Abstract
Objectives:
To evaluate the cost-effectiveness of MitraClip, an interventional procedure for patients with chronic severe mitral regurgitation.
Methods:
A decision analytic model with a lifetime horizon was developed to assess the cost-effectiveness of MitraClip vs conventional medical management in patients with severe mitral regurgitation, ineligible for surgery. The analysis was performed from a UK NHS perspective and the estimates for mortality, adverse events, and cross-sectional NYHA class were obtained from the EVEREST II High Risk Study (HRS). Utility decrements were obtained from a heath technology assessment on Cardiac Resynchronization Therapy, while unit costs were obtained from national databases. The concept model was clinically validated. Costs (2011 £UK) and benefits were discounted at an annual rate of 3.5%.
Results:
Compared to medical management, over 2- and 10-year periods MitraClip had incremental Quality Adjusted Life Year (QALY) gains of 0.48 and 2.04, respectively. The Incremental Cost-Effectiveness Ratios for MitraClip at 2 and 10 years are £52,947 and £14,800 per QALY gained. Overall, the model was most sensitive to the choice of time horizon, the discount rate applied to benefits, the starting age of cohort, the utility decrement associated with NYHA II, and cost of the MitraClip procedure. The model was insensitive to changes in all other parameters. MitraClip was also found to be cost-effective, regardless of the modelling approach, and insensitive to the key assumptions of the procedure cost.
Study limitations:
The primary limitation of the analysis is the reliance on aggregate data from a modestly sized non-randomized study with a short-term follow-up period. Aligned to this was the need to extrapolate survival well beyond the study period in order to generate meaningful results. The impact of both of these limitations was explored via extensive sensitivity analyses.
Conclusion:
Compared to medical management, MitraClip is a cost-effective interventional procedure at conventional threshold values.
Introduction
Chronic severe mitral regurgitation (MR) is a progressive valvular lesion that leads to LV dysfunction, heart failure (HF), high morbidity, and excess mortalityCitation1–3. The economic and humanistic burdens are substantial due to repeated HF hospitalizations and negative impacts on health-related quality-of-life including physical, emotional, and energy/vitality aspectsCitation4–7. Approximately 60% of the $39 billion in annual costs of HF in the US are associated with hospitalizations, many associated with MRCitation8.
Established treatment options for MR include mitral surgery (replacement or repair) and medical therapy, although patients at high risk for surgery receive only medical therapy. Importantly, medication impacts symptoms but does not treat the underlying disease. Clinical practice guidelinesCitation9,Citation10 recommend mitral valve surgery as the gold standard for degenerative disease, but surgical therapy for functional MR is less well established. A European Society of Cardiology survey found that severe, symptomatic MR patients with major risk factors including LV dysfunction, significant comorbidities, or advanced age are unlikely to be referred for surgery at allCitation11.
In 2008, Abbott Vascular obtained CE approval for the MitraClip system, a novel first-in-class, minimally invasive catheter based repair technique for moderate-to-severe MR. The intervention treats the mechanical problem of leaflet malcoaptation, by approximating the leaflets of the mitral valve. The MitraClip reduces MR less than surgery but it is safer, and offers a percutaneous approach rather than thoracotomy and cardiopulmonary bypass.
Evidence indicates that the MitraClip procedure reduces MR and HF hospitalizations while improving clinical symptoms and quality of life in selected patients who are high-risk surgical candidatesCitation12–27 as determined by a multi-disciplinary heart team. In particular, symptomatic high risk, or otherwise inoperable patients with severe MR (degenerative or functional) are the most appropriate candidates for the MitraClip, when the echocardiographic criteria of eligibility are also metCitation28. The MitraClip procedure has been investigated in clinical trials including EVEREST (Endovascular Valve Edge-to-Edge Repair study) I and EVEREST II conducted in 464 individuals. In both studies and clinical practice, MitraClip has been used in over 10,000 patients from the US and Europe.
The current cost-effectiveness model is concerned with the symptomatic (NYHA 3+ or 4+) high risk (surgical mortality of at least 12%) or otherwise inoperable patients with severe MR (degenerative or functional). To represent this population the analysis has been based on the single arm EVEREST II High Risk Registry Study of patients treated with MitraClip (n = 78) in comparison with a concurrent control group of patients undergoing standard of care treatment (medical management (MM), n = 36)Citation15. To be included, patients had severe symptomatic (3+ or 4+) MR. High surgical risk was determined as a predicted surgical mortality of at least 12%.
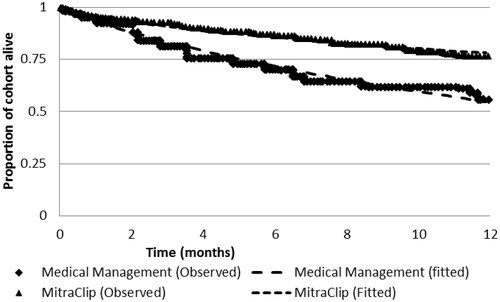
In this study, the 12-month survival rate was 76% in the MitraClip patients and 55% in the concurrent control (p < 0.05). Significant improvements from baseline in LV end-diastolic volume, end-systolic volume, NYHA class, quality-of-life and HF hospitalizations were observed for the MitraClip patients.
Despite these clear clinical benefits, individuals in the EVEREST II HRS were old and infirm and the MitraClip implant cost is incurred on day one. Hence, from a reimbursement perspective it is important to know whether or not these individuals accrue enough benefit to overcome this initial expenditure. In this context, benefit is taken to mean a composite of the amount of additional life gained and the quality of that life.
The objective of the current economic study performed at an early stage of therapy development is to assess the cost-effectiveness of the MitraClip therapy compared to medical management in patients with severe MR, for whom surgery is not an option due to high operative risk, with the primary data source being the EVEREST II High Risk StudyCitation15.
Methods
Model description
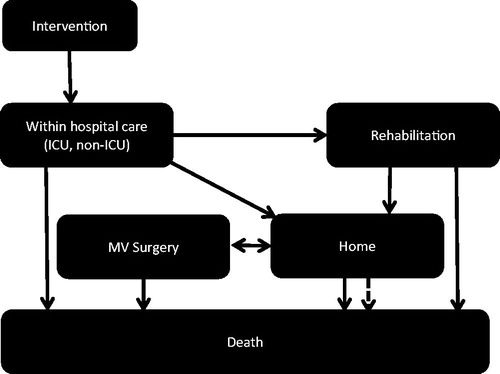
For the cost-effectiveness analysis of MitraClip, two interlinked Markov modelsCitation29 were developed in Microsoft Excel (Microsoft Corporation, Redmond, WA) for the post-procedure period (). The short-term model comprised of the health states including surgery, short-term subsequent MitraClip procedure, intensive care, non-intensive care (‘within hospital care’), home, rehabilitation, and death, while the long-term model incorporated the health states of home, subsequent MitraClip-related procedures, and death. The model was constructed on the best available evidence, and its underlying assumptions were validated by an advisory panel of experienced clinicians.
The short-term model accounted for a time horizon of 30 days post-MitraClip procedure with a time unit of 1 day. Health states were based around location of care and post-procedural rehabilitation. Death was possible from all health states. Individuals in the MitraClip arm entered the model in the ‘Intervention’ state and those in the medical management arm in the ‘Home’ state. Contingent on survival through 30 days, all patients entered the long-term model in the ‘Home’ state and remained there until death or requirement of an additional procedure. Individuals in the medical therapy arm remained in the Home state until death. In the long-term model, a time horizon of five years was used with the unit of time set to one month.
The analysis was performed from a UK National Health Service perspective. Regardless of treatment option, patients incurred costs and accrued benefits for each day/month alive as they passed through the health states in both models. Costs and benefits were discounted at the rate of 3.5% per year. The results of the model are presented as an Incremental Cost Effectiveness Ratio (ICER) and benefits expressed in terms of Quality Adjusted Life Years (QALYs).
The methods used to estimate key model parameters are summarized below. In general, actual results from the EVEREST II HRS were used wherever possible and were supplemented by information in selected publications identified through a systematic review of Embase and Medline.
Modelling change in NYHA class
In the absence of patient level data, aggregate values from the EVEREST II HRS were used to model treatment-related changes in NYHA class from baseline to 30 days, 12 months, and 24 monthsCitation15,Citation30,Citation31 (Supplementary material Table S1). The changes in the NYHA mix were based on the assumption of linear change between time points in NYHA mix over time, thus allowing calculation of monthly estimates over the first 2 years. After 2 years, NYHA mix was assumed to be constant. For patients in the medical management arm, the baseline NYHA mix was assumed to be constant.
The rationale for this approach was that, due to the high mortality rate associated with NYHA IV heart failure, there would not be a build-up of patients in this stage over time. It, thus, followed that, on an aggregate level, the assumption of a constant case mix was not unreasonable.
Mortality
The primary source of mortality data used in the base case analysis was from the EVEREST II HRSCitation15. Reported Kaplan-Meier curves were utilized over the first 12 months for both patient groups as the basis for parametric survival analysis using a Weibull function which was found to be the best fit using standard statistics. Separate curves were used for each arm, meaning that no proportional hazard assumption has been made. The cumulative survival estimates were extrapolated into the future and used to derive daily or monthly transition probabilities using standard formulae during all model cyclesCitation29. The EVEREST II HRS clinical study reportCitation30 showed no intra-procedural mortality in the MitraClip arm.
Estimating other clinical events
The rates of other treatment-related events such as subsequent MitraClip procedures, major stroke, emergency CV surgery, post-procedure length of stay, and Heart failure-related hospitalization were obtained from the EVEREST II HRSCitation30 (Supplementary Material Table S1).
Heart failure hospitalizations for patients who did not undergo interventional procedures were obtained from the published literature, which reflects a conservative estimate given the high severity of the EVEREST II HRS treatment populationCitation32. For mild disease hospitalization (NYHA I/II), the derived value corresponded to a monthly probability of 1.69%. For NYHA III/IV a monthly hospitalization probability of 8.46% was derived from 12-month pre-recruitment data from all individuals in the EVEREST II HRSCitation30. Transition rates between hospital-based states (ICU, non-ICU, non-hospital) were based on mean length of stay and derived using standard formulaeCitation29.
Cost and HRQoL weights
Drug costs and other resource uses were obtained from the British National FormularyCitation33 and UK National Service Schedule of Reference CostsCitation34, Hospitalization costs were calculated using weighted averages of the events (ICU, non-ICU, stroke, CV surgery, myocardial infarction, renal failure, deep wound infection, etc.). The total cost of thr MitraClip delivery system was provided by Abbott (£20,000). Where necessary the values were inflated to 2011 prices using an appropriate indexCitation35. The MitraClip procedure cost was the subject of several sensitivity analyses (Supplementary material). Estimates of background medication were based on expert opinion and assumed constant across both treatment options.
The model incorporated Health Related Quality-of-Life (HRQoL) as utilities, expressed on a linear scale, with a value of one equivalent to perfect health and zero to death. Gender-adjusted utility value for the representative UK populationCitation36 and MR decrements (−0.193) for all patients in the base case were based on literatureCitation4,Citation6. NYHA class-specific utility decrements for all four classes were derived from a representative sample of the UK population with a mean age of 45 yearsCitation37. Decrements relating to ICU and non-ICU stay and treatment-related adverse events were obtained from the literatureCitation38,Citation39. Utility for non-ICU stays was obtained from the literature (0.712) and then age and gender adjusted using the population norm of 0.837, resulting in a utility decrement of 0.125 for non-ICU stays. Similarly, for the ICU stay, the utility of 0.532 was adjusted using 0.837, leading to a decrement of 0.305 (Supplementary material).
Sensitivity analyses
A probabilistic sensitivity analysis was performed to characterize uncertainty in the model parameters (utility decrements, short-term adverse events, length of stay, procedure costs, resource use patterns, mortality estimates/other transition probabilities, baseline NYHA mix, all scaling factors) using statistical distributions rather than point estimates). Results were represented using a cost-effectiveness acceptability curve (CEAC) representing the probability of an intervention being cost-effective over a range of different willingness-to-pay thresholds.
Furthermore, deterministic analyses around the following parameters: choice of time horizon, discount rate applied to benefits, starting age of the cohort, utility decrement associated with NYHA II, and cost of MitraClip procedure were performed. In addition, threshold analyses were considered around key parameters the model was sensitive to.
We also conducted analyses looking at the impact of different structural assumptions on the cost-effectiveness of the MitraClip. These analyses included alternative data sources for baseline mortality, different methods for including utility and also for estimating all decrements.
Results
The Weibull parametric survival functions were a good fit to the HRS mortality data (R2 > 0.97, ) Predicted lifetime survival in the treatment and control arms is 5.1 and 1.9 years, respectively. Expected survival in an equivalent age- and gender-adjusted member of the general public is 10.6 years.
Compared to medical management, the average MitraClip patient received an incremental 0.48 QALYs (95% CrI = 0.46–0.51) compared to those on MM over a 2-year time horizon, but incurred total additional costs of £25,565 (95% CrI = £17,502–£37,316). The resulting ICER was £52,947 per QALY gained (95% CrI = £36,359–£79,109) ().
Table 1. Cost-effectiveness results (Mean, 95% CrI) at a range of time horizons.
Using a 5-year time horizon, MitraClip generated an ICER relative to MM of £22,200 per QALY gained (95% CrI = £15,600–£32,300). The average MitraClip patient received an incremental 1.22 QALYs (95% CrI = 1.17–1.27) compared to those on MM, but incurred total additional costs of £27,000 (95% CrI = £18,900–£38,700). ().
The major incremental cost drivers in the model were lifetime implant costs (+£20,500), short-term hospitalizations (+£3,800), and background medication (+£1400). The latter arises as a result of the extended survival associated with MitraClip (+1.4 discounted years). Similarly, while the average monthly cost of hospitalization is lower in the MitraClip arm, as a result of this additional life the total costs in both arms are very similar.
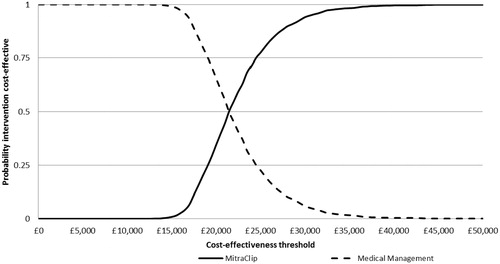
The results from the analysis are presented graphically in . At the thresholds used by the National Institute for Health and Clinical Excellence (NICE) in the UK for reimbursement decisions (£20,000 and £30,000 per QALY gained), the probability that MitraClip is cost-effective is approximately and 37% and 93%, respectively.
Sensitivity analyses
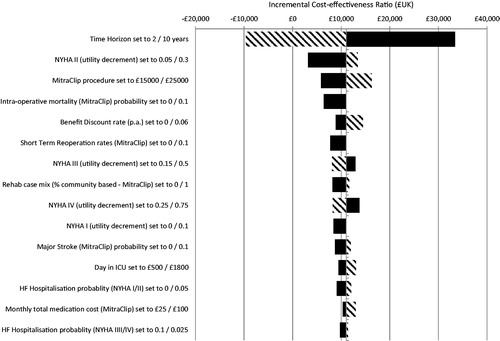
The results from the univariate deterministic sensitivity analyses are presented in , with a negative value corresponding to an ICER above £30,000 per QALY gained and a positive value to an ICER below this threshold. Overall, the model was most sensitive to the choice of time horizon, the utility decrement associated with NYHA II and cost of the MitraClip procedure. The model was insensitive to changes in all other parameters.
A threshold analysis was performed on the time horizon indicated to identify the level of extrapolation of trial data required to demonstrate cost-effectiveness at threshold values of £30,000 and £20,000 per QALY gained (). For the former the model had to be run for ∼3 years and for the latter 5 years. For ease of comparison, predicted survival in the general population has also been generated. Therefore, when accounting for all other parameter uncertainty only 2 years of extrapolation of the HRS data is required to demonstrate value for money.
Table 2. Deterministic sensitivity analysis: predicted survival and cost-effectiveness at a range of alternative time horizons.
A similar analysis was performed on the key assumption in the model, namely the total MitraClip procedure cost. Setting the cost to £25,000 (+25%) resulted in a modest change to the ICER, with the value ranging from £16,000–£25,000. The model is, therefore, insensitive to the assumption made.
Finally, alternative structural assumptions for baseline mortality, impact of MR on HRQoL, and long-term re-operation were explored) (Supplementary Material). Regardless of choice, the model was insensitive to the alterations, with the ICER being below £30,000 for nearly all options. Hence, MitraClip remained overall cost-effective, regardless of the modelling approach.
Discussion
Conceptually, interventional procedures incur the majority of relevant costs on day 1, but accrue benefits over time. As such, the key economic question is whether or not treatment offers enough benefit to offset the additional costs incurred by treatment. This question is particularly relevant for individuals such as those in the EVEREST II HRS since, due to a combination of age and co-morbidities, they are too high risk for conventional surgery.
The model presented in this paper is the first to assess the cost-effectiveness of MitraClip in this high risk medically managed population. The model was based on the best available evidence and the structure was very similar to that used in a published cost-effectiveness study of TAVICitation40. The model was also developed to be fully compliant with the requirements laid down by NICECitation41.
Overall, when all parameter uncertainty was included, MitraClip was shown to be dependent on the length of time the model is run for, and relatively insensitive to the device and procedure cost. The cost-effectiveness ratio was ∼£22,500 per QALY gained considering a 5-year time horizon and, as such, lies between the threshold values typically used by NICE in their reimbursement decision-making. Agencies such as NICE, however, require that the lifetime costs and benefits of each intervention are evaluated. After 5 years 47% of the MitraClip and 8.9% of the medical management cohorts are still alive.
Thus, when viewed from a reimbursement agency perspective, a 5-year time horizon is too short. When the model was run for 10 years, the ICER generated was well below the lower threshold used by NICE (£14,800 per QALY gained, ). Using this time horizon, there was a 95% probability that MitraClip was cost-effective compared to MM at £20,000 per QALY gained. Extensive sensitivity analyses showed that the results were robust to changes in model parameters as well as alternative approaches to including key clinical parameters. Thus, were NICE to formally appraise MitraClip, it is highly likely that they would conclude it is cost-effective; individuals with baseline characteristics comparable to those in the EVEREST II HRS do indeed live long enough to accrue enough benefit to justify the initial procedure-related costs.
An inevitable consequence of the need to generate long-term costs and benefits is that data from clinical trials will require extrapolation. The process is predicated on the use of mathematical models to identify trends in the clinical trial data and to project these trends forwards. Numerous examples of this process exist within the area of cardiology (see, for example, Linde et al.Citation42, Watt et al.Citation40, Gada et al.Citation43, Briggs et al.Citation44, Henriksson et al.Citation45, and Sculpher et al.Citation46).
In the current analysis, the survival data from the EVEREST II HRS only need be extrapolated beyond last follow-up for ∼2 years to be considered cost-effective ( and Supplementary material). Similarly, while the ICER generated using a 2-year time horizon is higher than accepted in the UK, it is potentially acceptable in other countries ($83,300 per QALY gained, ).
On a technical level, we have not assumed any form of mortality treatment effect associated with MitraClip and have modelled the two arms of the study data independently; we have not viewed the single arm study data as if it were RCT data. This assumption was based on an analysis of the extracted survival data. In order to overcome perceived limitations associated with the use of parametric functions to model mortality we have incorporated an entirely different approach into the model—the assumption that mortality is a multiple of the general population estimate. This approach was the only analysis undertaken that generated in an ICER well in excess of £30,000 per QALY gained and it should be noted that the scaling factor was derived using a fairly crude calculation. Hence, by looking to move beyond the use of parametric functions we may have created more problems than we solved.
The key driver of cost-effectiveness relates to the benefits associated with treatment and not to costs. Using a 2-year time horizon, patients receiving MitraClip experience a projected survival gain of ∼0.5 discounted QALYs compared to medical management. The equivalent value for a 10-year horizon is 2.75 life years and 2 QALYs. Hence, when using a long-term time horizon, even when the up-front cost of either the procedure or the probability of heart failure hospitalization is increased by up to 25%, the impact on the cost-effectiveness of the MitraClip is minor. The sensitivity analysis around the procedure cost can be interpreted to cover a number of real world events such as a small proportion of patients actually undergoing an intra-operative repair/replacement, more than one clip being required in a small proportion of patients or both.
The tornado plot showed the relative importance (or otherwise) of alterations to other parameters. An increase in the intra-operative mortality to 5% resulted in a £1300 increase in the ICER. When this rate was combined with a 10% increase in the MitraClip procedure cost, the ICER rose by £3100. These analyses show that the cost-effectiveness conclusions are not reliant on operator experience.
Contextually, the results from the model are similar to those derived for other cardiovascular procedures offering patients markedly increased survivalCitation40,Citation42,Citation47,Citation48. Internationally, MitraClip has been the subject of two formal appraisals in the surgically ineligible population, with an FDA Advisory Panel recommending regulatory approval in carefully selected patientsCitation49 and the Australian Medical Services Advisory Committee noting it had a place in the treatment pathway for these patientsCitation50. Formal reimbursement and funding has also been established in various countries such as Germany, Switzerland and Turkey. A different view was taken by the UK NHS who, in a commissioning policy statement, stated that they would not routinely fund the treatment citing lack of evidence relating to benefit as justificationCitation51. Crucially, the cost-effectiveness of MitraClip in our model was robust as to the methods used to model mortality.
Study limitations
The key limitation of this model is reliance on aggregate data from one study of 78 treated and 36 control patients in place of patient level data from a large randomized clinical trial. The EVEREST HRS publication identifies several weaknesses in the data. First, although the comparator group was similar in risk scores and baseline characteristics to the HRS group, a large proportion of the control patients may not have been anatomically eligible for MitraClip. The inclusion of such patients may not then provide a relevant and appropriate comparator arm. Also, the 12 month analysis reported the ECG and functional data only for surviving patients, possibly inflating the true benefit provided by MitraClip. Finally the reporting of the number of MitraClip devices used was incomplete, EVEREST I reported that 29% of patients received more than one device during the index procedure. While the use of multiple clips might have substantial implications to the cost-effectiveness, it has been Abbott’s policy to charge per procedure rather than per MitraClip device, so the use of two or more devices in a single patient would be cost neutral. As such, the conclusions drawn can be applied with confidence to individuals who meet the criteria of this study and there remains a need to conduct well designed randomized controlled trials to confirm the effects of treatment.
The model is also limited by the need to extrapolate survival data from these patients to generate lifetime costs and benefit estimates. To confront this limitation we undertook extensive sensitivity analyses and assessed the impact of different structural assumptions to explore what would happen if the real world data looked different. In particular, we used data from a landmark phase III trial (CARE-HF)Citation52 as an alternative proxy for baseline risk and explored the impact of assuming that patients with severe MR die ∼3-times quicker than the general population. In both cases, MitraClip remained cost-effective at conventional thresholds.
Similarly, there is a paucity of data on long-term, post-12 month MitraClip replacement rates and we had to rely on data from an Italian modelling studyCitation53. We also had 1-year follow-up data on additional MV surgery from the EVEREST II HRS which showed no additional procedures. However, a number of clinical studies on the use of MitraClip in high risk patients have documented short-term rates in excess of 3%Citation54–57. Whilst it is unlikely that there will be a substantial number of repeat procedures in the target patients group, additional or repeat procedures may have an impact on the cost-effectiveness of the MitraClip procedure.
Finally, we chose our ‘decision point’ to be the point at which a patient enters the theatre and as such have not included pre-surgical work-up costs. Intuitively, patients who are ineligible for surgery would be expected to consume fewer such costs than those who undergo surgery. This omission was made for pragmatic reasons. In the context of the long-term (5-year) cost-effectiveness results, if the UK is willing to pay £30,000 for a QALY then the difference in work-up costs in the two arms would have to be £9600 to alter the cost-effectiveness decision. Using the long-term (lifetime) results, this difference would have to be greater again. Hence, the omission of these costs is unlikely to have any meaningful impact on cost-effectiveness.
Conclusions
From a UK reimbursement perspective, in patients with severe symptomatic MR who are deemed ineligible for either surgical repair/replacement due to having to a predicted surgical mortality risk in excess of 12%, MitraClip represents a cost-effective treatment option compared to medical management over a 10-year time frame at conventional reimbursement thresholds. The clinical effectiveness of this procedure in this patient group will need to be confirmed through appropriately positioned randomized controlled studies.
Transparency
Declaration of funding
The work reported in this manuscript was funded via a consultancy agreement between Oxford Outcomes Ltd. and Abbott Vascular.
Declaration of financial/other relationships
TF has received grants and also consultancy/advisory payments from Abbott Vascular. All other authors have no declarations.
Supplementary Material
Download PDF (37.1 KB)References
- Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 Guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:676-85
- Grigioni F, Tribouilloy C, Avierinos JF, et al; MIDA Investigators. Outcomes in mitral regurgitation due to flail leaflets a multicenter European study. JACC Cardiovasc Imaging 2008;1:133-41
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83
- Goldsmith IR, Lip GY, Patel RL. A prospective study of changes in the quality of life of patients following mitral valve repair and replacement. Eur J Cardiothorac Surg 2001;20:949-55
- Rimington H, Weinman J, Chambers JB. Predicting outcome after valve replacement. Heart 2010;96:118-23
- Steptoe A, Mohabir A, Mahon NG, et al. Health related quality of life and psychological wellbeing in patients with dilated cardiomyopathy. Heart 2000;83:645-50
- Supino PG, Borer JS, Franciosa JA, et al. Acceptability and psychometric properties of the Minnesota Living with Heart Failure Questionnaire among patients undergoing heart valve surgery: validation and comparison with SF-36. J Card Fail 2009;15:267-77
- Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace 2011;13(2 Suppl):ii13-ii17
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:1-148
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65
- Alegria-Barrero E, Chan PH, Paulo M, et al. Edge-to-edge percutaneous repair of severe mitral regurgitation. Circ J 2012;76:801-8
- Ussia GP, Cammalleri V, Scandura S, et al. Update on percutaneous mitral valve therapy: clinical results and real life experience. Minerva Cardioangiol 2012;60:57-70
- Ussia GP, Cammalleri V, Sarkar K, et al. Quality of life following percutaneous mitral valve repair with the MitraClip System. Int J Cardiol 2012;155:194-200
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair the EVEREST-II (Endovascular Valve Edge-to-Edge Repair) high risk study. J Am Coll Cardiol 2012;59:130-9
- Rudolph V, Knap M, Franzen O, et al. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 2011;58:2190-5
- Auricchio A, Schillinger W, Meyer S, et al; PERMIT-CARE Investigators. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011;58:2183-9
- Maisano F, La Canna G, Colombo A, et al. The evolution from surgery to percutaneous mitral valve interventions: the role of the edge-to-edge technique. J Am Coll Cardiol 2011;58:2174-82
- Schillinger W, Athanasiou T, Weicken N, et al. Impact of the learning curve on outcomes after percutaneous mitral valve repair with MitraClip and lessons learned after the first 75 consecutive patients. Eur J Heart Fail 2011;13:1331-9
- Maisano F, Godino C, Giacomini A, et al. Patient selection for MitraClip therapy impaired left ventricular systolic function. Minerva Cardioangiol 2011;59:455-71
- Maisano F, Godino C, Giacomini A, et al. Clinical trial experience with the MitraClip catheter based mitral valve repair system. Int J Cardiovasc Imaging 2011;27:1155-64
- Maisano F, Taramasso M, Cioni M, et al. Review of the MitraClip clinical evidence. Minerva Cardioangiol 2012;60:85-93
- Rogers JH, Franzen O. Percutaneous edge-to-edge MitraClip therapy in the management of mitral regurgitation. Eur Heart J 2011;32:2350-7
- Franzen O, van der Heyden J, Baldus S. MitraClip® therapy in patients with end-stage systolic heart failure. Eur J Heart Fail 2011;13:569-76
- Tamburino C, Immè S, Barbanti M, et al. Reduction of mitral valve regurgitation with Mitraclip® percutaneous system. Minerva Cardioangiol 2010;58:589-98
- Tamburino C, Ussia GP, Maisano F, et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J 2010;31:1382-9
- Feldman T, Kar S, Rinaldi M, et al; EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686-94
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406
- Briggs A, Claxton K, Schulpher M. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press, 2006
- Abbott Vascular. A study of the evalve cardiovascular valve repair (MitraClip) System Endovascular Valve Edge-Edge REpair STudy (EVEREST-II): EVEREST -II High Risk Registry Clinical Report. (Data on file)
- Feldman T, Foster E, Kar S, et al; on behalf of the EVEREST II Investigators. Randomized comparison of percutaneous mitral valve repair and surgery for mitral regurgitation. 2 year results of the EVEREST II randomized controlled trial. Presented at ACC 2011. San Francisco, California, USA. June 29--July 1, 2011. http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425339.pdf. Accessed February 2012
- Aronson D, Goldsher N, Zukerman R et al. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med 2006;166:2362-8
- British National Formulary. UK department of health. www.bnf.org
- National Health Service Schedule of Reference costs 2008/2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591
- Eurostat - Histological EU inflation rates (HICP - all items). 2011. Available by the European Commission
- Kind P, Hardman G, Macran S. UK Population Norm for EQ-5D - CHE discussion paper. 1999. http://www.york.ac.uk/media/che/documents/papers/discussionpapers/CHE%20Discussion%20Paper%20172.pdf. Available from the University of York, UK website
- Kirsch J, McGuire A. Establishing health state valuations for disease specific states: an example from heart disease. Health Econ 2000;9:149-58
- Edwards SJ, Campbell HE, Plumb JM. Cost-utility analysis comparing meropenem with imipenem plus cilastatin in the treatment of severe infections in intensive care. Eur J Health Econ 2006;7:72-8
- Saborido CM. Systematic review and cost effectiveness evaluation of ‘pill-in-the-pocket’ strategy for paroxysmal atrial fibrillation compared to episodic in-hospital treatment or continuous antiarrhythmic drug therapy. Health Technol Assess 2010;14:iii-iv, 1-75
- Watt M, Mealing S, Eaton J, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart 2012;98:370-6
- National Institute for Health and Care Excellence. Methodological guideline for assessing cost-effectiveness. London, UK: NICE. http://www.nice.org.uk/media/68D/29/The_guidelines_manual_2009_-_Chapter_7_Assessing_cost_effectiveness.pdf
- Linde C, Mealing S, Hawkins N, et al. Cost-effectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure: insights from the European cohort of the REVERSE (Resynchronization Reverses remodeling in Systolic Left Ventricular Dysfunction). Eur Heart J 2011;32:1631-9
- Gada H, Kapadia SR, Tuzcu EM, et al. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol 2012;109:1326-33
- Briggs A, Mihaylova B, Sculpher M, et al. Cost effectiveness of perindopril in reducing cardiovascular events in patients with stable coronary artery disease using data from the EUROPA study. Heart 2007;93:1081-6
- Henriksson M, Epstein DM, Palmer SJ, et al. The cost-effectiveness of an early interventional strategy in non-ST-elevation acute coronary syndrome based on the RITA 3 trial. Heart 2008;94:717-23
- Sculpher MJ, Lozano-Ortega G, Sambrook J, et al. Fondaparinux versus Enoxaparin in non-ST-elevation acute coronary syndromes: short-term cost and long-term cost-effectiveness using data from the Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators (OASIS-5) trial. Am Heart J 2009;157:845-52
- Calvert MJ, Freemantle N, Yao G, et al. Cost-effectiveness of cardiac resynchronization therapy: results from the CARE-HF trial. Eur Heart J 2005;26:2681-8
- Griffin SC, Barber JA, Manca A, et al. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ 2007;334:624
- United States Food and Drug Administration. 20th March, 2013. US Food and Drug Agency. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM345235.pdf. Accessed 13 April, 2013
- Medical Services Advisory Committee. 2013. Australian Medical Services Advisory committee. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/01C3008A7A465AEACA25794F001FB36E/$File/MSAC-App-1192-Minutes-Nov2012-redacted.pdf. Accessed 13th April, 2013
- NHS Commissioning Board. Clinical Commissioning Policy Statement: MitraClip. www.engage.commissioningboard.nhs.uk. Accessed 13th April, 2013
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11
- Garcia Meta. Evaluación económica del tratamiento de la del tratamiento de la insuficiencia de la válvula mitral. Sevilla, Spain: Spanish Ministerio De Santidad Y Consumo, 2008. http://www.juntadeandalucia.es/salud/servicios/contenidos/nuevaaetsa/up/AETSA_2006-31_Insuf_valv_mitral.pdf
- Baldus S, Schillinger W, Franzen O, et al. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050-5
- Conradi L, Treede H, Franzen O, et al. Impact of MitraClip™ therapy on secondary mitral valve surgery in patients at high surgical risk. Eur J Cardiothorac Surg 2011;40:1521-6
- Rudolph V, Knap M, Franzen O, et al. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 2011;58:2190-5
- Treede H, Schirmer J, Rudolph V, et al. A heart team’s perspective on interventional mitral valve repair: percutaneous clip implantation as an important adjunct to a surgical mitral valve program for treatment of high-risk patients. J Thorac Cardiovasc Surg 2012;143:78-84
- Cleland JG, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J 2006;27:1928-32
- Ara R, Brazier J. Deriving an algorithm to convert the eight mean SF-36 dimension scores into a mean EQ-5D preference-based score from published studies (where patient level data are not available). Value Health 2008;11:1131-43