. Br J Haematol 2005;131:22-8. [Erratum, Br J Haematol 2006;132:665.]
- Simon A, Besuden M, Vezmar S, et al. Itraconazole prophylaxis in pediatric cancer patients receiving conventional chemotherapy or autologous stem celltransplants. Support Care Cancer 2007;15:213-20
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348-59
- Winston DJ, Maziarz RT, Chandrasekar PH, et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients: a multicenter, randomized trial. Ann Intern Med 2003;138:705-13
- Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 2004;103:1527-33
- Morgenstern GR, Prentice AG, Prentice HG, et al.; for the U.K. Multicentre Antifungal Prophylaxis Study Group. A randomized controlled trial of itraconazole versus fluconazole for the prevention of fungal infections in patients with haematological malignancies. Br J Haematol 1999;105:901-11
- Cecil JA, Wenzel RP. Voriconazole: a broad-spectrum triazole for the treatment of invasive fungal infections. Expert Rev Hematol 2009;2:237-54
- Herbrecht R, Denning D, Patterson T, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Eng J Med 2002;347:408-15
- Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 2005;366:1435-42
- Marks DI, Pagliuca A, Kibbler C, et al. Voriconazole versus itraconazole for antifungal prophylaxis following allogeneic haematopoietic stem-cell transplantation. Br J Haematol 2011;155:1365-2141
- Jiang H, Zhou XH. Bootstrap confidence intervals for medical costs with censored observations. Stat Med 2004;23:3365-76
- Singh N. Antifungal prophylaxis in solid-organ transplant recipients: considerations for clinical trial design. Clin Infect Dis 2004;39:S200-6
- Johnson E, Gilmore M, Newman J, et al. Preventing fungal infections in immunocompromised patients. Br J Nurs 2000;9:1154-6, 1158–64
- Epstein JB, Truelove EL, Hanson-Huggins K, et al. Topical polyene antifungals in hematopoietic cell transplant patients: tolerability and efficacy. Support Care Cancer 2004;12:517-25
- Dixon S, McKeen E, Tabberer M, et al. Economic evaluations of treatments for systemic fungal infections: a systematic review of the literature. Pharmacoeconomics 2004;22:421-33