Abstract
Objective:
This retrospective claims study investigated the rates of all-cause hospitalization among chronic obstructive pulmonary disease (COPD) patients initiating treatment with short-acting beta agonists (SABA) or long-acting beta agonists (LABA).
Methods:
Data from the 5% national sample of Medicare enrollees for 2006–2008 were used. Patients initiating COPD therapy were identified as those with no COPD therapy for ≥ 6-months prior to initiating SABA or LABA (administered via dry-powder inhalers, metered-dose inhalers, or nebulizer) treatment. All patients were continuously eligible for Medicare Parts A, B, and D for 18 months. Those enrolled in Medicare Advantage, who had asthma, or were < 65 years old were excluded. Differences in the rates of all-cause hospitalizations and time to all-cause hospitalization during the 6-month follow-up period were examined, while adjusting for demographics, clinical indicators, and health service use.
Results:
Among 3017 COPD patients who met the inclusion criteria, 883 (30%) were LABA users and 2134 (70%) were SABA users. Overall, 21% of patients (16% [144/883] of LABA and 23% [492/2134] of SABA) had a hospitalization during the follow-up period. Mean time to hospitalization was 86 days for LABA vs 64 days for SABA patients (p < 0.05). The adjusted hazard ratio for hospitalization in a Cox proportional hazards model was 0.74 (95% CI = 0.62–0.90) for patients treated with LABA vs. SABA.
Limitations:
The analysis was adjusted for multiple background characteristics, but important measures of severity in COPD, such as measures of lung functioning, were not available and may have differed between patients treated with LABA or SABA.
Conclusions:
The results of this analysis indicate COPD patients initiating LABA treatment had a longer time to all-cause hospitalization and a 26% lower risk of hospitalization during the 6-months follow-up period compared to those initiating SABA therapy.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease marked by dyspnea, cough, and sputum production. The worldwide prevalence of COPD has been estimated at 7.6%; however, due to the progressive nature of COPD, the prevalence increases with age: in adults age 40 and older the prevalence was 9.9% and in those age 65 and older it was 14.2%Citation1.
The goals of COPD treatment are to reduce symptoms, decrease the frequency and severity of COPD exacerbations, and improve exercise tolerance and health outcomesCitation2. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) treatment guidelines, updated in 2011, recommend bronchodilators as the primary pharmaceutical treatments for COPD. The two main types of bronchodilators are beta-agonists and anticholinergics, available as short-acting (for rescue or as needed treatment) and long-acting (for maintenance treatment). Monotherapy with short-acting bronchodilators is only recommended in mild COPDCitation2. However, long-acting treatments are preferred because, by the time most patients are diagnosed, their COPD has progressed beyond the mild phaseCitation3,Citation4. Other treatments that are recommended as second-line or combination use as the disease progresses include inhaled corticosteroids, methylxanthines, and phoshodiestrase-4 inhibitors. Supplemental oxygen therapy is used for more severe patientsCitation2.
In usual clinical care, COPD treatment guideline recommendations are not always followed and many patients do not appear to receive the recommended maintenance treatmentCitation5. A recent study using administrative claims data found that 66% of commercial and 71% of managed Medicare patients received no maintenance treatment for COPDCitation6. When examining only the moderate-to-severe COPD patients, 59% of commercial and 69% of managed Medicare patients still received no maintenance therapyCitation6. Another study reported that 38% of patients did not receive any COPD medicationCitation7.
Lack of maintenance therapy appears to have negative consequences. One study reported that COPD patients with no maintenance treatment had a significantly greater risk of hospitalization and significantly higher treatment costsCitation8. In randomized controlled trials, long-acting anticholinergics (LAAC) have been found to reduce hospitalizations when compared with short-acting anticholinergics or placebo treatmentCitation9. However, few reports have specifically examined the effects of long-acting beta agonists (LABA) on reducing all-cause hospitalizations. Exacerbations of COPD, particularly severe exacerbations which require hospitalizationCitation10, may result in progressing disease severityCitation11, lowered health statusCitation12, increased likelihood of rehospitalizationCitation13,Citation14, and increased mortalityCitation13,Citation14.
In the US, individuals aged 65 and older are eligible for Medicare coverage, which makes Medicare administrative claims data particularly relevant for studying COPD hospitalization outcomes in usual clinical care. The prevalence of COPD in Medicare has been reported at 10.9%, with enrollees having an average of 1.25 inpatient discharges per yearCitation15. The objective of this study was to investigate the relative risk of all-cause hospitalization among Medicare COPD patients initiating treatment with LABA vs short-acting beta agonists (SABA).
Methods
Database description
This retrospective cohort analysis used the Medicare 5% sample for years 2006–2008. The database consisted of all the administrative claims during this period for a randomly selected sample of 5% of the Medicare beneficiariesCitation16. The analytical database included inpatient facility claims (Part A), outpatient medical service claims (Part B), and prescription drug claims (Part D). The analysis was restricted to patients who had a fee-for-service Medicare plan and did not include beneficiaries enrolled in the Medicare Advantage supplemental insurance (Part C). Administrative claims database analyses allow for the unobtrusive observation of usual clinical care. The study proposal was submitted to the Institutional Review Board at the University of Southern California and a waiver was received prior to study initiation.
Inclusion/exclusion criteria
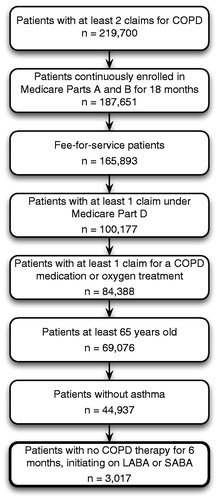
All patients included in the analysis were required to have data during the full study period based on at least 18 months of continuous enrollment in Medicare Parts A and B and at least one Medicare Part D drug claim. Patients with COPD were identified as those with at least two claims with ICD-9-CM diagnostic codes for COPD (491.x, 492.x, 494.x, or 496.x) in 2006 and treatment for COPD based on at least one claim for a COPD medication under Medicare Parts B or D or one claim for oxygen treatment. The analysis was further restricted to patients aged 65 and older who did not have any asthma diagnoses. Finally, patients who initiated treatment with either LABA or SABA treatment were identified; each patient had no COPD therapy during the 6-month baseline period and then initiated treatment with LABA or SABA. The patient flow through the selection criteria is documented in .
Study design
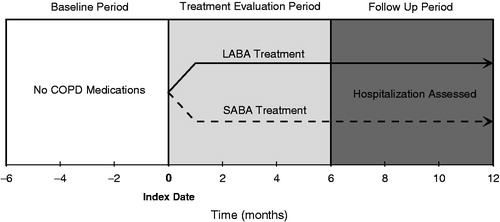
Each patient was followed over an 18-month period that was divided into three 6-month study periods (see ). The date when each patient initiated LABA or SABA treatment was designated as the patient’s index date. The 6-month period preceding the index date, when the patients had no treatments for COPD, was labeled as the baseline period. The 6-month period following the index date was labeled the treatment evaluation period. In the absence of disease severity information for these patients initiating COPD pharmacotherapy, the treatment evaluation period provided additional early treatment information that could reflect potential underlying differences between patients and was controlled for in the analyses. Any patient who initiated treatment with LABA and was treated with both LABA and SABA during the treatment evaluation period was classified as a LABA patient. Patients who started on SABA, but switched to LABA during the treatment evaluation period were excluded from the analyses. All outcome variables were assessed during the follow-up period, which was the final 6-month period in the study (months 6–12 following the index date). All patients were followed regardless of continuing or discontinuing LABA or SABA treatment or the addition of other COPD treatments.
Measures
The primary outcome variable, time to all-cause hospitalization, was measured from the first day of the follow-up period until the first day of an inpatient hospitalization. All-cause hospitalization was chosen instead of COPD-related hospitalization because COPD can play a significant role in the severity and mortality of other common causes of hospitalization, such as cardiopulmonary disease and pneumoniaCitation17,Citation18. To adjust for potential differences between the LABA and SABA patient groups, a large number of covariates were included in the models. The covariates were comprised of demographic information, clinical indicators, and health service use. The demographic information included age at the index date, gender, and race. The clinical indicators included number of spirometry tests, primary diagnosis (based on the first 3 digits of ICD-9-CM COPD diagnosis), the number and type of comorbid conditions, Charlson Comorbidity IndexCitation19, and concomitant oral corticosteroid (OCS) use. Finally the health service utilization covariates included any physician office visit, any specialist (pulmonologist) office visits, any emergency department (ER) visits, and the total number of days hospitalized. The covariates were calculated during the baseline and treatment evaluation periods. Descriptive information on the average length of stay and cost of treatment for the two cohorts during the follow-up period was provided for exposition purposes. All costs represent the amount paid to the provider as reported in the database.
Statistical methods
Comparisons between the LABA and SABA patients at baseline were conducted using t-test for continuous variables and chi-square tests for categorical variables. Descriptive information for continuous variables was displayed as mean and standard deviation (SD). A Cox proportional hazards model was used to compare the risk of first hospitalization for patients treated with LABA and SABA while controlling for the covariates defined above. Among the subset of LABA and SABA patients who were hospitalized during the follow-up period, the mean time to hospitalization was compared using a multivariate regression model while controlling for the previously defined covariates. The average inpatient cost and length of stay during the follow-up period was presented as unadjusted means and no significance tests were conducted on these variables. The alpha level was set at 0.05. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Sample description
The final sample consisted of 3017 Medicare beneficiaries, who were treated with either SABA (70.7%; n = 2134) or LABA (29.3%; n = 883). During the baseline period, the LABA group had a lower mean age, fewer comorbidities, more spirometry tests and physician visits, and less ER and hospitalization use (see ).
Table 1. Patient characteristics during the baseline and treatment evaluation periods.
Time to hospitalization
The Kaplan-Meier curves of time to all-cause hospitalization for the LABA and SABA treated patients are depicted in . During the follow-up period, 21% (636/3017) of the patients (16% [144/883] of LABA vs 23% [492/2132] of SABA; p < 0.05) had a hospitalization. The Cox proportional hazards model found the LABA-treated patients had a lower risk of hospitalization relative to SABA patients (hazard ratio = 0.74; p = 0.002) after correcting for background characteristics (see ). For the 636 patients who were hospitalized, the mean time to all-cause hospitalization was 86 (SD = 52) days for the LABA-treated patients (n = 144) and 64 (SD = 49) days for the SABA-treated patients (n = 492). In a linear regression model, after correcting for the same covariates as the Cox model, the time to hospitalization was 13 days longer for the LABA-treated patients than the SABA-treated patients (p = 0.005).
Figure 3. Kaplan-Meier curve for time to all-cause hospitalization. After correcting for background characteristics, the LABA treated patients had a significantly lower risk of hospitalization (HR = 0.74, p = 0.002).
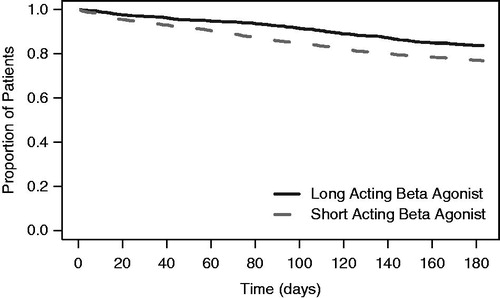
Table 2. Results of Cox proportional hazards model for time to hospitalization.
Inpatient costs and inpatient days
The average inpatient cost during the 6-month follow-up period was $2018 for the full LABA group and $3416 for the full SABA group. Among the subset of patients who were hospitalized, the average inpatient cost was $12,277 and $14,821 for the LABA and SABA groups, respectively. The average number of total inpatient days during the follow-up period among the subset of patients who were hospitalized was 19.7 days for the LABA group and 28.0 days for the SABA group.
Discussion
In this retrospective analysis of Medicare claims, LABA treatment for COPD was associated with a longer time to all-cause hospitalization relative to SABA treatment. The results were comparable between the Cox proportional hazards model (HR = 0.74) and the linear regression model comparing the days until hospitalization among those who were hospitalized. The unadjusted inpatient treatment costs during the follow-up period were $569 and $336 per month for the SABA and LABA treated patients, respectively. The use of LABA maintenance treatment instead of SABA rescue treatment appears to reduce or delay hospitalizations, which could potentially reduce healthcare expenses and improve outcomes for patients.
The use of LABA is consistent with the GOLD treatment guidelines once a patient’s COPD has progressed beyond the mild phaseCitation2. Although no direct information about disease severity was available in the claims, previous research has reported that nearly all patients' COPD had progressed by the time of diagnosisCitation3. For the majority of patients in this study, the SABA monotherapy treatment was probably not consistent with the GOLD treatment guidelines.
Greater hospitalization risks have been previously reported for patients with COPD in the Medicare population who were not treated with any maintenance treatmentCitation8. A study using data from the Medicare Current Beneficiary Survey from 1997–2005, prior to the Medicare Part D drug benefit implementation, compared outcomes for COPD patients who were treated or not treated with any COPD maintenance therapyCitation8. Any maintenance therapy use was associated with a decreased likelihood of hospitalization (Odds Ratio [OR] = 0.70), rehospitalization within 30 days (OR = 0.74) and $3916 in reduced total annual medical expenditures. Even though this previous study relied on self-report medication use, combined all COPD maintenance therapies into a single category and contrasted the treated patients with patients potentially receiving no COPD treatments, the results were similar to the current study comparing LABA and SABA treatments on hospitalization risk.
Randomized controlled studies have also examined risk of hospitalization for patients treated with specific long acting bronchodilators. In a Cochrane meta-analysis, tiotropium (a LAAC) was found to be more effective than short acting anticholinergic bronchodilators or placebo in reducing exacerbations and hospitalizationsCitation9. There were no meaningful differences between tiotropium and the LABA medication, salmeterol, in preventing exacerbations or hospitalizationsCitation9. Similarly, another Cochrane review found that combination treatment with LABA and LAAC medications was effective in reducing hospitalizations vs placeboCitation20. Finally, a randomized study of the LABA medication, salmeterol, vs placebo found that salmeterol treatment resulted in reduced hospitalization costsCitation21. Although this study represents the first comparative research on the hospitalization risk for patients treated with LABA vs SABA medications in usual care, the findings of the current study are consistent with meta-analyses of randomized controlled trials contrasting treatment with long-acting bronchodilators relative to short-acting bronchodilators or placebo. Additional research examining formulation (nebulizer vs dry-powder inhaler) is needed in order to determine potential differences based on formulation.
In usual care, patients with COPD are often not treated with long-acting bronchodilators despite the recommendations in treatment guidelinesCitation2,Citation6. The findings of the current study and the broader literature suggest patients who do not receive long-acting bronchodilators experience higher rates of COPD exacerbations that could lead to hospitalizations. COPD is a progressive disease and exacerbations may speed up disease progressionCitation11. Increased rates of exacerbation have also been linked to lowered health statusCitation12 and increased risk for rehospitalizationCitation13,Citation14. Prevention of further deterioration of lung function is one of the primary goals of treatmentCitation22.
Patients who are hospitalized have increased mortality risk and have a higher likelihood of readmissions. A study by Jencks et al.Citation23 found that 22.6% of Medicare patients hospitalized for COPD were readmitted within 30 days of discharge. To reduce preventable hospital readmissions, Section 3025 of the Patient Protection and Affordable Care Act gave the Centers for Medicare and Medicaid Services (CMS) the authority to penalize hospitals with higher than expected 30-day readmission ratesCitation24. Greater use of LABA instead of SABA treatment in patients diagnosed with COPD in Medicare may reduce hospitalization rates and reduce hospitalization expenses.
Limitations
The administrative claims data used in this study had several limitations. Important measures of severity in COPD, such as measures of lung functioning, were not available and may have differed between patients treated with LABA or SABA. The analysis was adjusted for a large number of potential confounds and comorbidities, but measures for some important differences may not have been available and could have confounded the results. In addition, the majority of the LABA patients were treated with inhaled combination LABA/corticosteroids, which have a demonstrated benefit in preventing COPD exacerbations. Information on adherence with daily or as needed therapy was not available. The findings from this analysis need to be considered in light of the findings from randomized controlled trials. Patients with diagnoses in administrative claims may not always meet COPD diagnostic criteria if assessed using a formal diagnostic interview and spirometry. Consistent with some, but not all, previous administrative claims research, the definition of COPD included bronchiectasisCitation25–28. Among the 3017 individuals in the analysis, only 36 individuals (1.2%) met the operational definition of COPD based solely on diagnostic codes for bronchiectasis (ICD-9-CM = 494.x). The analysis examined all-cause hospitalizations because hospitalizations for COPD may be attributed to one of the common comorbiditiesCitation17,Citation18; the results may not generalize to the risk of COPD-related hospitalizations. The majority of LABA patients were treated with dry powder inhaled LABAs; additional analyses are needed to assess for potential differences in outcomes based on drug delivery system (dry powder inhaler/metered dose inhaler vs nebulizer).
Conclusions
The results from this retrospective database analysis suggest COPD patients initiating LABA treatment had a longer time to hospitalization and had a 26% lower risk of hospitalization during the 6-months follow-up period compared to those initiating SABA treatment. Greater use of LABA instead of SABA in usual care, which is consistent with treatment guidelines, may reduce hospitalizations.
Transparency
Declaration of funding
Funding for this study was provided by Sunovion Pharmaceuticals, Inc.
Declaration of financial/other relationships
Vamsi Bollu, Krithika Rajagopalan, and John Karafilidis are full time employees of Sunovion Pharmaceuticals, Inc., and were involved with the design, interpretation, and drafting of the manuscript, but not the data analysis. Flavia Ejzykowicz and Joel Hay have no relevant financial or other relationships to disclosure. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Technical writing support was funded by Sunovion Pharmaceuticals, Inc and provided by Michael D. Stensland, PhD of Agile Outcomes Research, Inc. Rochester, MN 55902.
References
- Halbert RJ, Natoli JL, Gano A, et al. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-32
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of COPD (Revised 2011). Global Initiative for Chronic Obstructive Lung Disease, Inc, 2011. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed September 17, 2012
- Lindberg A, Bjerg A, Bjerg-Bäcklund A, et al. Prevalence and underdiagnosis of COPD by disease severity and the attributable fraction of smoking Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med 2006;100:264-72
- Sandelowsky H, Ställberg B, Nager A, et al. The prevalence of undiagnosed chronic obstructive pulmonary disease in a primary care population with respiratory tract infections – a case finding study. BMC Fam Pract 2011;12:122-30
- Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed 2007;9:24-40
- Make B, Dutro MP, Paulose-Ram R, et al. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis 2012;7:1-9
- Heins-Nesvold J, Carlson A, King-Schultz L, et al. Patient identified needs for chronic obstructive pulmonary disease versus billed services for care received. Int J Chron Obstruct Pulmon Dis 2008;3:415-21
- Stuart BC, Simoni-Wastila L, Zuckerman IH, et al. Impact of maintenance therapy on hospitalization and expenditures for Medicare beneficiaries with chronic obstructive pulmonary disease. Am J Geriatr Pharmacother 2010;8:441-53
- Barr RG, Bourbeau J, Camargo CA. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005;(2):CD002876 . DOI: 10.1002/14651858.CD002876.pub2
- Andersson F, Borg S, Jansson SA, et al. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med 2002;96:700-8
- Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52
- Spencer S, Calverley PMA, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698-702
- Connors AF, Jr Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67
- McGhan R, Radcliff T, Fish R, et al. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007;132:1748-55
- Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Outcomes 2009;7:82-92
- Research Data Assistance Center Website. School of Public Health, Health Policy and Management, Minneapolis, MN: University of Minnesota, 2012. http://www.resdac.org Accessed September 17, 2012
- Holguin F, Folch E, Redd SC, et al. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005;128:2005-11
- Camilli AE, Robbins DR, Lebowitz MD. Death certificate reporting of confirmed airways obstructive disease. Am J Epidemiol 1991;133:795-800
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83
- Nannini L, Cates CJ, Lasserson TJ, et al. Combined corticosteroid and long-acting beta-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007;(4):CD003794 . DOI: 10.1002/14651858.CD002876.pub2
- Jones PW, Wilson K, Sondhi S. Cost-effectiveness of salmeterol in patients with chronic obstructive pulmonary disease: an economic evaluation. Respir Med 2003;97:20-6
- Celli BR. Update on the management of COPD. Chest 2008;133:1451-62
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418-28
- United States Congress. Patient Protection and Affordable Care Act. Sect. 3025, 2010. Washington, DC: United States Congress. 111-48. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf. Accessed September 17, 2012]
- Chan FWK, Wong FYY, Yam CHK, et al. Risk factors of hospitalization and readmission of patients with COPD in Hong Kong population: analysis of hospital admission records. BMC Health Serv Res 2011;11:186
- Moretti AM, Tafuri S, Parisi D, et al. Epidemiology and costs of hospital care for COPD in Puglia. Multidiscip Respir Med 2011;6:299-304
- Lorenz KA, Asch SM, Yano EM, et al. Comparing strategies for United States veterans’ mortality ascertainment. Popul Health Metr 2005;3:2
- Macchai A, Monte S, Romero M, et al. The prognostic influence of chronic obstructive pulmonary disease in patients hospitalised for chronic heart failure. Eur J Heart Fail 2007;9:942-8