Abstract
Objective:
To evaluate the impact of universal vaccination with a pentavalent rotavirus vaccine (RV5) on the healthcare burden and costs associated with rotavirus gastroenteritis (RGE) in Japan.
Methods:
The model included a hypothetical cohort of 1,091,156 children followed for their first 5 years of life. In the absence of universal vaccination, there were 19 deaths, 78,000 hospitalizations, and 678,000 outpatient visits due to RGE. The efficacy of RV5 is based on international clinical trial data, which was similar to the efficacy observed in clinical trials conducted in Japan. The primary outcome measure is the cost per quality-adjusted-life-year (QALY) gained. In the base case, the QALY loss per 1000 RGE episodes included 2.2 for children and 1.8 per parent.
Results:
Universal vaccination is projected to reduce hospitalizations by 92%, outpatient visits by 74%, and work-loss days by 73%. For the base case analysis, the total vaccination cost was ¥26 billion. The estimated reduction in medical costs was ¥16 billion. Of 2500 QALYs gained with the vaccination program, approximately half are directly attributed to the child. In the base case analysis, the incremental cost-effectiveness ratio (ICER) for vaccination vs no vaccination is ¥4 million and ¥2 million per quality-adjusted life year (QALY) gained from the healthcare payer and societal perspectives, respectively. The ICERs are ¥8 million and ¥4 million if parental disutilities are excluded.
Key limitation:
The QALY decrements for children and parents were evaluated using different instruments, and the QALY decrements do not vary based on episode severity. Given the interdependence between children and their parents, excluding parental disutilities may under-estimate the impact of RGE.
Conclusion:
Universal vaccination with RV5 in Japan is projected to have a substantial public health impact and may be cost-effective from both the payer and societal perspectives if parental disutilities are included in the cost-effectiveness ratios.
Keywords::
Introduction
Rotavirus is the most common cause of severe gastroenteritis in infants and young children worldwide. Prior to the availability of safe and effective vaccines, virtually all children were known to acquire a rotavirus infection by 5 years of age regardless of socioeconomic statusCitation1. Rotavirus gastroenteritis (RGE) often results in diarrhea, vomiting, fever, and/or lethargy lasting from a few days up to a couple of weeks. Severe episodes of RGE are more likely to involve a combination of these symptoms which may lead to dehydration if untreatedCitation2,Citation3. Rotavirus associated deaths are common in developing countries where access to care is limitedCitation4, and are responsible for greater than 450,000 deaths per year in children under the age of 5Citation5. Although deaths are rare in industrialized countries, the healthcare burden of RGE-related hospitalizations and emergency department visits is substantialCitation6–8. In addition to the healthcare burden, the impact that RGE has on the family can be considerableCitation9–11.
In Japan, rotavirus is known to be associated with substantial healthcare utilization and medical costs. Prior to the introduction of rotavirus vaccines, RGE was associated with over 50% of all hospitalizations for acute gastroenteritis in children younger than 5 years of age. In this age group, it is estimated that RGE is responsible for 78,000 hospitalizationsCitation12 and 800,000 outpatient visitsCitation13.
Two rotavirus vaccines are currently available in Japan, the monovalent vaccine (Rotarix, GlaxoSmithKline, Research Triangle Park, SC) and the pentavalent rotavirus vaccine (RotaTeq, Merck Sharp & Dohme Corp, West Point, PA). Both products have been shown in clinical trials and post-vaccine introduction studies to significantly reduce rotavirus disease when widely adoptedCitation14–16. Although RGE is not associated with a high rate of mortality in developed countries, universal rotavirus immunization has been shown to be a cost-effective health intervention in many economic analyses due to reductions in healthcare utilization and parental work lossCitation17–28. Rotavirus vaccines have been recommended as part of the infant vaccination schedule in many countries and are recommended by the World Health OrganizationCitation20,Citation29–34.
Historically, the Japanese government only provided reimbursement for a small number of mandatory vaccines due to concerns about vaccine safety in generalCitation35. All other vaccines were regarded as voluntary and, overall, coverage rates were low. Recently, the Vaccination Subcommittee of the Health Sciences Council was formed to re-evaluate the National Immunization Program. Within this past year, seven vaccines have received a recommendation to be added to the list of mandatory vaccinesCitation36.
The objective of this study is to describe the projected public health and economic impact of the pentavalent rotavirus vaccine (RV5) in Japan and provide Japanese decision-makers with information to support inclusion of rotavirus vaccination in the National Immunization Program as a mandatory vaccine. Inclusion in the National Immunization Program will result in government funding for rotavirus vaccination and should increase coverage. In the Sato et al.Citation19,Citation37 cost-effectiveness analysis for rotavirus vaccination which was previously published in Japan, the number of hospitalizations estimated was based on a combination of prospective and retrospective data. However, research has shown that retrospective studies under-report the burden of rotavirus because physicians generally do not test for RGECitation38–40. The Sato et al. study also included QALY decrements derived from the EQ-5D based on a study of physicians in the UK who served as proxies for children with RGECitation41. Recent research has shown that physicians tend to over-estimate the health status of their patients, and this could lead to an under-estimate of the QALY decrements, particularly for acute conditionsCitation42,Citation43. The cost-effectiveness analysis presented here gives more weight to published data based on prospective hospital surveillance studiesCitation12, and uses a different approach to determining the decrement in QALYs for episodes of rotavirus gastroenteritis.
Methods
Model structure
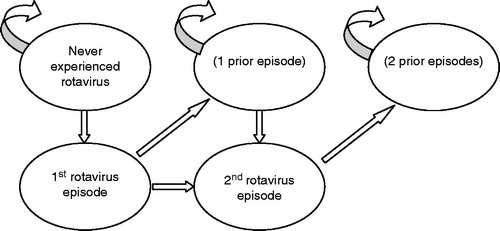
A Markov model developed in Microsoft Excel® (Version 2003) tracks two hypothetical birth cohorts with and without rotavirus vaccination during the first 5 years of life because infants may develop multiple rotavirus infections. The model consists of monthly cycles through 6 months before they are fully vaccinated and then quarterly cycles through year 5.
As shown in , the model allows for movement between different health states over each Markov cycle. Transition probabilities reflect the likelihood of moving from one health state to anotherCitation44. The different health states reflect whether the child has ever experienced a rotavirus infection, the number of prior rotavirus infections, the serotypes causing the episodes, and whether the child survives or dies from the illness. The model allows children to contract up to two symptomatic episodes of RGE after which all subsequent infections are assumed to be asymptomatic. This assumption is consistent with a study conducted in Mexico that illustrated the natural history of rotavirus infection in a cohort of 200 childrenCitation45. This study was unique because study personnel collected stool specimens from all children born in the catchment area during the study period, regardless of symptoms, every 2 weeks for the first 2 years of life. Almost all other studies are conducted in a specific healthcare setting after symptoms developCitation45,Citation46. The transition probabilities or the likelihood that the child will move from one health state to another, reflect the age-specific probability of developing first and second episodes of rotavirus and the percentage of episodes that are symptomatic.
Analytic perspectives and outcome measures
This cost-effectiveness analysis is conducted from the healthcare payer and societal perspectives based on the recommendations of the Panel on Cost-EffectivenessCitation47. The healthcare perspective is limited to direct medical care costs and the cost of the vaccination program. The societal perspective encompasses all RGE-related costs including travel to healthcare facilities and parental work days missed to care for sick children. The outcome measure is the cost per quality adjusted life year (QALY) gained by universal vaccination compared to no vaccination. The benchmark used to determine the cost-effectiveness of universal vaccination is consistent with the recommendations of the Vaccine Evaluation Sub-Committee of the Health Science Council Vaccination Committee in JapanCitation36. Vaccines are considered cost-effective if the ICER is ¥5 million per QALY gained or less from the payer perspective.
Model inputs
General overview
lists the key model inputs for the base case scenario along with a range of estimates for one-way sensitivity analyses and the distributions on which the probabilistic sensitivity analysis was based. All medical and non-medical care costs are presented in Japanese Yen (¥) adjusted for inflation to 2011 dollars using the Consumer Price Index for JapanCitation48. All costs and QALYs were discounted at 3% per annum in future years. RV5 is administered as a 3-dose vaccine at 2, 3 and 4 months of age, which is the schedule recommended by the Japan Pediatric SocietyCitation49.
Table 1. Model inputs used in analysis.
The size of the birth cohort in Japan, based on recent census data, is 1,091,156Citation50. Rotavirus incidence is assumed to vary by age. Age-specific incidence of all infections (both symptomatic and asymptomatic) is calculated based on the observed age distribution of outpatient visitsCitation13, with the majority of cases occurring during the first 2 years of life. Secondary infection rates are calculated assuming that 80% of those who get a primary infection are at risk of getting a second infectionCitation45. The model then estimates the percentage of all infections within each age group that are symptomatic.
Vaccine efficacy estimates used in this analysis are from published clinical trial data, the Rotavirus Efficacy and Safety Trial (REST), which includes an evaluation of vaccine efficacy based on healthcare utilization end-pointsCitation14. REST was conducted in 11 countries and enrolled over 70,000 infants who were followed through the first 2 years of life. Although Japan was not one of the countries included in REST, the efficacy estimates for RV5 from a recent clinical trial conducted in Japan were similar to RESTCitation51. Since the Japanese study did not include healthcare resource utilization end-points, data from REST was used to populate the model.
Since there are no efficacy data against death, the effect of vaccination on hospitalizations is also applied to RGE deaths. The efficacy prior to completion of the 3rd dose shown in is consistent with post-hoc analyses of the clinical trial data from RESTCitation52. Given that there is very little data from the trial regarding the efficacy against RGE for those receiving only one or two doses, efficacy assumptions for hospitalizations and deaths for an incomplete series are based on a recent non-randomized studyCitation53,Citation54. Efficacy for genotypes other than G1–G4 and G9 is assumed to be 50% of the efficacy for the G1–G4 and G9 genotypes.
Base case scenario for a single cohort of children born in Japan
For the base case scenario we rely on a prospective surveillance study conducted by Nakagomi et al.Citation12 for the number of rotavirus hospitalizations as well as the cost of a hospitalization for children under five in JapanCitation55. The number of deaths is based on the number of deaths due to acute gastroenteritis and the proportion of those events that are due to rotavirusCitation56,Citation57. The number of outpatient visits and the cost of a visit are consistent with Sato et al.Citation19. Finally, the non-medical and indirect costs including the number of work loss days associated with episodes of rotavirus gastroenteritis are derived from a study conducted by KawamuraCitation58. The percentage of all RGE episodes that are caused by G1, G2, G3, G4, and G9 rotavirus genotypes is assumed to be 91.4%Citation59. Vaccination coverage for infants in the vaccinated cohort is assumed to follow coverage rates consistent with published rates of DTP vaccination (94% for all three doses, 3% for two doses, and 3% for one dose).
The QALY data is based on a Canadian study where the QALY decrements for RGE were based on the Health Utilities Index 2 for children and the EuroQol 5D for parentsCitation10,Citation11. RGE mainly affected two domains in children—emotion and pain/discomfort. The main EQ-5D health domains affected in parents of children with RGE were usual activities, pain/discomfort, and anxiety/depression. The QALY lost per 1000 cases of RGE in children ≤ 3 years of age was estimated to be 2.2 based on the HUI2 and 1.8 for each parent based on the EQ5D. In total there were 5.8 QALYs lost per 1000 RGE cases assumed in the base case scenario or 0.0058 for each episode of rotavirus gastroenteritis or ∼2.2 days on an annual basisCitation10. The QALYs lost due to the morbidity associated with RGE were then added to the life years lost associated with each RGE death estimated in the base case scenario.
The total cost of the 3-dose RV5 series is based on the catalogue price (¥5700 per dose) plus administration fee (¥2500 per dose) for a total series cost of ¥24,600Citation60,Citation61.
Analyses based on children born in five hypothetical municipalities
A set of analyses were also run to examine the public health and economic impact of RV5 on five hypothetical municipalities in Japan. Birth cohorts of 1000, 3000, 5000, 10,000, and 20,000 were evaluated.
Sensitivity analyses
Various one-way and probabilistic sensitivity analyses (PSA) were performed to check the robustness of model results. One-way sensitivity analyses were performed for: frequency and cost of RGE hospitalizations and outpatient visits, number of RGE deaths, vaccine administration fee, and QALY loss assumptions because these parameters were likely to be the most influential. We also conducted a sensitivity analysis to examine the impact of adding minor adverse events as reported in REST. The only statistically significant difference in adverse events between placebo recipients and vaccinees was a slightly higher rate of diarrhea and vomiting in the first week after the first doseCitation62.
The PSA included efficacy of the three-dose series, efficacy of incomplete regimens, number of deaths, hospitalizations, and outpatient visits attributable to RGE, mean number of work loss days per RGE episode, proportion of working parents who miss work, the QALY loss per RGE episode, medical care costs, and the age-specific distribution of healthcare contacts by type. provides detail on the data sources and the distributions assumed for each of the parameters included in the PSA. The model was run 1000 times, and, for each simulation, each PSA variable was set to a different value drawn from its assigned probability distribution. Probability distributions were chosen according to recommendations from a previous study that indicated that different distributions are most appropriate depending on the type of data from which the probabilities are derivedCitation63.
Results
Base case scenario for a single cohort of children born in Japan
The model predicts that in the absence of vaccination there would be 606,285 symptomatic episodes of RGE in a single birth cohort over 5 years with rotavirus. Universal vaccination would avert 71,985 hospitalizations, 503,933 outpatient visits, and 281,412 parental work loss days. This results in reductions of 92%, 74%, and 73% for hospitalizations, outpatient visits, and parental work loss days, respectively.
In the absence of vaccination, RGE-related costs are expected to be ¥26,043,595,288, including ¥18,860,561,942 in direct medical costs, of which 53.1% are hospitalization costs (). If a universal vaccination program were implemented, the ¥26,037,164,472 in vaccination costs would be offset by lower costs to treat rotavirus, which would be ¥2,857,625,197 in direct medical costs or ¥5,043,846,303 if indirect costs are included.
Table 2. Public health and economic impact of a universal vaccination program vs no vaccination program for the base case scenario.
Of the 2500 QALYs gained with the vaccination program, 1269 are directly attributed to the effects on the child including 516 life years lost due to the 17 RGE deaths avoided. The ICERs would be ¥4,014,001 and ¥2,015,122 per QALY gained from the payer and societal perspectives, respectively ().
Table 3. Cost-effectiveness results for universal vaccination vs no vaccination in a single birth cohort in Japan from birth through 5 years of age (in ¥).
Analyses based on children born in five hypothetical municipalities
The results that show the public health and economic impact from the perspective of five municipalities with different birth cohort sizes are shown in . The table provides the number of hospital admissions and outpatient visits as well as direct medical and societal costs for the vaccination and no vaccination strategies.
Table 4. Public health and economic impact of universal RV5 for five birth cohorts in municipalities of varying sizes.
Sensitivity analyses
Of the one-way sensitivity analyses explored, the model was found to be most sensitive to QALY loss per episode followed by vaccine administration fee, number of hospitalizations, and hospitalization cost. The model was least sensitive to number of outpatient visits, outpatient visit costs, and number of deaths. The results of the one-way sensitivity analyses from the medical care and societal perspectives are shown in .
Figure 2. Results of one-way sensitivity analysis: impact of variations in key parameters on ICER, (a) payer perspective; (b) societal perspective: base case value (range).
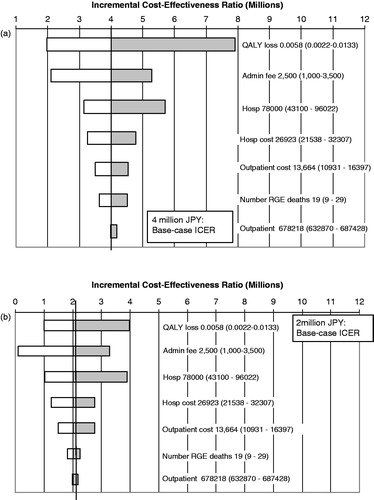
If we conservatively assume the same disutility associated with an episode of rotavirus gastroenteritis for the adverse events involving diarrhea or vomiting, the ICERs would increase by ∼¥136,000 and ¥68,000 from the healthcare or payer and societal perspectives, respectively.
If all of the base case assumptions are maintained but parental disutilities are excluded from the QALY calculation, the ICERs would be ¥7,907,648 and ¥3,969,823 from the payer and societal perspectives, respectively.
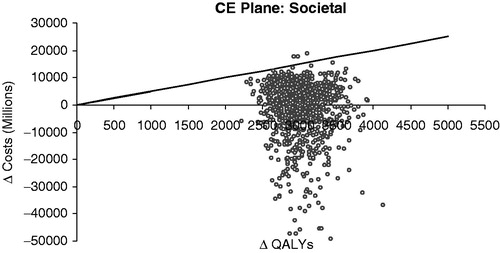
The PSA results are shown in . Each point in the figure represents incremental costs and QALYS for vaccination vs no vaccination from the societal perspective and the diagonal line indicates the ICER threshold of ¥5 million per QALY gained. Of the 1000 PSA simulations, 99% fall below the 5 million per QALY gained line.
Discussion
The results of this study indicate that RV5 has the potential to be a cost-effective public health intervention in Japan, when evaluated from both the societal and payer perspectives. Although there is no formal published threshold in Japan at which health interventions are assumed to be cost-effective, a recent report has indicated that interventions with ICERs below ¥5 million would be considered cost-effective from the healthcare payer perspectiveCitation36.
The economic impact of rotavirus vaccines in Japan has also been described in a previous studyCitation19. This analysis estimated that implementation of rotavirus vaccines on a national level would be considered cost-effective in Japan at ¥9 million and ¥900,000 per QALY gained from the payer and societal perspectives, respectively.
There are several model assumptions and data inputs that differ between the two studies. Our model includes inputs for rotavirus-associated hospitalizations generated from a prospective studyCitation12, while the previous analysis used a combination of hospitalization estimates from prospective and retrospective studiesCitation12,Citation37. A recent comparison of RGE surveillance data from prospective and retrospective studies indicated that, in the US and Hong Kong, rotavirus healthcare utilization estimates generated from studies utilizing retrospective study designs may under-estimate the true burden of rotavirus diseaseCitation38,Citation39. Prospective study designs that are able to capture and test all cases of acute gastroenteritis may provide a more complete understanding of the burden of RGE. This difference in hospitalization estimates accounts for part of the difference in results between our study and the previous cost-effectiveness study.
Sato et al.Citation19 also used a different source for the QALY decrement assumptions. The QALY decrements were derived from the EQ-5D based on a study of physicians in the UK who served as proxies for the children, but physicians often over-estimate the health status of their patients. Use of physician judgement as a proxy for patient preferences may, therefore, under-estimate the decrement in QALYsCitation42,Citation43. Recent studies have recognized the problems associated with applying the existing guidelines from the Panel on Cost-Effectiveness for pediatric studiesCitation64–66. Generating health state preferences and subsequent QALY decrements for temporary health states in children is quite complex and there is not currently a single accepted methodology. Although the use of proxies may potentially introduce bias into the analyses, they are inevitable when young infants are involved. Some researchers have recommended a family model to elicit preferences for conditions affecting very young children in view of the interdependence between young children and their parentsCitation67–69. We believe the approach taken in this study is consistent with these recent recommendations. Although the QALY decrements for the children and their parents were collected separately using two different instruments, the exclusion of parental disutilities is likely to under-estimate the impact of rotavirus gastroenteritis. The estimates used in our analysis are based on prospective surveys of parents and caregivers of children with RGE over a 2-week period, so they may more accurately reflect the quality-of-life impact on the child and family than the physician-proxy survey. We chose a mid-range value for QALY loss per RGE episode and varied the QALY loss assumptions in sensitivity analysis over the range cited in the Brisson et al.Citation10 publication to account for the different elicitation instruments and inclusion of QALY loss for caregivers. As Brisson et al. noted, the QALY decrements derived for rotavirus are reasonable given the QALY decrements reported for other childhood preventable vaccines.
Several other parameters differed between the two models, which may impact differences in results including percentage of working parents projected to miss work, and costs associated with parental work loss. Vaccine cost in our model is greater than the previous model. Healthcare cost inputs for our model and the previous publication, as well as vaccine efficacy estimates, are similar.
The generation of a probabilistic sensitivity analysis is a significant contribution of this analysis that was not completed as part of the previous published analysis. The probabilistic sensitivity analysis allows for the assessment of the impact of parameter uncertainty on model results. The PSA results indicate that, despite significant uncertainty in some parameter estimates such as QALY decrements, the qualitative conclusions that may be drawn from this analysis are robust to model assumptions. RV5 would be considered a cost-effective health intervention under a wide range of model assumptions.
This analysis indicates that universal vaccination with RV5 is projected to have a substantial impact on RGE burden of disease and would be considered a cost-effective healthcare intervention from the payer perspective, as well as the societal perspective, but the economic benefits extend beyond medical care costs saved. When evaluated from the societal perspective, it is projected that parental work loss associated with RGE episodes would also be substantially reduced by universal vaccination. The ICER values generated in this analysis are well within the range of other recently adopted childhood vaccines in JapanCitation70. Exclusion of parental disutilities is likely to under-estimate the impact of rotavirus and decrease the estimated value of a vaccination program. In order to reduce the substantial burden of rotavirus-related disease in Japan, efforts to improve rotavirus vaccine coverage and reduce financial barriers should be considered and implemented in the near future.
Transparency
Declaration of funding
This study was funded by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc.
Declaration of financial/other relationships
All authors have disclosed that they are employed by and own stock or stock options in Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55:1-13
- Staat MA, Azimi PH, Berke T, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J 2002;21:221-7
- Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics 2008;122:1235-43
- Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003;9:565-72
- Tate JE, Burton AH, Boschi-Pinto C, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012;12:136-41
- Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: a national survey. Pediatrics 2002;109:259-61
- Mast TC, Chen PY, Lu KC, et al. Epidemiology and economic burden of rotavirus gastroenteritis in hospitals and paediatric clinics in Taiwan, 2005–2006. Vaccine 2010;28:3008-13
- Mast TC, Walter EB, Bulotsky M, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J 2010;29:e19-e25
- Mast TC, DeMuro-Mercon C, Kelly CM, et al. The impact of rotavirus gastroenteritis on the family. BMC Pediatr 2009;9:11
- Brisson M, Senecal M, Drolet M, et al. Health-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. Pediatr Infect Dis J 2010;29:73-5
- Senecal M, Brisson M, Lebel MH, et al. Measuring the Impact of Rotavirus Acute Gastroenteritis Episodes (MIRAGE): a prospective community-based study. Can J Infect Dis Med Microbiol 2008;19:397-404
- Nakagomi T, Nakagomi O, Takahashi Y, et al. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis 2005;192(1 Suppl):S106-10
- Yokoo M, Arisawa K, Nakagomi O. Estimation of annual incidence, age-specific incidence rate, and cumulative risk of rotavirus gastroenteritis among children in Japan. Jpn J Infect Dis 2004;57:166-71
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354:23-33
- Vesikari T, Itzler R, Matson DO, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis 2007;11(2 Suppl):S29-S35
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11-22
- Wu CL, Yang YC, Huang LM, et al. Cost-effectiveness of childhood rotavirus vaccination in Taiwan. Vaccine 2009;27:1492-9
- Itzler RF, Chen PY, Lac C, et al. Cost-effectiveness of a pentavalent human-bovine reassortant rotavirus vaccine for children </=5 years of age in Taiwan. J Med Econ 2011;14:748-58
- Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn J Infect Dis 2011;64:277-83
- Lorgelly PK, Joshi D, Iturriza GM, et al. Exploring the cost effectiveness of an immunization programme for rotavirus gastroenteritis in the United Kingdom. Epidemiol Infect 2008;136:44-55
- Newall AT, Beutels P, Macartney K, et al. The cost-effectiveness of rotavirus vaccination in Australia. Vaccine 2007;25:8851-60
- Rheingans RD, Constenla D, Antil L, et al. Potential cost-effectiveness of vaccination for rotavirus gastroenteritis in eight Latin American and Caribbean countries. Rev Panam Salud Publica 2007;21:205-16
- Widdowson MA, Meltzer MI, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics 2007;119:684-97
- Goossens LM, Standaert B, Hartwig N, et al. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine 2008;26:1118-27
- Melliez H, Levybruhl D, Boelle PY, et al. Cost and cost-effectiveness of childhood vaccination against rotavirus in France. Vaccine 2008;26:706-15
- Ho AM, Nelson EA, Walker DG. Rotavirus vaccination for Hong Kong children: an economic evaluation from the Hong Kong Government perspective. Arch Dis Child 2008;93:52-8
- Milne RJ, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value Health 2009;12:888-98
- Jit M, Bilcke J, Mangen MJ, et al. The cost-effectiveness of rotavirus vaccination: comparative analyses for five European countries and transferability in Europe. Vaccine 2009;27:6121-8
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009;58:1-25
- Bernaola IE, Gimenez SF, Baca CM, et al. [Vaccination schedule of the Spanish association of pediatrics: recommendations 2009]. An Pediatr (Barc) 2009;70:72-82
- Nohynek H, Salo H, Renko M, et al. Finland introduces rotavirus vaccine into the national vaccination programme in September 2009. Euro Surveill 2009;14:19322
- Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J 2011;30:S25-9
- Zeller M, Rahman M, Heylen E, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010;28:7507-13
- World Health Organization. Rotavirus Vaccines WHO Position Paper – January 2013. Wkly Epidemiol Rec 2013;88:49-64
- Saitoh A, Okabe N. Current issues with the immunization program in Japan: can we fill the “vaccine gap”? Vaccine 2012;30:4752-6
- Health Sciences Council IDS-CVC. Report of the Vaccine Evaluation Sub-committee. 2011. Tokyo, Japan: The Ministry of Health Labor and Welfare
- Kamiya H, Nakano T, Inoue M, et al. A retrospective evaluation of hospitalizations for acute gastroenteritis at 2 sentinel hospitals in central Japan to estimate the health burden of rotavirus. J Infect Dis 2009;200(Suppl 1):S140-6
- Matson DO, Staat MA, Azimi P, et al. Burden of rotavirus hospitalisations in young children in three paediatric hospitals in the United States determined by active surveillance compared to standard indirect methods. J Paediatr Child Health 2012;48:698-704
- Nelson EA, Tam JS, Bresee JS, et al. Estimates of rotavirus disease burden in Hong Kong: hospital-based surveillance. J Infect Dis 2005;192(Suppl 1):S71-9
- Glass RI, Bresee JS, Parashar UD, et al. First rotavirus vaccine licensed: is there really a need? Acta Paediatr Suppl 1999;88:2-8
- Martin A, Cottrell S, Standaert B. Estimating utility scores in young children with acute rotavirus gastroenteritis in the UK. J Med Econ 2008;11:471-84
- Suarez-Almazor ME, Conner-Spady B, Kendall CJ, et al. Lack of congruence in the ratings of patients' health status by patients and their physicians. Med Decis Making 2001;21:113-21
- Muhlbacher AC, Juhnke C. Patient preferences versus physicians' judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy 2013;11:163-80
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409
- Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996;335:1022-8
- Velazquez FR, Calva JJ, Guerrero L, et al. Cohort study of rotavirus serotype patterns in symptomatic and asymptomatic infections in Mexican children. Pediatr Infect Dis J 1993;12:54-61
- Gold MR, Siegel JE, Russell LB, Weinstein MC (eds). Cost-effectivness in health and medicine. New York, NY: Oxford University Press, 1996
- Statistics Bureau, Portal Site of Official Statistics of Japan: Subgroup Index for Japan 2011. 2013. Tokyo, Japan: Ministry of Internal Affairs and Communications
- Japan Pediatric Society Vaccine Schedule [in Japanese]. 2012. 3–26–2013. Tokyo, Japan: The Japan Pediatric Society
- Vital Statistics for Japan 2008. Tokyo, Japan: Statistics Bureau, the Ministry of Internal Affairs and Communications. 2009
- Iwata S, Nakata S, Ukae S, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother 2013;9. [Epub ahead of print]
- Dennehy PH, Vesikari T, Matson DO, et al. Efficacy of the pentavalent rotavirus vaccine, RotaTeq(R) (RV5), between doses of a 3-dose series and with less than 3 doses (incomplete regimen). Hum Vaccin 2011;7:563-8
- Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J 2010;29:1133-5
- Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010;125:e199-207
- Ministry of Health Labor and Welfare. Diagnosis Procedure Combination 2010 [in Japanese]. 2010. Tokyo, Japan: The Ministry of Health Labor and Welfare
- Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010;375:1969-87
- Kawai K, O'Brien MA, Goveia MG, et al. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine 2012;30:1244-54
- Kawamura N. Economic burden derived from rotavirus enteritis on families of pediatric patients [abstract] Kawamura N. 41st Annual Meeting of the Japanese Society for Pediatric Infectious Diseases 2009. Fukui, Japan
- Kitahori Y, Inoue Y, Takebe H, et al. Serotyping of human group A rotaviruses in Nara Prefecture, Japan. Jpn J Infect Dis 2003;56:39-41
- Rotavirus and Vaccination. Nagoya, Japan: Nagoya City's official information site http://www.city.nagoya.jp/kenkofukushi/page/0000034344.html [in Japanese]. [Accessed on 26 Dec 2012]
- Asada N. Nagoya city announced the 50% financial support for rotavirus vaccination from October. Chunichi Shimbun, 2012. Nagoya, Japan
- Dennehy PH, Goveia MG, Dallas MJ, et al. The integrated phase III safety profile of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine. Int J Infect Dis 2007;11(2 Suppl):S36-42
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. USA: Oxford University Press, 2008
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600-14
- Grange A, Bekker H, Noyes J, et al. Adequacy of health-related quality of life measures in children under 5 years old: systematic review. J Adv Nurs 2007;59:197-220
- Happich M, von Lengerke T. Valuing the health state ‘tinnitus': differences between patients and the general public. Hear Res 2005;207:50-8
- Ungar WJ. Challenges in health state valuation in paediatric economic evaluation: are QALYs contraindicated? Pharmacoeconomics 2011;29:641-52
- Prosser LA, Hammitt JK, Keren R. Measuring health preferences for use in cost-utility and cost-benefit analyses of interventions in children: theoretical and methodological considerations. Pharmacoeconomics 2007;25:713-26
- Basu A, Meltzer D. Implications of spillover effects within the family for medical cost-effectiveness analysis. J Health Econ 2005;24:751-73
- Hoshi SL, Kondo M, Okubo I. Economic evaluation of vaccination programme of 7-valent pneumococcal conjugate vaccine to the birth cohort in Japan. Vaccine 2012;30:3320-8
- Van der Wielen M, Giaquinto C, Gothefors L, et al. Impact of community-acquired paediatric rotavirus gastroenteritis on family life: data from the REVEAL study. BMC Fam Pract 2010;11:22