Abstract
Background:
Growing financial pressure on US dialysis providers requires economic efficiency considerations. The objective of this study was to examine short-term economic efficiencies of a cinacalcet-based treatment approach for secondary hyperparathyroidism.
Methods:
This study retrospectively assessed cost per biochemical response of the OPTIMA trial. OPTIMA was conducted in end-stage renal disease patients to compare biochemical control in patients receiving cinacalcet in addition to vitamin D sterols and phosphate binders vs patients receiving vitamin D sterol and phosphate binders alone. It explored three laboratory measurement response definitions from baseline to week 23: (1) decreases in parathyroid hormone (PTH) ≥30%; (2) PTH ≤ 300 pg/ml; and (3) PTH ≤ 300 pg/mL, calcium <9.5 mg/dL and phosphorus <5.5 mg/dL. Medication use and costs were measured to calculate average costs and incremental cost per responder. Stratification by lower and higher baseline PTH assessed cost per response by disease severity.
Results:
There were 38–77% more responders with cinacalcet vs control, depending on response definition. Mean (SD) per patient total medication costs were $5423 ($3698) for cinacalcet and $2633 ($2334) for control, leading to a mean difference of $2790 over 23 weeks. When response was defined as a decrease in PTH ≥ 30% from baseline, the average cost per responder was $11,266 for control vs $7027 for cinacalcet. The incremental cost per incremental responder ranged from $5186–$9168. Across all response measures, cost per responder was lower in patients with lower baseline PTH.
Conclusions:
Representing a more efficient allocation of economic resources over the short-term, cinacalcet-based treatment algorithm led to a lower cost per biochemical response, particularly in patients with lower disease severity, vs vitamin D sterols and phosphate binders alone. These findings should be interpreted alongside the study limitation of converting international trial-based medication utilization into US costs.
Introduction
The evolving reimbursement policy for dialysis-related services in the US introduces a shift in the paradigm for clinical decision-making that may impact clinical as well as economic incentivesCitation1. In 2012 the Centers for Medicare and Medicaid Services (CMS) launched a pay-for-performance evaluation of the quality-of-care called the End-Stage Renal Disease (ESRD) Quality Incentive Program (QIP). The revised program is designed to track and incentivize quality based on attainment of targets for the recognized elements of dialysis care including, for example, dialysis adequacy and anemiaCitation2,Citation3. However, abnormalities of bone and mineral metabolism, such as secondary hyperparathyroidism (SHPT), are not currently included in the QIP creating a risk of sub-optimal short-term and long-term outcomes for SHPT.
SHPT is a common consequence of ESRD that can result in a host of clinical consequencesCitation4–12, including increased rates of fractureCitation13–17, vascular calcification and cardiovascular morbidity and mortalityCitation11,Citation18–20. This leads to high direct medical costs as well as decreased quality-of-lifeCitation15,Citation16,Citation21,Citation22. Studies have also shown that, by controlling PTH, calcium and phosphorus levels, the systemic consequences of disordered mineral metabolism can be attenuatedCitation5,Citation6,Citation8,Citation23–25, although the extent of this relationship remains uncertain as a more recently completed outcomes trial did not show treatment effects on clinical outcomes despite improved biochemical controlCitation26. Cinacalcet (Sensipar®, Amgen Inc, Thousand Oaks, CA) is an allosteric modulator of the calcium-sensing receptor effective in decreasing and maintaining PTH levels within proven therapeutic target ranges, while decreasing calcium and phosphorus levelsCitation23. Whereas vitamin D sterols are often used to treat SHPT, the benefits have a diminishing biochemical response that may result in increased disease severityCitation4,Citation25,Citation27. Furthermore, the narrow therapeutic window of vitamin D sterols requires incrementally increased dosing that is associated with hypercalcemia and hyperphosphatemiaCitation28.
The evolving reimbursement policy for dialysis services aims to curtail provider costs on the backdrop of budget constraintsCitation29; therefore, the question of health economic efficiency for treatment allocation from the provider perspective will be critical since it concerns the optimization of health benefits for a fixed cost from US treatment decision-makersCitation30. In the absence of the fully capitated payment system, the budgets and incentives of dialysis providers are fragmented, and under such conditions the most relevant time horizon for treatment of SHPT and its clinical and economic consequences for the US provider remains short, but guidelines for the optimization of medication use are absent, owing in part to the lack of consensus on the appropriate therapeutic targets for SHPT treatment. Data on US medication costs paired with clinical consequences for different SHPT treatment algorithms from the provider perspective (i.e., not including the long-run consequences of treatment) are not well understood. This is, in part, due to the issue that typical clinical trials tend to focus more on biological effects of therapies than on the economic efficiency of the treatment algorithms. Studies on cinacalcet, including ACHIEVE (Optimizing the Treatment of Secondary Hyperparathyroidism: A Comparison of Sensipar® and Low Dose Vitamin D vs Escalating Doses of Vitamin D Alone) and OPTIMA (Open-Label, Randomized Study Using Cinacalcet to Improve Achievement of KDOQI, Kidney Disease Outcomes Quality Incentive, Targets in Patients with End-Stage Renal DiseaseCitation31), sought to describe treatment algorithms optimized for biochemical control of SHPT. In the ACHIVE study, the primary end-point did not reach statistical significanceCitation25,Citation32; however, this may have been related to the primary clinical end-point used in the study. A PTH target range (150–300 pg/mL) and calcium × phosphorus product were used in ACHIEVE, whereas PTH levels below a specific level were the primary end-point in most other early studies with cinacalcetCitation23. A portion of cinacalcet treated subjects in ACHIEVE had over-suppressed PTH levels <150 pg/mL which contributed to the failure to meet the primary end-point. While ACHIEVE had a relatively small sample size (<100 patients per arm), the OPTIMA study was a more robust pragmatic trial helping to further elucidate the clinical benefits of cinacalcet and to describe potentially cost-containing treatment algorithms.
The objective of our study was to assess the short-term cost-efficiency of cinacalcet from the US provider perspective using data from the OPTIMA study. Specifically, we assessed cost, incremental cost per patient response, and incremental cost per time in response (weeks) to two SHPT medication regimens
Patients and methods
We conducted a post-hoc analysis of the OPTIMA study that compared a cinacalcet-based treatment algorithm containing cinacalcet, vitamin D sterol, and phosphate binders (n = 368) with vitamin D sterol and phosphate binders alone (n = 184)Citation31. All randomized patients were included in this analysis. Patients from the OPTIMA trial were ≥18 years of age with a diagnosis of SHPT who were on maintenance dialysis for more than 1 month and intact PTH (iPTH) between 300–800 pg/ml (bio-intact PTH ≥ 150 pg/ml and <410 pg/ml) and albumin-corrected serum calcium ≥8.4 mg/dl. Exclusion criteria are listed in the original publication of the OPTIMA studyCitation31.
Laboratory measurements were taken over an ∼6-month period from baseline to week 23. In this study, we used several definitions of response to therapy that have been broadly considered acceptable in assessing clinical efficacy in treating patients with SHPT based on (a) FDA-approved end-points in registrational trialsCitation33, and (b) attainment of KDOQI targets. Thus, we used:
A PTH decrease of ≥30% from baseline value to week 23 (this is consistent with the FDA approved primary end-point in the registrational trials);
PTH ≤ 300 pg/mL during the efficacy assessment phase (this response marker was consistent with KDOQI guidelines);
PTH ≤ 300 pg/mL, calcium <9.5 mg/dL, and phosphorus <5.5 mg/dL during the efficacy assessment phase (EAP) (the definition of response was recommended by KDOQI and captured the treatment response in a more comprehensive way).
The last observation carried forward method was used to account for missing data; for example, when a subject discontinues the study prior to reaching the EAP. This method provides a conservative estimate of the treatment benefit for patients in the cinacalcet group because patients who discontinue the treatment early might not have reached the optimal dose. Consistent with the OPTIMA trial design and clinical findings, the response definitions were assessed during the efficacy phase of the trial (7 weeks in duration during weeks 16–23). For each of the definitions of response, we also measured the number of weeks in response during the entire 23 weeks of follow-up. This expanded the definition of response beyond a dichotomous measure to include a description of the duration of response.
The average weekly dose was calculated for the following medications over 23 weeks: cinacalcet, vitamin D sterols (oral and [intravenous] IV calcitriol, oral and IV alfacalcidol, IV paricalcitol), and phosphate binders (calcium-containing supplements and sevelamer). If subjects withdrew early from the study, their medication use, up to the time of discontinuation, was included in the calculation of the total use. The USD costs of medications were calculated using 2012 wholesale acquisition cost (WAC) prices, consistent with the provider perspective where the pharmacy costs are born by the provider.
Analysis
The costs of medication use and responders were summarized for the cinacalcet and control groups as the mean per patient total cost of all medications and the total number, and percentage, of responders for the various definitions of response. The mean cost per responder was calculated as the mean per patient medication total cost divided by the percentage of patients responding. Incremental cost per incremental responder was calculated as the change in the mean per-patient cost between cinacalcet and control arms divided by the change in the percentage of patients responding between cinacalcet and control arms. Response over time was described as the mean number of response weeks by treatment arm. Finally, a sub-group analysis was conducted whereby cost per responder was calculated for patients within each treatment arm stratified by baseline PTH to PTH 300–500 pg/ml (lower) and PTH > 500 to PTH ─<800 pg/mL (higher).
In our analyses, we followed the International Society for Pharmacoeconomics and Outcomes Research good research practices for the transferability of economic evaluations across jurisdictionsCitation34.
Results
From the OPTIMA trial, 61% of patients randomized to the cinacalcet arm were male, 95% were white race, with a mean (SD) age of 58.5 (14.5) years, and a mean (SD) duration of dialysis of 64.1 (72.1) months. Results were comparable in the control arm in that 64% were male, 93% white race, with mean (SD) age of 58.3 (14.5), and mean (SD) duration of dialysis 69.4 (73.6) months. Application of the cinacalcet-based OPTIMA treatment algorithm resulted in significant reductions in mean iPTH values, with 71% of patients achieving the primary end-point of a mean iPTH value ≤300 pg/mL (compared with 22% in the control arm)Citation31. Additionally, PTH, calcium, phosphorus and Ca × P product levels decreased with cinacalcet therapy, while the control arm either increased or showed no change.
As reported in OPTIMACitation31, the most common cinacalcet doses, used by 65% of patients, were 60 mg/day or less. A total of 6% of patients used the maximum 180-mg/day dose most often vs other dosages throughout the efficacy assessment phase. The mean and median doses were 56 mg/day and 30 mg/day, respectively. The proportion of patients receiving vitamin D sterols increased from 66% to 81% at the end of the study in the control arm and from 68% to 73% in the cinacalcet arm. The costs (and utilization) of the intervention arm medications are summarized as a result of the present study.
summarizes the number and percentage of patients within each treatment arm who responded using the three different definitions of response. For each definition of response, more patients in the cinacalcet arm responded (ranging from 38–71%) than patients in the control arm (ranging from 7–23%). The incremental percentage ranged from 30–54%. Mean (SD) time spent as a responder was also higher for the cinacalcet arm compared to control for the three definitions of response (bottom half of ). When response was defined as PTH ≤ 300 pg/ml, cinacalcet arm patients on average (SD) had 13.4 (7.3) weeks of control compared to 4.1 (5.7) weeks, or the difference in means of 9.3 weeks between the cinacalcet and control arms. Depending on the definition of response, the incremental difference in mean time spent as a responder ranged from 6.5–10.1 weeks between cinacalcet and control arm patients.
Table 1. Response summary (number and percentage of responders for cinacalcet and control groups).
Medication unit costs, average per-patient weekly doses and the difference in mean weekly dose between cinacalcet and control arms are presented in . Average weekly dose for each type of vitamin D sterol was higher for the control than cinacalcet arm. Mean relative dose of vitamin D sterols decreased by 22% in the cinacalcet arm from baseline to week 23, but was virtually unchanged (3%) in the control armCitation32. Similarly, mean weekly dose of sevelamer was higher for control than cinacalcet arm patients, but patients in the cinacalcet arm received more calcium containing phosphate binders than patients in the control arm. Mean (SD) cinacalcet weekly dose over the 23 week period was 334.7 (213.8) mg.
Table 2. Medication wholesale acquisition cost (in US$) and trial-based utilization.
presents the mean (SD) medication utilization per-patient costs by medication type, as well as overall costs. While mean average costs for vitamin D sterols and sevelamer in the cinacalcet arm were lower than for control arm, cinacalcet and calcium containing phosphate binder costs were higher. Mean (SD) per patient total medication costs were $5423 ($3698) for the cinacalcet arm and $2633 ($2334) for the control arm, leading to the difference in means of $2790 over 23 weeks. That is, patients in the cinacalcet arm, on average, cost $2790 more over 23 weeks of treatment compared to the control arm.
Table 3. Medication trial-based costs ($)*.
Cost per responder and incremental cost per incremental responder
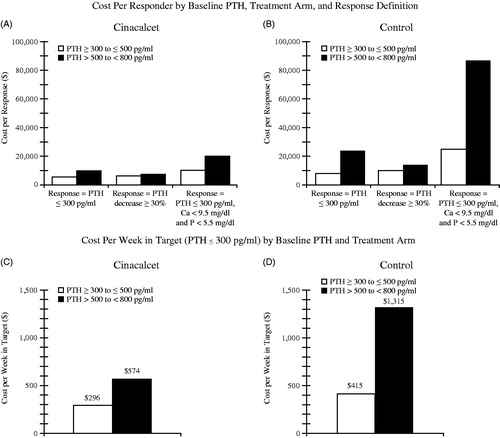
Average cost per responder by treatment arm and average incremental cost per incremental response for the three definitions of response is summarized in . For the responder definition of PTH ≤ 300 pg/ml, the average cost per responder was $11,815 for the control arm compared to no therapy. Similarly, the average cost per responder was $7617 for the cinacalcet arm compared to no therapy. The average incremental cost per incremental response was $5704 using the PTH ≤ 300 pg/ml responder definition. The interpretation of the average incremental cost per incremental response value is the average additional cost ($5704) required to achieve one additional responder when treating with the cinacalcet strategy as compared to the control strategy. Similar patterns were found with the other definitions of response ().
Table 4. Average cost ($) per response by definition of response across the 23 week trial period.
Average cost per week spent as a responder is also presented in where average cost per week spent as a responder was the lowest for cinacalcet patients vs control patients ($708, $2250, respectively; response defined as the control of all three biomarkers PTH, calcium and phosphorus). The incremental cost per response week for the three biomarkers definition was $430 for cinacalcet vs control.
Overall, while average medication costs were higher for patients in the cinacalcet arm than for patients in the control arm, a higher percentage of patients responded in the cinacalcet arm, yielding a lower average cost per responder in these patients.
Sub-group analyses
summarizes the results of the sub-group analysis by PTH at baseline. Overall, cinacalcet had lower cost per responder within each stratum. For each definition of response, the cost per response was lower for patients with a lower baseline PTH (300–500 pg/ml) than for patients with higher baseline PTH (501–< 800 pg/ml). For example, within the cinacalcet arm, the cost per responder (PTH decrease at least 30% from baseline) in the lower baseline PTH sub-group was $6299 compared to the higher baseline PTH sub-group where cost per responder was $7561. Likewise, within the cinacalcet arm, the average cost per response-week (a week with PTH remaining at or below 30% reduction from baseline) was lower for the lower PTH baseline sub-group ($296) than for the higher baseline PTH sub-group ($574).
Figure 1. Cost per response results by treatment arm and disease severity. *Incremental cost per incremental responder was calculated as the change in the mean per patient costs between cinacalcet and control arms divided by the change in the percentage of patients responding between cinacalcet and control arms. The incremental (cinacalcet – control) cost per incremental responder ranged from $5186–$9168. The incremental increase in mean time spent as a responder ranged from 6.5–10.1 weeks between the cinacalcet and control arms.
Discussion
Our study emphasizes the clinical and economic efficiency of treatment options according to patient response to (i.e., benefit from) medications for the treatment of SHPT. In using commonly accepted treatment targets for the disease utilizing a cinacalcet-centered treatment algorithmCitation31, we found cinacalcet to be less costly per patient-response than vitamin D sterols and phosphate binders alone. The cost per response and cost per week in response was lower in the cinacalcet arm for the lower baseline PTH (300–500 pg/ml) sub-group as compared to that for the higher baseline PTH (501–< 800 pg/ml) sub-group, suggesting that treating patients earlier in the disease severity continuum is more cost efficient with a cinacalcet-based treatment resulting in more time within the response target. Much of this was driven by the higher doses of vitamin D needed to drive a response and greater use of sevelamer in the sub-group with higher baseline SHPT severity. The average cost per responder with cinacalcet was lower than the corresponding average cost per responder with current therapy alone (control). These ordered findings were consistent across different definitions of biomarker response, time-in-response as well as dichotomous response, and for different sub-groups according to baseline PTH levels. Drivers of cost per response included the relative response rate and the trade-off of relative resources used within the treatment arms. Costs due to adding cinacalcet were somewhat off-set by savings in vitamin D sterols and phosphate binders, i.e., greater use of less expensive calcium containing binders in the cinacalcet arm. In the OPTIMA study, while the percentage of subjects receiving vitamin D increased slightly over the duration of the study in both arms, the average weekly dose of vitamin D decreased in the cinacalcet arm, while the mean dose of vitamin D remained unchanged in the control arm.
The cost per responder calculation assumes that each treatment is compared to not treating the patient at all. In addition to the cost per responder calculations, this study examined the average incremental cost per incremental response between cinacalcet and control arm patients as a way to analyze the relative economic efficiency gained from one treatment vs another. The incremental cost estimates indicate that the vitamin D sterols and phosphate binders alone treatment strategy is dominated by the cinacalcet treatment algorithm through extended dominance. In other words, given the provider has sufficient budget to afford cinacalcet, the provider will on average achieve higher response rates per dollar spent utilizing the cinacalcet treatment algorithm as compared to the response rates per dollar spent when utilizing vitamin D sterols and phosphate binders alone.
Two studies from the US societal perspective using incremental cost per quality adjusted life years as their outcome found cinacalcet to be cost-effective as compared to flexible vitamin D aloneCitation35 and as compared to parathyroidectomy up until 7.5 months of treatment, while parathyroidectomy dominated cinacalcet in the long-runCitation36. In a 2011 pharmacoeconomic review of cinacalcet used to treat SHPT, PloskerCitation37 suggested that cinacalcet plus standard therapy (vitamin D and/or phosphate binders) is cost effective relative to standard therapy alone based on a number of pharmacoeconomic analyses across various country settings. Due to limitations on indirect treatment comparisons, further evidence is needed to compare cinacalcet to other interventions, including parathyroidectomy.
The important and novel contribution of our study is in addressing economic efficiency of a cinacalcet-based algorithm from the dialysis provider perspective assuming a short-term time horizonCitation35,Citation38. Our study describes a pragmatic approach that the dialysis providers may take when making day-to-day treatment allocation decisions for patients with SHPT selecting from a variety of plausible treatment options. Because there are currently no required or endorsed biomarker targets for SHPT treatment as quality performance metrics, the evolving CMS reimbursement policy has the potential of discouraging dialysis providers from adequately treating patients with SHPT. Without some type of targeted measure, providers may take a cost minimization approach rather than a health economic efficiency approach in making clinical decisions and developing treatment algorithms to address SHPT.
In addition to the US provider perspective, we recognize that evidence is needed to answer separate and complementary questions on the cost-effectiveness of cinacalcet from the US societal perspective. The important and novel contribution of our study is in addressing economic efficiency of a cinacalcet-based algorithm from the dialysis provider perspective assuming a short-term time horizon as opposed to the prior studies that take a societal perspective with long-term time horizons and quality-adjusted life yearsCitation35,Citation38. With evidence on hard clinical end-points now available from other trials, the US societal perspective cost-effectiveness evidence should be recalibratedCitation26.
The Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial was the largest study in the ESRD population (3883 subjects) to test the hypothesis that treatment with cinacalcet would reduce the risks of death and non-fatal cardiovascular events among patients with SHPT who were undergoing dialysisCitation26. In an unadjusted intention-to-treat analysis, cinacalcet did not significantly reduce the risk of death or major cardiovascular events—there was a non-significant 7% reduction in risk of the primary composite end-point with cinacalcet useCitation26. After adjustment for baseline characteristics, there was a nominally significant 12% reduction in riskCitation26. Therefore, uncertainty remains in understanding the relationship between cinacalcet and long-term end-points requiring additional rigorous studies.
We presented the short-term clinical and economic value of cinacalcet from the provider perspective and used a flexible format with various definitions of biochemical response. Hard clinical end-points may remain the long-term goal, but are outside the provider’s relevant time horizon, thus enhancing the present cost per responder analysis on an array of biochemical response definitions.
Our study has limitations. Because our analysis was based on a controlled clinical trial there is a threat to external validity, or generalizability, of the results. The OPTIMA study, however, was one of the more pragmatic studies in which a cinacalcet based algorithm was used in a manner consistent with current clinical practice patterns. Likewise, since the OPTIMA study was conducted in countries outside of the US, the application of its results for the US-based practice may be challenging considering differences in patient characteristics (e.g. ∼95% of patients in OPTIMA were white) and therapeutic mix (e.g. paricalcitol is used much more frequently in the US than was seen in OPTIMA). However, because the commonly prescribed vitamin D sterols and phosphate binders in the US were also used in the OPTIMA study, and in the absence of compelling comparative efficacy data for different vitamin D compounds, we do not feel that this would impact the validity of our clinical conclusions. On the cost side, a higher than observed use of paricalcitol in the control arm as compared to the cinacalcet arm would have further reduced the difference in cost between the two arms; thus, making our current cost estimates conservative against cinacalcet. Lastly, despite heterogeneity in vitamin D use in OPTIMA (), different vitamin D treatments were not the key drivers of the cost per responder results given the limited impact that different vitamin D analogues had on cost ().
Importantly, since the completion of the OPTIMA study, the updated clinical guidelines for SHPTCitation39 suggest that PTH be maintained in the range of 2–9 times the upper limit of normal. Utilizing a greater than 30% reduction in PTH as a measure of efficacy allowed us to assess responsiveness across a broad spectrum of baseline SHPT disease state severity. This also allows extrapolation to PTH target ranges that might differ than that utilized in the OPTIMA study.
The last observation carried forward method was used to account for missing data; for example, when a subject discontinues the study prior to reaching the EAP. This method provides a conservative estimate of the treatment benefit for patients in the cinacalcet group because patients who discontinue the treatment early might not have reached the optimal dose. Therefore, a per-protocol analysis would likely have found even more favorable cost-efficacy results for cinacalcet as compared to control.
Lastly, and importantly, the costs that were used in our analyses are publicly available WAC prices. This may be different from the prices faced by the providers in light of the contracting and discounting programs whose details are not publically available.
Conclusion
Our findings suggest that the use of a cinacalcet treatment algorithm in which cinacalcet is added to standard of care (vitamin D and/or phosphate binders) results in both higher levels of biochemical response and, from the provider perspective, more efficient use of economic resources when compared to treating SHPT with standard of care alone. Therefore, additional resources devoted to the cinacalcet treatment algorithm may yield higher clinical returns for the dollars spent when the cost per response of all SHPT therapies are considered. Future studies are needed to reduce uncertainty around the long-term costs and outcomes associated with cinacalcet compared to its alternatives. When providers are faced with making treatment decisions in a cost-constrained environment, it will be important that SHPT treatment choices are evaluated based on both clinical and economic considerations in order to ensure that patients receive optimal treatment, and that the attainment of treatment targets for SHPT is incentivized through a QIP program to ensure adequate attention to this important complication of ESRD.
Transparency
Declaration of funding
Resources for this study were supported by Amgen Inc.
Declaration of financial/other relationships
VB and ST are employees and stockholders in Amgen Inc. AL and SC were employees and stockholders in Amgen Inc. at the time the study was conducted. JC is a consultant for Amgen Inc. JME Peer reviewers on this manuscript have no relevant financial or other relationships to disclose. Vasily Belozeroff and Andrew Lee participated in the study design, data interpretation, and writing of the manuscript. Spring Tseng was responsible for the statistical analysis, interpretation, and contributed to the writing of the manuscript. Silvia Chiroli contributed to the data interpretation and writing of the manuscript drafts. Jonathan D. Campbell is a consultant for Amgen Inc who participated in the data analysis, data interpretation, and writing of the manuscript. All authors provided their approval of the final manuscript draft.
| Abbreviations | ||
| ACHIEVE, | = | Optimizing the Treatment of Secondary Hyperparathyroidism: A Comparison of Sensipar and Low Dose Vitamin D vs Escalating Doses of Vitamin D Alone |
| CMS, | = | Centers for Medicare and Medicaid Services |
| ESRD, | = | End-Stage Renal Disease |
| EVOLVE, | = | Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events |
| HPT, | = | Hyperparathyroidism |
| KDOQI, | = | Kidney Disease Outcomes Quality Incentive |
| OPTIMA, | = | Open-Label, Randomized Study Using Cinacalcet to Improve Achievement of KDOQI Targets in Patients with End-Stage Renal Disease |
| PPS, | = | Prospective Payment System |
| PTH, | = | Parathyroid Hormone |
| QIP, | = | Quality Incentive Program |
Acknowledgments
The authors wish to thank Holly Tomlin and Larry Kovalick (employees and stockholders, Amgen Inc.) for medical writing, editing, and journal formatting assistance. During the course of manuscript development, each author had complete access to all the data and contributed to the writing of the manuscript according to the ICMJE criteria as well as Amgen publication policies.
References
- Services CfMM. 42 CFR Parts 410, 413, and 414 Medicare Program; End-Stage Renal Disease Prospective Payment System; Final Rule and Proposed Rule: Centers for Medicare & Medicaid Services; Federal Register. August 12, 2010
- Watnick S, Weiner DE, Shaffer R, et al. Comparing mandated health care reforms: The Affordable Care Act, Accountable Care Organizations, and the Medicare ESRD Program. Clin J Am Soc Nephrol 2012;7:1535-43
- Fishbane S, Miller I, Wagner JD, et al. Changes to the end-stage renal disease quality incentive program. Kidney Int 2012;81:1167-71
- Block GA, Zeig S, Sugihara J, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant 2008;23:2311-8
- Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 2010;26:1948-55
- Frazao JM, Messa P, Mellotte GJ, et al. Cinacalcet reduces plasma intact parathyroid hormone, serum phosphate and calcium levels in patients with secondary hyperparathyroidism irrespective of its severity. Clin Nephrol 2011;76:233-43
- Goodman WG, Hladik GA, Turner SA, et al. The Calcimimetic agent AMG 073 lowers plasma parathyroid hormone levels in hemodialysis patients with secondary hyperparathyroidism. J Am Soc Nephrol 2002;13:1017-24
- Kilpatrick R, Gill K, Block GA. Exploring the relationship between temporal trends in PTH, P and Ca in HD patients. Am Soc Nephrol 2010. Abstract and Poster
- Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 2011;26:1368-76
- Moe SM, Chertow GM, Coburn JW, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int 2005;67:760-71
- Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 2011;26:1938-47
- Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Nephrol Dial Transplant 1996;11(3 Suppl):130-5
- Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:396-9
- Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006;70:1358-66
- Salaffi F, Cimmino MA, Malavolta N, et al. The burden of prevalent fractures on health-related quality of life in postmenopausal women with osteoporosis: the IMOF study. J Rheumatol 2007;34:1551-60
- Shi N, Foley K, Lenhart G, et al. Direct healthcare costs of hip, vertebral, and non-hip, non-vertebral fractures. Bone 2009;45:1084-90
- Stehman-Breen CO, Sherrard DJ, Alem AM, et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:2200-5
- Moe SM, Reslerova M, Ketteler M, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 2005;67:2295-304
- Slinin Y, Foley RN, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 2005;16:1788-93
- Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002;39:695-701
- Doan QV, Gleeson M, Kim J, et al. Economic burden of cardiovascular events and fractures among patients with end-stage renal disease. Curr Med Res Opin 2007;23:1561-9
- Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm Jun 2007;13:397-411
- Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004;350:1516-25
- Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62:245-52
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718-25
- Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012;367:2482-94
- Palmer SC, McGregor DO, Craig JC, et al. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2009;4:CD008175
- Kilpatrick RD, Danese MD, Belozeroff V, et al. The association of vitamin D use with hypercalcemia and hyperphosphatemia in hemodialysis patients: a case-crossover study. Pharmacoepidemiol Drug Saf 2011;20:914-21
- Feldman RL, Desmarais MP, Muller JS. Orals in the bundle: a policy framework. Clin J Am Soc Nephrol 2013;8:1043-7
- Drummond M. Methods for the economic evaluation of health care programmes. 3rd edn. Great Claredon St, Oxford: Oxford University Press, 2005
- Messa P, Macario F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 2008;3:36-45
- Shireman TI, Almehmi A, Wetmore JB, et al. Economic analysis of cinacalcet in combination with low-dose vitamin D versus flexible-dose vitamin D in treating secondary hyperparathyroidism in hemodialysis patients. Am J Kidney Dis 2010;56:1108-16
- Amgen Inc. Thousand Oaks, Ca. Sensipar(R) Package Insert
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health: J Int Soc Pharmacoecon Outcomes Res 2009;12:409-18
- Boer R, Lalla AM, Belozeroff V. Cost-effectiveness of cinacalcet in secondary hyperparathyroidism in the United States. J Med Econ 2012;15:509-20
- Narayan R, Perkins RM, Berbano EP, et al. Parathyroidectomy versus cinacalcet hydrochloride-based medical therapy in the management of hyperparathyroidism in ESRD: a cost utility analysis. Am J Kidney Dis 2007;49:801-13
- Plosker GL. Cinacalcet: a pharmacoeconomic review of its use in secondary hyperparathyroidism in end-stage renal disease. Pharmacoeconomics 2011;29:807-21
- Eandi M, Pradelli L, Iannazzo S, et al. Economic evaluation of cinacalcet in the treatment of secondary hyperparathyroidism in Italy. Pharmacoeconomics 2010;29:1041-54
- Moe SM, Drueke TB. The KDIGO guideline on dialysate calcium and patient outcomes: need for hard evidence. Kidney Int 2011;79:478; author reply 478-9