Abstract
Objective:
The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial demonstrated that apixaban was effective in reducing the risk of stroke and major bleeding in non-valvular atrial fibrillation (NVAF) patients. Medical cost avoidance studies for oral anticoagulants have used warfarin event rates from clinical trials, which may not reflect the real-world (RW) setting. This study aimed to estimate the difference in medical costs associated with apixaban instead of warfarin in RW NVAF patients.
Methods:
This study selected patients with NVAF diagnosis during 2007–2010 from a Medco population of US commercial and Medicare health plans. Stroke and major bleeding excluding intracranial hemorrhage (MBEIH) were identified using diagnosis codes. Pharmacy claims were used to define warfarin exposure periods. Rates of stroke and MBEIH were calculated during warfarin exposure. To estimate the absolute risk reduction (ARR) between warfarin and apixaban in RW, the relative risk reductions (RRR) from ARISTOTLE were multiplied by the event rates observed in RW during warfarin exposure. Medical cost reductions associated with apixaban were calculated by applying the ARR to the 1-year incremental cost for each event. Stroke and MBEIH costs were obtained from the literature and adjusted to 2011 levels.
Results:
During a patient year, the use of apixaban instead of warfarin resulted in medical cost reductions of $493 for stroke and $752 for MBEIH and $1245 for the combined outcome of both events. The medical costs avoided were greater as baseline stroke risk increased.
Conclusion:
If RRRs demonstrated in ARISTOTLE persist in RW, the use of apixaban will be associated with lower medical costs vs warfarin. Main limitations of this study were: identification of clinical events using administrative codes rather than confirmatory clinical data, inability to evaluate the level of international normalized ratio (INR) control, and not including INR monitoring and drug costs.
Introduction
Atrial fibrillation (AF) affects more than 5 million Americans and is characterized by symptoms including an irregular heart rhythm, heart palpitations, shortness of breath, and overall weaknessCitation1,Citation2. Primarily a condition of the elderly, the prevalence of AF doubles with each decade of life after the age of 60 years and occurs in ∼10% of the population by the age of 80Citation3. AF patients have a 5-fold increased risk of ischemic strokeCitation4,Citation5. In addition, strokes caused by AF are fatal in 20% of cases and result in serious disability in 60%Citation6. Non-valvular atrial fibrillation (NVAF), or AF in the absence of mitral stenosis or valvular prostheses, account for up to 70% of AF casesCitation7. The prevalence of both stroke and NVAF are increasing due to aging populationsCitation8. NVAF and its resulting complications are associated with significant mortality, morbidity, and increased use of healthcare resources. Costs to manage AF in the US are estimated at $6.65 billion, including $4.88 billion in hospitalization expenses and $1.53 billion in outpatient management costs (in 2005 US Dollars [USD])Citation9. Costs of hospitalizations for acute care are a major cost driver, accounting for 50–70% of total direct costsCitation10,Citation11. A recent study examining the costs of NVAF in a Medicare population found that the incremental costs for patients experiencing a complication relative to those with no events were $34,201 for ischemic stroke, $29,965 for major bleeding, and $44,716 for hemorrhagic stroke (HS) per year (in 2006 USD). Three years after the events, costs remained elevated by $3156–$5400 per yearCitation12.
The principal anticoagulant used to reduce the risk of stroke among patients with NVAF has been warfarin. Multiple randomized clinical trials have demonstrated the effectiveness of warfarin in reducing the risk of stroke, particularly among those with a moderate-to-high riskCitation13–17. The use of warfarin, however, has several practical limitations, such as a narrow therapeutic range, as measured by the international normalized ratio (INR), the need for frequent INR monitoring, and multiple interactions with drugs and food. Apixaban is a novel oral anticoagulant approved for use in the US, Europe, Canada, and Japan as an alternative to warfarin for NVAF patients. Results of a recent randomized controlled trial, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), demonstrated that patients treated with apixaban had a 21% relative risk reduction (RRR) in stroke or systemic embolism (SE) (primary efficacy end point) and a 31% RRR in major bleeding (primary safety end point) compared with patients receiving warfarinCitation18. These relative reductions in risk observed in the trial were consistent across a range of baseline stroke and bleeding risks, and, indeed, the absolute incremental benefit associated with apixaban was larger in those with a higher risk of stroke or bleedingCitation19.
The reduction in clinical events associated with apixaban is likely to impact the medical costs of patients with NVAF. A recent study found that, in a patient year (PY), the medical cost reduction associated with using each novel oral anticoagulant instead of warfarin was −$179, −$89, and −$485 for dabigatran, rivaroxaban, and apixaban, respectivelyCitation20. In this study, the medical cost reduction was calculated using the absolute risk reduction (ARR) for each novel oral anticoagulant. This measure was derived by applying the RRR associated with each novel oral anticoagulant to a weighted-average of warfarin event rates obtained from the clinical trials, which served as a reference risk. While the RRRs from clinical trials are believed to be generalizable to actual practice, the reference risk may not be, as trial investigators tend to select patients more likely to tolerate the effects of the study medicationsCitation21,Citation22. This may mean excluding elderly patients or those at higher risk of having adverse eventsCitation21,Citation22. Therefore, the reference risk among patients in actual clinical practice is often higher compared to patients in clinical trials. If one assumes that the RRR demonstrated in a trial persists in the real world, then both a greater absolute risk reduction and a greater medical cost reduction would be expected from adopting a new drug in clinical practice.
The objective of this study was to evaluate the absolute numbers of stroke and major bleeding excluding intracranial hemorrhage (MBEIH) events avoided and the associated medical cost reductions that may be expected with the use of apixaban as an alternative to warfarin in NVAF patients in clinical practice, from a US payer perspective. Reference event rates for stroke and MBEIH were estimated using patient data from the Medco research database and applied to the corresponding RRRs from ARISTOTLE to establish estimates for patients treated in real-world (RW) clinical practice.
Methods
A retrospective cohort analysis using the Medco research database was conducted to estimate the reference event rates of stroke and major bleeding, excluding intracranial hemorrhage (MBEIH), among real-world NVAF patients.
Data source
The Medco research database was used to obtain data for NVAF patients receiving warfarin in an actual clinical setting. These data were collected by Medco Health Solutions, formerly a large pharmacy benefits management company in the US. The Medco data integrate both pharmacy and medical claims and, as of 2011, data were available for ∼12.7 million covered lives. We conducted this study using both the eligibility files and the medical and pharmacy claims. The eligibility file contained dates of health plan eligibility and demographic information. The medical claims included service dates, primary and secondary International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes, and Healthcare Common Procedure Coding System (HCPCS) procedure codes for hospital inpatient and outpatient medical services provided to health plan members. Prescription drug information including dispense dates, National Drug Codes, quantity, and days supplied were obtained from the pharmacy claims.
Sample selection
Patients 18-years or older were eligible for study inclusion if they had two or more medical claims separated by at least 30 days, with a primary or secondary ICD-9-CM diagnosis code indicating AF (427.31) between January 1, 2007 and June 30, 2010. The date of the first medical claim for AF was defined as the study index date. Only patients with continuous enrollment in pharmacy and health benefits for a minimum of 1 year prior to the index date (baseline period) were included. Stroke risk was estimated using the CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke) risk score during the baseline period. Patients with a CHADS2 score ≥1 were included to match the inclusion criteria for baseline stroke risk used in the ARISTOTLE trial. Finally, patients were excluded if they had any medical claims during the study period (baseline and follow-up) for cardiac valvular disease in order to select NVAF patients only and a population consistent with ARISTOTLE.
The study period began on the study index date and continued until disenrollment from either the pharmacy or medical benefits or the end of follow-up (June 30, 2011), whichever occurred earlier. Reasons for disenrollment were not available in the data source and may have included death. Supplementary Appendix Table A1 shows how the risk factors included in the CHADS2 scores were identified using the Medco database. The point values associated with each risk factor are also presented. Scores were calculated for each patient by summing up the points as determined by the presence of the risk factor during the patient’s baseline period.
Assessment of warfarin exposure periods
Warfarin exposure was determined by identifying prescriptions for warfarin following the study index date in the pharmacy claims. A period of warfarin exposure began with the date that the prescription was filled and continued until there was a ≥60 day gap between the end of one prescription and the next date that warfarin was dispensed. Patients receiving warfarin before the study index date and who had a remaining warfarin supply after study index had their warfarin exposure measured from the index date based on the estimated number of days supply remaining from their last prescription prior to study index.
Identification of stroke and MBEIH in Medco population
We identified stroke in the Medco database using the primary or secondary ICD-9-CM diagnosis codes appearing on inpatient hospital or emergency room (ER) claims and included ischemic, hemorrhagic and unknown types (Supplementary Appendix Table A2). Because of the limitations of ICD-9 diagnosis coding, it was not possible to distinguish between intracranial hemorrhage (IH) and hemorrhagic stroke as in the ARISTOTLE trial. Therefore, we assumed that claims with a code for intracranial hemorrhage occurred because a patient ultimately sought care due to a neurological deficit as a result of the hemorrhage and classified these claims as hemorrhagic stroke. MBEIH was identified using primary or secondary diagnosis and procedure codes. We did not include intracranial hemorrhage in our definition of major bleeding to avoid double-counting events. Bleeding associated with inpatient care, blood transfusion, decreased hemoglobin or hematocrit, or physician-guided medical or surgical treatment were considered major (Supplementary Appendix Table A2).
Estimation of stroke and major bleeding reference rates
Rates of stroke and MBEIH during periods of warfarin exposure were calculated for the Medco population as follows:
The stroke and MBEIH rates estimated for the Medco population were unadjusted and expressed per 100 PYs of warfarin exposure. The rates of stroke and MBEIH calculated from the Medco database were compared to those in the warfarin group of ARISTOTLE. Stroke was combined with SE (e.g., not reported separately) for stratified analyses according to CHADS2 score in the trial publications. As SE was a relatively rare occurrence in the trials, we used the combined stroke/SE event rates from ARISTOTLE to compare with the stroke rates observed in the Medco population for stratified comparisons according to CHADS2 score.
Medical cost reduction associated with apixaban in real-world
The absolute numbers of stroke and MBEIH events that might be avoided with the use of apixaban in place of warfarin in actual practice ARR were estimated by multiplying the relative risk reductions (RRR) (0.21 for stroke and 0.21 for MBEIH) from ARISTOTLE by the event rates obtained from patients in the Medco population during periods of warfarin exposure (). As a secondary analysis, the absolute risk reduction for HS was calculated using the RRR from ARISTOTLE (0.49) and HS rates derived from the Medco population.
Table 1. Relative risk and cost inputs for stroke and major bleeding (excluding intracranial hemorrhage) outcomes.
The ARRs for each event were then multiplied by their respective incremental medical costs in order to calculate the medical cost reductions associated with preventing stroke and major bleedings. The incremental medical costs, defined as the incremental costs to a US payer as a result of an NVAF patient experiencing a stroke or major bleeding event during 1 year following the event, were obtained from Mercaldi et al.Citation12 (). The costs of ischemic and hemorrhagic stroke were reported separately in Mercaldi et al.Citation12 Therefore, a combined cost of stroke was calculated by applying a weighted average to the separate cost estimates based on the assumption that strokes would occur in a ratio of 31 hemorrhagic to 69 ischemic. This assumption was based on the ratio observed in the warfarin arm of the ARISTOTLE trialCitation18. Costs were inflated to 2011 levels using the medical care component of the Consumer Price Index (CPI). The medical costs avoided with apixaban relative to warfarin were calculated separately for stroke and MBEIH; a combined cost estimate for the two outcomes was also estimated. Medical cost reductions were calculated for the overall study sample, as well as by CHADS2 score and age groups (≥65-years-old, <65-years-old). To estimate the medical cost reduction associated with preventing HS, the ARR for HS was multiplied by the incremental 1 year cost associated with HS ($53,467) from Mercaldi et al.Citation12 Since these were secondary analyses, they were conducted for the overall study sample only. This study focused on the medical cost reduction associated with the clinical outcomes of stroke and major bleeding and excluded drug costs and INR monitoring-related expenses.
Sensitivity analyses
Univariate sensitivity analyses were conducted to determine whether the effect of varying the ARR for an event, the corresponding incremental medical cost, or the reference event rates would significantly affect the medical cost reductions associated with apixaban. The ARR for events were varied across the ranges of their respective 95% confidence intervals. Incremental cost values were varied ±30% and the reference event rates were varied ±50%.
Results
Baseline characteristics
shows a comparison of the baseline characteristics of NVAF patients receiving warfarin from the Medco database with those randomized to warfarin in the ARISTOTLE clinical trial. The median age of patients in the Medco population was 78.0 years compared with a median age of 70.0 for patients enrolled in ARISTOTLE. There were a higher proportion of female patients (44.6%) in the Medco population compared to 35.0% of patients enrolled in ARISTOTLE. The mean (SD) CHADS2 score for the Medco population was 1.8 (1.0) and it was 2.1 (1.1) for ARISTOTLE patients. The mean (SD) duration of warfarin use was 662 (452) days for the Medco population.
Table 2. Baseline characteristics of NVAF patients from the Medco database compared with patients from ARISTOTLE.
Stroke and MBEIH event rates
The rates of stroke and MBEIH following a diagnosis of NVAF during warfarin exposure in the Medco population were more than 3-times higher than the warfarin group of ARISTOTLE for both stroke (5.3 per 100 PY vs 1.5 per 100 PY) and MBEIH (10.0 per 100 PY vs 2.3 per 100 PY) (). Similarly, in analyses stratified according to baseline stroke risk (CHADS2), rates of stroke and MBEIH among real-world Medco patients were also more than 3-times higher compared to patients receiving warfarin in ARISTOTLE. The rate of HS was 1.1 per 100 PY among Medco patients during warfarin use compared with 0.5 for those randomized to the warfarin arm of ARISTOTLE (data not shown).
Table 3. Stroke and major bleeding (excluding intracranial hemorrhage) event rates per 100 person-years of follow-up for NVAF patients during warfarin exposure: Real-world clinical practice (Medco population) vs ARISTOTLE.
Absolute number of clinical events avoided with apixaban in real-world
We estimate that, in the real-world, 1.1 stroke events and 2.1 MBEIH events would be avoided per 100 PYs of treatment with apixaban compared to warfarin () (Stroke events avoided = Reference stroke rate for patients exposed to warfarin in Medco population [5.3] × RRR from ARISTOTLE for stroke [0.21]; MBEIH events avoided = Reference MBEIH rate for patients exposed to warfarin in Medco population [10.0] × RRR from ARISTOTLE for MBEIH [0.21]). Similar to the observation in ARISTOTLE, the benefit associated with apixaban is estimated to increase across higher levels of baseline stroke risk (CHADS2) as a greater number of stroke and MBEIH events will be avoided. The number of clinical events avoided is also projected to be higher among those ≥65-year-old vs patients <65-years-old. It is estimated that 0.54 HS events would be avoided per 100 PYs of apixaban therapy vs warfarin (data not shown) (HS events avoided = Reference HS rate for Medco population [1.1] × RRR from ARISTOTLE for HS [0.49]).
Table 4. Estimated number of stroke and major bleeding (excluding intracranial hemorrhage) events avoided per 100 years of follow-up in actual clinical practice with apixaban vs warfarin.
Medical cost reductions associated with apixaban in real-world
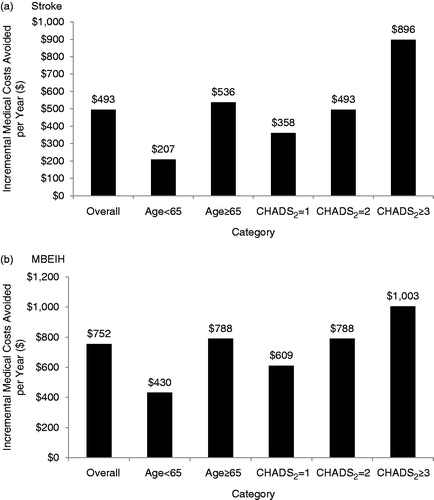
The medical cost reductions associated with the use of apixaban instead of warfarin in a PY were estimated at $493 for stroke and $752 for MBEIH (). For the combined outcome of stroke and MBEIH, the medical cost reduction was $1245. The medical costs avoided were greater as baseline stroke risk increased. The costs avoided for patients in the highest baseline risk category (CHADS2 ≥ 3) were 2.5-times higher for stroke and 1.7-times higher for MBEIH compared with patients in the lowest risk category included in the study (CHADS2 = 1). The medical cost reductions associated with apixaban among patients ≥65-years-old vs patients <65-years-old were 1.8- and 2.6-times higher for MBEIH ($788 vs $430) and stroke ($536 vs $207), respectively. The medical cost reduction associated with apixaban treatment in place of warfarin was $289 for HS in a PY.
Figure 1. Incremental medical costs avoided per year with apixaban vs warfarin in real-world clinical practice for (a) stroke and (b) major bleeding excluding intracranial hemorrhage outcomes, overall and by age, CHADS2 scores. Note: Incremental 1-year cost of stroke = $42,792, incremental 1-year cost of major bleeding = $35,829, costs adjusted to 2011 values using the medical care component of the CPI, Medical costs avoided = incremental 1-year cost of event × number of events avoided per year. MBEIH, major bleeding excluding intracranial hemorrhage.
Sensitivity analyses
Results of univariate sensitivity analyses demonstrate consistent medical cost reductions associated with apixaban relative to warfarin following variations in key study parameters. Varying the ARR had the greatest impact on the medical cost reduction associated with reducing stroke events (range $134–$806) (). Variations in the incremental 1-year medical cost of stroke had a smaller effect on the medical cost reduction related to stroke (range $345–$641).
Figure 2. Univariate sensitivity analyses examining the influence of variations in the absolute risk reduction, incremental event costs, and reference event rates on the medical cost reductions associated with avoiding (a) stroke and (b) major bleeding excluding intracranial hemorrhage events for apixaban relative to warfarin. Note: Annual cost of event varied from −30% to +30%, ARR varied using the ranges of the 95% confidence intervals of event rates from ARISTOTLE, reference event rate varied from −50% to +50% from baseline values calculated from the Medco database. ARR, Absolute Risk Reduction.
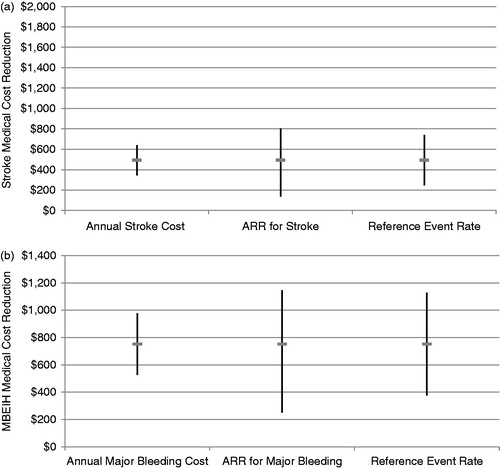
Varying the reference event rate had the greatest impact on the medical cost reduction related to avoiding MBEIH events (range $373–$1129) (). Variations in the incremental 1-year MBEIH medical cost and ARR had a smaller effect on the medical cost reduction (range $527–$978 and $251–$1147 after varying MBEIH cost and ARR, respectively).
Discussion
Results of our economic analysis show that there are likely substantial 1-year medical cost reductions (or savings) associated with avoiding stroke and MBEIH events when apixaban is used, instead of warfarin, in the real world. The medical cost reduction for stroke was $493 and $752 for MBEIH during a PY. The use of reference event rates from real-world patients receiving warfarin in calculating the absolute numbers of stroke and MBEIH events avoided and associated medical cost reductions may be helpful in determining the potential economic impact of apixaban, from a US payer perspective. Deitelzweig et al.Citation20 evaluated the medical cost reductions associated with using different novel oral anticoagulants, including apixaban, instead of warfarin, based on data from the clinical trials. This current study builds upon those analyses by using the reference event rates from a real-world patient population exposed to warfarin, rather than obtaining these data from the clinical trial. The use of the Medco database allowed us to estimate the medical cost reductions in a real-world setting where patients may have more advanced disease with less monitoring than clinical trial participants. Indeed, the medical cost reduction associated with apixaban for major bleeding (−$282), ischemic (−$39), and hemorrhagic (−$110) stroke reported in Deitelzweig et al.Citation20 were much lower than the medical cost reductions estimated in this study.
Our estimation of the clinical events avoided and the medical cost reductions associated with apixaban treatment in real-world patients was based on the assumption that the RRRs observed in the ARISTOTLE trial would persist in an actual clinical practice setting. While it is widely accepted that the RRRs from clinical trials are generalizable to the real world and preferable to RRR estimates derived from real-world observational studies, the reference risks from clinical trials may not beCitation22. In fact, the risks of stroke and major bleeding are often reported to be higher in the real world. Therefore, the number-needed-to-treat (NNT) with apixaban in order to avoid stroke and major bleeding events in actual practice is likely to be substantially lower than estimates using clinical trial data alone. We estimate a NNT of 91 for stroke and a NNT of 48 for MBEIH events using the real-world event rates from the Medco population. This is in contrast to NNTs of 313 and 210 for stroke and MBEIH events, respectively, using the baseline risks from the ARISTOTLE trial. The higher reference event rates with corresponding lower NNTs are due to several factors. Primarily, clinical trials tend to enroll patients who are at lower risk by excluding those with advanced disease or who have a higher risk of adverse events. Participants in clinical trials also must follow strict treatment protocols and are therefore evaluated more frequently by physicians and other healthcare workers. Indeed, within the Medco population, despite having a lower overall baseline CHADS2 score, compared to ARISTOTLE, event rates were higher than in the clinical trial setting. This may be due to risk factors not captured in CHADS2, poorer INR control, less stringent management of other disease risk factors (e.g., less use of guideline-recommeded medications), or other unmeasured factors.
To explore the potential for variation in our results, due to differences between various patient populations, sensitivity and sub-group analyses were performed. Results of our sub-group analyses indicate that the use of apixaban would result in a medical cost reduction for patients across a range of baseline stroke risks, as well as in those aged ≥65 years and <65 years. In addition, our sensitivity analyses suggest that the economic results would be consistent across a range of plausible scenarios and real-world settings, where there may be significant variation in medical costs and reference risks of events.
This study had several limitations concerning the estimation of stroke and major bleeding rates. Administrative ICD-9-CM diagnosis and current procedural terminology (CPT)-4 codes were used to identify stroke and major bleeding events rather than clinical data reported by the investigator. Diagnostic and procedure coding errors are possible, while rightfully recorded codes may have represented ‘rule-out’ diagnoses. The administrative coding definitions applied to the Medco data were selected to provide the closest match to the definitions used in the ARISTOTLE trial. In ARISTOTLE, stroke was defined as a non-traumatic abrupt onset of a focal neurologic deficit lasting at least 24 hCitation18. Major bleeding was defined according to the International Society on Thrombosis and Haemostasis (ISTH) definition of major bleeding: acute or sub-acute clinically overt bleeding accompanied by ≥1 of the following: (1) a decrease in hemoglobin level of ≥2 g/dL over a 24-h period, (2) a transfusion of ≥2 Units of packed red blood cells, and (3) bleeding that is fatal or occurs in at least one of the following critical sites: intracranial, intraspinal, intraocular, pericardial, intrarticular, intramuscular with compartment syndrome, or retroperitonealCitation23. Outcomes created using the Medco data were defined using codes appearing on hospital admission or emergency room (ER) visit claims for stroke and for major bleeding codes appearing on either hospital admission, outpatient hospital visit, or ER claims. It should be noted that, despite our best efforts to match Medco event definitions to those used in ARISTOTLE, the data were subject to the following limitations. Some stroke events may not have been counted in the Medco data if they did not result in an ER visit or hospital stay. Thus, there is the possibility that some event rates were under-estimated. Conversely, some claims for follow-up care for events occurring prior to study index may have been misclassified as a new stroke or bleeding event resulting in possible over-estimation of events. These limitations are common to administrative data sets where data are collected primarily for providing payments to healthcare providers, rather than for research purposes. In clinical trials, biases associated with the misclassification of study events are less problematic because they are captured using precise clinical definitions by trained study investigators. In addition, the study outcomes are usually independently adjudicated. The validity of the algorithms used to identify study events using the Medco database were not assessed as confirmatory clinical data were not available. Thus, it is not known to what extent study events were over-estimated (or potentially under-estimated) due to biases associated with misclassification. The identification of clinical events included in the calculation of CHADS2 scores may have also been subject to similar coding limitations.
Second, only medical costs associated with stroke and MBEIH events were considered in our evaluation of the medical cost reductions associated with apixaban treatment. INR monitoring and drug costs for warfarin and apixaban were not taken into account. At the time of analysis, apixaban was not approved by the FDA in the US and the price of the medicine was not established. Apixaban will likely have a higher price relative to warfarin as dabigatran and rivaroxaban are currently priced higher in the US. It is also expected that the costs for dose adjustments and routine monitoring would be higher for warfarin relative to apixaban. Further study is needed to more fully evaluate the total cost differences related to replacing warfarin with apixaban, while taking into account drug and monitoring costs. In fact, cost-effectiveness analyses of dabigatran and rivaroxaban have already shown, despite having a higher drug cost, that they are cost-effective relative to warfarin in NVAF populationsCitation24–27.
The study had additional limitations common to many retrospective database analyses. Warfarin exposure was inferred from the fill dates of warfarin prescription claims; we assumed patients were compliant with their prescriptions in assessing periods of warfarin use. In addition, the complexity of warfarin dose adjustments may have affected our ability to accurately assess periods of warfarin exposure, based on the prescription claims data alone. Thus, it is possible some major bleeding and stroke events were misclassified, as occurring during warfarin use. In addition, the level of anticoagulation control (target INR and time in therapeutic range [TTR]) could not be determined for the Medco patients. However, one can speculate that the level of control was probably inferior to patients enrolled in ARISTOTLE, as the stroke and major bleeding event rates in the Medco population were much higher compared to ARISTOTLE. Finally, due to ICD-9 diagnosis coding limitations, it was not possible to distinguish between IH and HS. Therefore, we assumed that claims for IH occurred because a patient sought care due to a neurological deficit, as a result of the haemorrhage, and classified these claims as HS. We were also conservative in estimating the number of stroke events avoided through our use of the RRR for stroke (0.21) from the ARISTOTLE trial. The RRR for stroke did not include IH, which was captured in a separate trial study measure. Had we adjusted the RRR to account for IH, the estimated number of stroke events avoided would have been higher.
Given the study limitations and the potential for significant variation in event rates across different patient sub-groups, clinicians may find it useful to compare rates of stroke and major bleeding during warfarin use derived from this study against estimates from their clinical practices. Clinicians could then measure the potential impact of apixaban for their unique patient populations using the methodology described in this study. This exercise could provide clinicians with valuable insight for the evaluation of their practices.
Conclusion
Results of this analysis suggest that apixaban is associated with a substantial reduction in medical costs (not including drug or INR monitoring costs) attributable to lower absolute numbers of stroke and MBEIH events, when used instead of warfarin in a real-world, NVAF, managed care patient population. Although real-world experience is needed to corroborate our findings, a careful examination of population characteristics in real-world settings will be important for evaluating the benefits and costs associated with apixaban therapy as a new era of anticoagulation emerges. In the absence of data on patients treated with apixaban in actual clinical practice, due to its approval in December 2012, modeling efforts extrapolating clinical trials to the real world for evaluating both the clinical and economic benefits are acceptable and practical approaches. Future research should focus on verifying our study conclusions once adequate data are available to allow for evaluation of therapies using phase IV reports and real-world data sources.
Transparency
Declaration of funding
This research was supported by Bristol-Myers Squibb and Pfizer.
Declaration of financial/other relationships
DM is an employee of Bristol-Myers Squibb and owns stock in the company. DW is an employee of Pfizer and owns stock in the company. LB, EG, MS, and NW are employees of United BioSource Corporation, which has received research funds from Bristol-Myers Squibb and Pfizer to conduct this study and develop this manuscript. AA is a paid consultant for United BioSource Corporation in connection with this research.
Supplementary Material
Download PDF (27.9 KB)References
- Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ 2008;11:281-98
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17-40, ix
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561-4
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8
- Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009;40:235-40
- Stettin GD. Treatment of nonvalvular atrial fibrillation. West J Med 1995;162:331-9
- Ali A, Bailey C, Abdelhafiz AH. Stroke prevention with oral anticoagulation in older people with atrial fibrillation - a pragmatic approach. Aging Dis 2012;3:339-51
- Coyne KS, Paramore C, Grandy S, et al. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health 2006;9:348-56
- Wodchis WP, Bhatia RS, Leblanc K, et al. A review of the cost of atrial fibrillation. Value Health 2012;15:240-8
- Wolowacz SE, Samuel M, Brennan VK, et al. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace 2011;13:1375-85
- Mercaldi CJ, Ciarametaro M, Hahn B, et al. Cost efficiency of anticoagulation with warfarin to prevent stroke in medicare beneficiaries with nonvalvular atrial fibrillation. Stroke 2011;42:112-8
- The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. N Engl J Med 1990;323:1505-11
- Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 1993;342:1255-62
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 1996;348:633-8
- Connolly SJ, Laupacis A, Gent M, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol 1991;18:349-55
- Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406-12
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Lopes RD, Al-Khatib SM, Wallentin L, et al. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet 2012;380:1749-58
- Deitelzweig S, Amin A, Jing Y, et al. Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ 2012;15:776-85
- Britton A, McKee M, Black N, et al. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy 1999;4:112-21
- Caro JJ, Migliaccio-Walle K. Generalizing the results of clinical trials to actual practice: the example of clopidogrel therapy for the prevention of vascular events. CAPRA (CAPRIE Actual Practice Rates Analysis) Study Group. Clopidogrel versus aspirin in patients at risk of ischaemic events. Am J Med 1999;107:568-72
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933-44
- Berg AM. Dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:570; author reply 570-1
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:2562-70
- Sorensen SV, Kansal AR, Connolly S, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908-19