Abstract
Objective:
To compare treatment persistence in attention-deficit/hyperactivity disorder (ADHD) of patients initiated on lisdexamfetamine (LDX) vs other ADHD medications.
Methods:
A large US administrative claims database was used to select ADHD patients who initiated an ADHD medication (index treatment) during/after 2007. Patients were classified, based on age and previous treatment status, as treatment-naïve or previously treated children and adolescents (6–17 years) and treatment-naïve or previously treated adults (18 years and older). Furthermore, patients were classified into seven mutually exclusive treatment groups, based on their index treatment: LDX, atomoxetine (ATX), osmotic-release methylphenidate hydrochloride long-acting (OROS MPH), other methylphenidate/dexmethylphenidate short-acting (MPH SA) and long-acting (MPH LA), and amphetamine/dextroamphetamine short acting (AMPH SA) and long-acting (AMPH LA). Treatment persistence, analyzed through discontinuation (interruption of the index treatment for ≥30 consecutive days), was compared between treatment groups using multivariate Cox proportional hazards. Patients were followed until first treatment discontinuation or up to 12 months after the initiation of the index treatment, whichever occurred first.
Results:
Among children and adolescents, LDX patients had a significantly lower discontinuation rate compared to other treatment groups (range hazard ratios [HRs]; 1.04–2.26; all p < 0.05), except when compared to treatment-naïve patients on ATX and OROS MPH, where no statistically significant differences were found and where LDX had a higher risk of discontinuation, respectively. Among adults, LDX patients had a significantly lower discontinuation rate compared to patients in other treatment groups (range HR; 1.14–1.86; all p < 0.05), except for the comparison with AMPH LA patients, where differences were not statistically significant.
Limitations:
This study did not control for ADHD severity.
Conclusion:
LDX-treated patients were associated with higher persistence compared to patients initiated on other ADHD medications, except for the comparisons with OROS MPH and ATX treated patients in treatment-naïve children and adolescents and AMPH LA-treated patients in adults.
Background
Attention-deficit/hyperactivity disorder (ADHD) is a behavioral disorder that affects both children and adults and is characterized by hyperactivity/impulsivity and inattentionCitation1–3. Untreated or sub-optimally treated ADHD can lead to significant long-term academic, professional, and psychosocial impairments, and results in reduced health-related quality-of-lifeCitation4. However, with treatments currently available, many people are able to minimize the impairments and improve their quality-of-life. Stimulant medication has been shown to be an effective treatment option to reduce and control ADHD symptomsCitation5–8. However, the benefit of a medication can be negatively affected by the discontinuation of the treatment, which might lead to resurfacing of symptoms and worse health-related quality-of-lifeCitation9. Moreover, frequent interruptions in the medication schedule may not permit adequate titration and prevent the patient from fully benefiting from the effects of the medication. It has also been reported that treatment discontinuation might lead to loss of medical care altogether, as some patients would stop visiting their physician’s office following treatment discontinuationCitation10.
In children, treatment discontinuation is an important consideration since uncontrolled ADHD symptoms can interfere with normal social and behavioral developmentCitation11. Similar concerns have been expressed regarding the impact of intermittent ADHD medication use on adolescent developmental stagesCitation12. Untreated or sub-optimally treated ADHD might also result in undesirable outcomes in older adolescents and young adults. For example, it has been shown that untreated ADHD patients are associated with lower driving performance compared to treated ADHD patients and that drivers with untreated or sub-optimally treated ADHD are associated with twice as many motor vehicle collisions than a comparable non-ADHD populationCitation13,Citation14.
ADHD treatment discontinuation has been frequently observed in children and adolescents and in adultsCitation12,Citation15,Citation16. Recent studies reported that the rate of overall treatment discontinuation exceeds the expected rate of patients who are no longer affected by ADHD symptoms, suggesting that many of those who stopped treatment would have benefitted from longer treatment durationCitation12,Citation17. Reasons for treatment discontinuation may vary significantly from one patient to another. Most common reasons include the availability of other treatment options, stigma related to medical treatment, side-effects, non-responsiveness or weak responsiveness to treatment, as well as patients’ or parents’ beliefs and preferences concerning reliability of diagnosis and safety of long-term useCitation12,Citation18,Citation19. Several other factors can influence ADHD treatment persistence, such as treatment settings, prescriber, or even high medication costs, which are sometimes not reimbursed by health insurance plansCitation20. In some instances, a planned discontinuation period (‘drug holiday’) might also be prescribed to allow physicians and patients to evaluate the state of the disease, and when growth is significantly affected by the ADHD treatment, a drug holiday may also be prescribed to allow ‘catch-up’ growth to occurCitation4,Citation10,Citation21,Citation22. However, the benefit of a drug holiday is unclear and treatment guidelines adopt different positions. For example, although NICE clinical guidelines mention the option of a treatment holiday in specific cases (e.g., to allow ‘catchup’ growth to occur in the case of impaired growth or to assess whether treatment is still needed), drug holidays are not routinely recommended and, when an acceptable treatment response is achieved, it is recommended to continue the ADHD medication as long as the treatment is deemed effectiveCitation4.
To the best of our knowledge, no study has yet compared treatment persistence in ADHD patients across the most commonly prescribed stimulant and non-stimulant medications among all age and all treatment groups (i.e., treatment-naïve and previously treated children, adolescents and adults) using a single methodology. To fill this knowledge gap, the goal of the present study is to characterize and compare rates of treatment persistence among the most common currently prescribed stimulant and non-stimulant medications for the treatment of ADHD using a large longitudinal population-level dataset of real-world data from the US. To provide a complete portrait of treatment persistence in patients with ADHD, treatment persistence was assessed in previously treated and treatment-naïve patients and across different age populations using the same methodology across study cohorts and populations. Outcomes were compared between patients initiated on lisdexamfetamine (LDX) and the most commonly prescribed stimulant and non-stimulant medications, osmotic-release methylphenidate hydrochloride long-acting (OROS MPH) and atomoxetine (ATX) (the only non-stimulant approved by the FDA at the time of the study), and between LDX and the other ADHD medication classes, including methylphenidate/dexmethylphenidate short-acting (MPH SA), methylphenidate/dexmethylphenidate long-acting (MPH LA), amphetamine/dextroamphetamine short-acting (AMPH SA), and amphetamine/dextroamphetamine long-acting (AMPH LA).
Findings from this study will provide information on the ADHD medications that are associated with higher persistence rates in specific ADHD patient cohorts.
Methods
Data source
This study was conducted using data from the Truven Health Analytics MarketScan® (MarketScan) database from 2006–2009. The MarketScan database is a private sector health data resource which reflects the healthcare experience of employees and dependents covered by the health benefit programs of large employers. The database includes enrollment history and claims for medical (provider and institutional) and pharmacy services for insured employees and their dependents, as well as for Medicare-eligible retirees with employer-provided Medicare Supplemental plans. All census regions are represented, though almost two thirds of enrollees are from the South and North Central (Midwest) regions. Data are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act.
Patient selection and construction of study cohorts
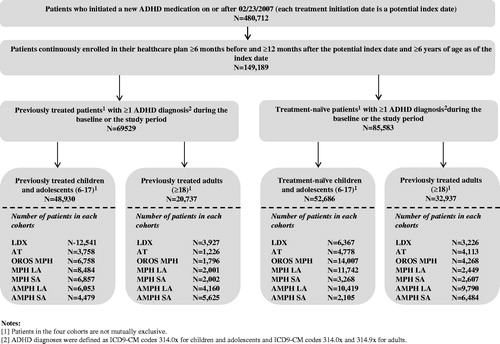
ADHD patients who initiated a new FDA-approved ADHD treatment between 2007–2009 were extracted from the MarketScan database. Based on their age and ADHD treatment status prior to the treatment initiation date (index date), patients were classified into two populations, children/adolescents (6–17 years old) and adults (≥18 years old), and into four cohorts: (1) treatment-naïve children and adolescents, (2) previously treated children and adolescents, (3) treatment-naïve adults, and (4) previously treated adults. For each cohort, patients were divided into seven mutually exclusive treatment groups with respect to the medication patients initiated at the index date; namely: LDX, ATX, OROS MPH, MPH SA, MPH LA, AMPH SA, AMPH LA (). A complete and detailed description of the sample selection and study design has been recently publishedCitation23, and a detailed list of the various drug categories evaluated in this study can be found in the Supplementary Appendix Table 5 of this publication.
The baseline period was defined as the 6-month period preceding the index date. The study period spanned from the index date up to 12 months following the index date. A retrospective design was used to compare outcomes between the LDX treatment group and each of the other treatment groups. For each cohort, results will be presented for the comparison of (1) LDX and the most commonly prescribed stimulant and non-stimulant medications (OROS MPH and ATX, respectively) and (2) LDX and the other ADHD medication classes (MPH SA, MPH LA, AMPH SA, and AMPH LA).
A retrospective design was used to compare outcomes between the LDX treatment group and each of the other treatment groups. For each cohort, results will be presented for the comparison of (1) LDX and the most commonly prescribed stimulant and non-stimulant medications (OROS MPH and ATX, respectively) and (2) LDX and the other ADHD medication classes (MPH SA, MPH LA, AMPH SA, and AMPH LA).
Outcomes and statistical analyses
Baseline characteristics
Patient baseline characteristics were compared between LDX and all the other treatment groups (i.e., ATX, OROS MPH, MPH LA, MPH SA, AMPH LA, and AMPH SA) using Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables. The following baseline variables were reported: age, gender, prior ADHD related psychotherapies, Charlson Comorbidity Index (CCI) (as defined in Quan et al.Citation24), physical and mental co-morbidities, healthcare resource utilization (having ≥1 inpatient visit, ≥1 emergency room visit, or ≥1 outpatient visit), and year of index date (2007–2009). For previously treated cohorts, ADHD medication used during the baseline period was also reported. Physical and mental comorbidities were evaluated based on claims related with conditions listed in the Agency for Healthcare Research and Quality Comorbidity SoftwareCitation25 and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [DSM-IV]Citation26, respectively. Only physical and mental comorbidities that were significantly different across treatment groups and that had a prevalence of at least 5% in each treatment group were reported.
Comparisons of baseline characteristics between the LDX treatment group and each one of the other treatment groups individually were also reported as additional information in Tables 1–4 in Supplementary Appendix I.
Treatment persistence of index medication
For the persistence analysis, treatment discontinuation was defined as an interruption of the treatment initiated on the index date (discontinuation of the index medication) for at least 30 consecutive days, that is a gap of at least 30 calendar days between the end of one prescription and the start of the next prescription for the index medication or the end of the study period. The discontinuation date was defined as the last day of supply of the index medication before the treatment gap. Patients were observed over the period spanning from the index date up to the occurrence of treatment discontinuation or the end of the 12-month study period, whichever occurred first. Persistence was compared among treatment groups by comparing discontinuation rates between LDX and each of the other treatment groups using log-rank tests. In addition, in order to adjust for confounding factors, multivariate Cox proportional hazards models were used to compare treatment discontinuation across treatment groups with LDX as the reference group. Results were reported as hazard ratios (HRs) with their 95% confidence intervals and p-values.
Covariates (considered as potential confounding factors) included in the multivariate Cox proportional hazards models were baseline demographics (age and gender), CCI, class and number of ADHD medications used at baseline (for previously treated patients), baseline healthcare resource utilization, baseline mental co-morbidities, and other baseline characteristics that showed statistically significant differences between cohorts and with occurrence of at least 5% in each cohort (including mental and physical co-morbidities, prior ADHD-related psychotherapy, year and month of treatment initiation, and most common non-ADHD medication classes used during the baseline period).
Sensitivity analyses
Four sensitivity analyses were performed. The first sensitivity analysis defined treatment discontinuation as an interruption of the index treatment for at least 90 consecutive days, that is, a gap of at least 90 calendar days between the end of one prescription and the start of the next prescription for the index medication or the end of the study period. Cox proportional hazards models were used to compare time to treatment discontinuation between the LDX and each of the other treatment groups. Since some treatment guidelines mention that treatment discontinuation may be considered during the summer holidays, a second sensitivity analysis was performed among the children and adolescents populationCitation4,Citation22, where discontinuation was defined as an interruption of at least 30 consecutive days excluding any treatment discontinuation occurring during the summer holiday period, that is, from May 15 to September 15. In the sensitivity analysis, because a patient could initiate the treatment at different calendar months and thus at different points in time from the summer holiday, logistic regressions were used to compare discontinuation rates. A third sensitivity analysis was conducted where multivariate models were adjusted for all covariates that showed statistically significant differences between cohorts and with an occurrence of at least 2% in each treatment group. Finally, the fourth sensitivity analysis, the Bonferonni-Holm adjustment method, was applied to adjust for multiple comparisons.
Treatment patterns after discontinuation of the index medication
To better understand treatment patterns following discontinuation of the index medication, a descriptive analysis of the treatment patterns among patients who discontinued (defined as an interruption of the treatment for at least 30 consecutive days) the index medication was conducted for each treatment group, and the following patterns were reported: (1) proportion of patients who initiated another ADHD medication following the discontinuation of the index medication, (2) proportion of patients who resumed the index ADHD medication by the end of the 12-month study period, and (3) proportion of patients who did not fill any prescriptions for any ADHD medication following the index treatment discontinuation. Treatment patterns were analyzed from the date of discontinuation of the index medication date up to the end of the 12-month study period.
Results
Among the 480,712 patients in the sample who initiated an ADHD treatment on or after 2007, a total of 149,189 patients met all of the selection criteria ().
Baseline characteristics
Overall, except for their ADHD diagnosis, patients had a relatively good health profile (i.e., low CCI and low prevalence of chronic conditions) ( and ). In terms of demographic characteristics, statistically significant differences (p < 0.05) were observed among all age and treatment groups.
Table 1. Baseline demographic and clinical characteristics: children and adolescents.
Table 2. Baseline demographic and clinical characteristics: adults.
Children and adolescents
The children and adolescent population was comprised of 52,686 treatment-naïve and 48,930 previously treated patients (). The average age of patients across treatment groups ranged from 10.1–11.9 years (p < 0.0001) and from 10.9–12.1 years (p < 0.0001) in the treatment-naïve and previously treated cohorts, respectively (). Across treatment groups, most of the patients were males with a proportion ranging from 65.8–70.7% (p < 0.0001) and from 70.3–73.4%, (p < 0.0001) in the treatment-naïve and previously treated cohorts, respectively. The prevalence of physical comorbidities was generally low; the average CCI ranged between 0.061–0.075 (p = 0.0582) and between 0.062–0.067 (p = 0.9432) across treatment groups in treatment-naïve patients and previously treated patients, respectively. Of the treatment-naïve cohorts, from 13.4–17.6% of the patients across treatment groups had at least one ADHD-related visit prior to the index date, whereas in the previously treated cohort, the proportions were approximately twice as high (ranging from 24.6–31.7%). Depressive disorder was the only mental comorbidity for which the prevalence was significantly different in both cohorts in the children and adolescents population (p < 0.0001). The main differences between treatment groups were observed in prior treatment used in the previously treated cohort; LDX- and AMPH SA-treated patients were most commonly previously prescribed AMPH LA (39.3% and 71.2%, respectively); ATX-, MPH LA-, and AMPH LA-treated patients were most commonly prescribed OROS MPH (35.9%, 39.4%, and 39.3%, respectively); and OROS MPH- and MPH SA-treated patients were most commonly previously prescribed MPH LA (38.2% and 50.8%, respectively).
Adults
Among the adult population, a total of 32,937 and 20,737 patients were included in the treatment-naïve and previously treated cohorts, respectively (). The average age across treatment groups ranged from 31.5–35.2 years (p < 0.0001) in the treatment-naïve cohort and from 32.0–34.3 years (p < 0.0001) in the previously treated cohort (). In contrast to the population of children and adolescents, most of the treatment groups in the adult population had an ∼1:1 ratio of males-to-emales; the proportions ranged from 45.3–50.2% (p < 0.0001) in the treatment-naïve cohort and from 44.0–52.8% (p < 0.0001) in the previously treated cohort. The prevalence of comorbidities was relatively low, with an average CCI ranging between 0.103–0.172 (p < 0.0001) and between 0.113–0.143 (p = 0.0130) across treatment groups in treatment-naïve and previously treated cohorts, respectively. Similarly to the children and adolescents population, the proportion of adult patients with at least one ADHD-related psychotherapy visit prior to the index date was relatively low and ranged from 8.5–16.5% in the treatment-naïve cohort and from 25.0–36.5% in the previously treated cohort. Depressive disorder, anxiety disorder, and adjustment disorder were the only reported mental comorbidities that presented statistically significant differences in both cohorts (p < 0.05). Hypertension was also found to be significantly different across treatment groups in the previously-treated cohort (p < 0.0001).
Similarly to children and adolescents, the main differences between treatment groups were observed in prior treatment used in the previously treated cohort; most LDX- (53.1%), ATX- (44.2%), OROS MPH- (33.6%), and AMPH SA (73.8%)-treated patients had been previously treated with AMPH LA; MPH LA-treated patients (37.0%) with MPH SA; MPH SA-treated patients (29.8%) with MPH LA; and most AMPH LA-treated patients (48.5%) with AMPH SA.
Treatment persistence
Key findings of the main analysis are summarized in .
Table 3. Summary table: comparison of treatment persistence across all cohorts.
Children and adolescents
In the children and adolescents population, LDX prescribed patients were more persistent compared to patients in each of the other treatment groups (p < 0.05), except when compared to OROS MPH and ATX patients in the treatment-naïve cohort. The highest persistence rates were generally observed in treatment-naïve patients.
In the treatment-naïve cohort, LDX patients had a significantly higher discontinuation rate compared to OROS MPH patients (85% vs 82%, p < 0.0001) and a similar rate when compared to ATX patients (85% vs 85%, p = 0.7568) (). Similarly, after adjusting for confounding factors, LDX patients had an 8% higher risk (1/0.93 = 7.5%) of discontinuation compared to OROS MPH patients (HR = 0.93, [0.90; 0.96], p < 0.0001), and no statistically significant difference was found when compared to ATX patients (HR = 1.03, [0.99; 1.07], p = 0.2090) ().
Figure 2. Comparison of hazard ratios: base case analysis (30-day gap). 1 HR > 1 indicates that patients initiated on other ADHD medications had a high risk to discontinue the index treatment over the observation period when compared to LDX patients. AMPH, amphetamine/dextroamphetamine; AMPH LA, amphetamine/dextroamphetamine long-acting; AMPH SA, amphetamine/dextroamphetamine short-acting; ATX, atomoxetine; LDX, Lisdexamfetamine; MPH, methylphenidate/dexmethylphenidate; MPH LA, methylphenidate/dexmethylphenidate long-acting; MPH SA, methylphenidate/dexmethylphenidate short-acting; OROS MPH, osmotic release methylphenidate hydrochloride long-acting.
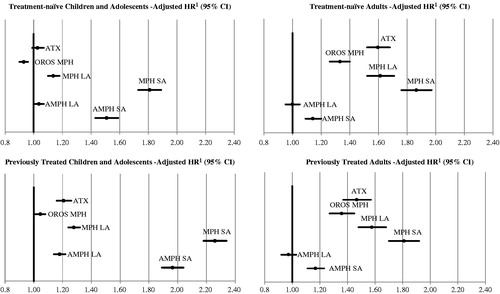
Table 4. Comparison of discontinuation rates: base case analysis (30-day gap).
Conversely, in the previously treated cohort, patients who were prescribed LDX had a significantly lower discontinuation rate compared to patients in the OROS MPH (74% vs 76%, p < 0.0001) and ATX treatment groups (74% vs 82%, p < 0.0001) (). After adjusting for confounding factors, compared to LDX patients, OROS MPH patients had a 5% higher risk of treatment discontinuation (HR = 1.05, [1.01; 1.08], p = 0.0128) and the risk of discontinuation was 21% higher in the ATX treatment group (HR = 1.21, [1.16; 1.26], p < 0.0001) ().
Overall, in the children and adolescents population, when compared to patients in each of the other treatment groups (MPH LA, MPH SA, AMPH LA, and AMPH SA), LDX patients had significantly lower discontinuation rates in both the treatment-naïve (85% vs range between 86–95%, all p < 0.0500) and the previously treated (74% vs range between 83–96%, all p < 0.0001) cohorts (). Consistently, after adjustment for confounders, LDX patients had a lower risk of discontinuation compared to patients in each of the other treatment groups (HRs ranged between 1.04–1.81 for treatment-naïve and between 1.05–2.26 for previously treated patients, all p < 0.0500).
In the sensitivity analysis where discontinuation was defined as a 90-day treatment gap, discontinuation rates were lower than those in the base case analysis, ranging from 50–82% across treatment groups (treatment naïve and previously treated patients) (). Results were consistent with those of the base case analysis except when the LDX treatment group was compared to the ATX and OROS MPH treatment groups in the treatment-naïve cohort, where the LDX treatment group had a statistically significant lower risk of discontinuation compared to ATX patients (HR = 1.25 [1.19; 1.31], p < 0.0001) and no statistically significant differences were found when compared to OROS MPH patients (HR = 0.98 [0.94; 1.02], p = 0.3200).
Table 5. Discontinuation rates: sensitivity analysis (90-day gap): children and adolescents.
For the second sensitivity analysis excluding treatment discontinuation occurring during the summer holiday period, results were consistent with those in the base case analysis, except for the treatment-naïve cohort, where patients in the ATX treatment group had a significantly higher risk of discontinuation compared to LDX patients (HR = 1.28 [1.17; 1.40], p < 0.0001) ().
Table 6. Comparison of discontinuation rates: sensitivity analysis without drug holiday (using a 30-day treatment gap definition): children and adolescents.
Results were also consistent with the base case in the sensitivity analysis where multivariate models were adjusted for all covariates that showed statistically significant differences between cohorts and with an occurrence of at least 2% in each treatment group (data not presented). Results were also consistent after Bonferroni-Holm adjustments for multiple comparisons with the exception of the comparison between the LDX and the AMPH LA treatment groups in the treatment-naïve cohort, where the difference was no longer significant (data not presented).
Adults
In the adult population, LDX patients were more persistent compared to patients in each of the other treatment groups, except when compared to AMPH LA patients where results from the multivariate regression analyses were not statistically significant.
In the treatment-naïve cohort, LDX patients had a significantly lower discontinuation rate compared to OROS MPH (76% vs 86%, p < 0.0001) and ATX patients (76% vs 90%, p < 0.0001) (). Similarly, compared to patients in the LDX treatment group, after adjusting for confounding factors, results showed a 33% (HR = 1.33, [1.26; 1.40], p < 0.0001) and a 60% higher risk of treatment discontinuation (HR = 1.60, [1.52; 1.68], p < 0.0001) for patients in the OROS MPH and ATX treatment groups, respectively ().
Consistently with the analysis on treatment-naïve adult patients, among previously treated adult patients, LDX patients also had a significantly lower discontinuation rate compared to patients in the OROS MPH (76% vs 85%, p < 0.0001) and ATX treatment groups (76% vs 89%, p < 0.0001) (). After adjusting for confounders, compared to LDX patients, the OROS MPH and ATX treatment groups had a 36% (HR = 1.36 [1.27; 1.45], p < 0.0001) and a 47% (HR = 1.47 [1.37; 1.57], p < 0.0001) higher risk of discontinuation of the index treatment, respectively ().
Overall, in the adult population, patients in each of the other treatment groups (MPH LA, MPH SA, AMPH LA, and AMPH SA) also had a significantly higher discontinuation rate compared to LDX patients for both the treatment-naïve (76% vs range between 79–91%, all p < 0.0001) and the previously treated cohorts (76% vs range between 78–93%, all p < 0.05), except for the AMPH LA treatment group in the treatment-naïve cohort, where the difference was not statistically significant (). After adjusting for confounding factors, compared to LDX patients, the risk of discontinuation remained significantly higher in each of the other treatment groups across both treatment-naïve and previously treated groups (HR ranged from 1.14–1.86, all p < 0.0001), except for AMPH LA patients where the difference was not statistically significant ().
In the sensitivity analysis where discontinuation was defined as a 90-day gap, the discontinuation rates were lower than those in the base case analysis, ranging from 55% (LDX treatment naïve) to 79% (ATX treatment-naïve and MPH SA previously treated) across treatment groups (). Results were consistent with the base case analysis, except for AMPH SA patients in the treatment-naïve cohort, where the difference was no longer statistically significant.
Table 7. Comparison of discontinuation rates: sensitivity analysis (90-day gap): adults.
Consistent results were also observed in the sensitivity analysis where multivariate models were adjusted for all covariates that showed statistically significant differences between cohorts and with an occurrence of at least 2% in each treatment group (data not presented).
Finally, results were also consistent after Bonferroni-Holm adjustments for multiple comparisons (data not presented).
Treatment patterns after discontinuation of the index medication
Among patients who discontinued their index medication, the majority resumed the index treatment or initiated another ADHD medication before the end of the 12-month study period. A small proportion of patients remained untreated following the discontinuation of the index medication.
Children and adolescents
Among children and adolescents who discontinued their index medication, between 67–85% of treatment-naïve patients initiated another ADHD medication before the end of the 12-month study period. In addition, between 79–95% of previously treated patients across treatment groups resumed their index medication by the end of the study period. Finally, between 5–33% of the patients did not fill a prescription for any ADHD medication after discontinuation of the index treatment ().
Table 8. Treatment patterns among children and adolescents who dscontinued the ADHD treatment initiated at the index date: over the 12-month period following the index date.
Adults
Similarly, in the adult population, most of the patients either switched/initiated another ADHD therapy or resumed the index treatment at some point before the end of the 12-month study period. More specifically, between 60–78% of treatment-naïve patients initiated another ADHD medication before the end of the study period. Between 73–90% of previously treated patients resumed treatment by the end of the study period. Across treatment groups, between 10–40% of the patients did not fill a prescription for any ADHD medication after discontinuation of the index treatment ().
Table 9. Treatment patterns among adults who discontinued the ADHD treatment initiated at the index date: over the 12-month period following the index date.
Discussion
For many patients, ADHD is a lifetime disorder, which, if untreated or sub-optimally treated, can cause significant long-term impairment in various aspects of an individual’s lifeCitation4. Pharmacologic treatment is one of the most effective options to reduce and control ADHD symptoms and impairmentsCitation5–8. However, these symptoms and impairments may resurface and interfere with social and behavioral development when treatment discontinuation occursCitation9,Citation11.
Findings from our study suggest that persistence on ADHD index medication in the 12 months following treatment initiation was consistently low among all treatment groups and age populations, regardless of the previous treatment status of the patients. The average discontinuation rates ranged between 74–96% across the different cohorts studied. However, only a small proportion of patients remained untreated following discontinuation of the index medication, with the lowest proportion generally observed among previously treated patients; the majority of patients (60–95% across the different cohorts) who discontinued the index treatment, resumed it, or initiated another ADHD medication at some point before the end of the 12-month study period.
Comparison of treatment persistence across most commonly prescribed medications showed that LDX patients were less likely to discontinue the index treatment when compared to patients initiated on other ADHD medications, except for in the following comparisons: (1) when compared to AMPH LA patients in the adult population, where no significant differences were found, (2) when compared to treatment-naïve children and adolescents on ATX where no statistically significant differences were found, and (3) when compared to OROS MPH treatment-naïve children and adolescents where LDX patients had a higher risk of discontinuation. The robustness of the results was confirmed by the overall convergence of the results between the base case analysis and the sensitivity analyses; results from the sensitivity analyses using a 90-day treatment gap definition, controlling for different baseline characteristics (using 2% threshold), excluding treatment discontinuation during summer holidays for children and adolescents, and applying Bonferroni-Holm adjustments were generally consistent with the base case analysis.
Although some studies have shown that the persistence and adherence profiles were better in ADHD patients treated with extended-release and stimulant medicationsCitation16,Citation27, to the best of our knowledge, no study has yet compared treatment persistence between the most commonly prescribed ADHD medications. However, studies reporting persistence rates also showed high discontinuation rates of ADHD treatments. In a study by Van den Ban et al.Citation20, it was found that ∼ 53% of the population (<45 years of age) treated in 2004–2005 discontinued (treatment gap ≥90 days) any ADHD medications within 1 year after treatment initiation. In another study conducted in patients younger than 20 years of age from a Medicaid population, only 26.9% of patients received ADHD treatment for at least 1 year, when continuous ADHD drug exposure was assumed as long as every follow-up month had at least one active ADHD drug claim, regardless of whether patients continued the index drug or switched to another ADHD treatmentCitation28. Other studies have also shown short persistence (from 95.4 days to 252.7 days) with various ADHD medication treatmentsCitation15,Citation16. Compared to the literature, the discontinuation rates found in our analysis appear to be slightly higher. However, this could be mainly explained by the differences in the definition of treatment discontinuation used; while treatment discontinuation was defined in the Van den Ban et al.Citation20 study as not having a refill for any ADHD prescription within 90 days following the end date of a previous prescription, our study used a 30-day treatment gap definition. Moreover, in contrast to the Van den Ban et al.Citation20 study and Winterstein et al.Citation28 studies, where the authors assessed treatment adherence to any ADHD treatment, our study assessed patients’ adherence to the index treatment. Accordingly, the discontinuation rates in our study include a significant proportion of patients who discontinued their index medication and switched to another ADHD medication. Nonetheless, results from both our study and from the literature show sub-optimal ADHD treatment persistence.
Patients may discontinue their medication for different reasons. Most common reasons reported in the literature include other treatment options, side-effects, non-responsiveness to treatment, and patients’ or parents’ preferences/beliefsCitation12,Citation18–20. Loss of follow-up, miscommunication to the patient of the benefits of treatment, prescribed treatment breaks to assess disease evolution or to treat an important comorbidity, and perception that medication is not working properly to manage symptoms or is no longer needed might all be other reasons for treatment discontinuationCitation12,Citation18–20. However, further studies would be warranted to confirm the main reasons for treatment discontinuation in ADHD patients and to better understand the potential avenues to improve long-term persistence in ADHD patients.
The limitations of the study include the usual caveats of retrospective claims data. First, the severity of ADHD symptoms as well as the ADHD sub-type may greatly vary across individuals, and this may affect patients’ treatment persistence. Claims databases only record procedural and diagnostic codes and do not include disease severity measurement, reason for treatment discontinuation, and other factors that may affect treatment persistence, including lifestyle measures, education, and physician prescribing behavior. Accordingly, our models could not be adjusted for these potential factors that are likely to affect treatment persistence. Second, although multivariate regression models were used to adjust for observable differences of baseline characteristics, an unobserved confounding effect may still exist such that the difference in treatment persistence between the treatment groups may be due to unobserved differences in disease profile. Third, treatment persistence was analyzed using claims that represent prescription fills, which does not guarantee the actual consumption of the medication by the patient. Fourth, the study was limited to privately insured ADHD patients in the US who had continuous health insurance coverage for the observation period of the study. Accordingly, the generalizability of our findings is limited to US populations of ADHD patients with commercial health insurance coverage and might be less generalizable to, for example, an ADHD Medicaid population, that is a lower socioeconomic status populationCitation29 with more prevalent mental disordersCitation30–33. Finally, retrospective databases are also subject to coding errors or data omissions. However, these are expected to affect all treatment cohorts to a similar extent and are unlikely to alter conclusions. Despite these limitations, retrospective claims data remain a valuable source of information, as they consist of a valid and large sample and have the advantage of reflecting patients’ behavior in a real world setting.
To the best of our knowledge, the present study is the first to compare treatment discontinuation of the index medication across the most commonly prescribed stimulant and non-stimulant medications among treatment-naïve and previously treated children and adults. Findings from this study provide a complete picture of treatment persistence among patients with ADHD in a real-world setting. Additional analyses are needed to determine potential factors (including psychological, educational, or social interventions) impacting treatment persistence in patients with ADHD and to compare direct and indirect costs of persistent vs non-persistent ADHD patients.
Conclusions
This study examined drug-specific persistence for both treatment-naïve and previously treated patients in a full spectrum of patients with ADHD, stratified by age (6–17 years old and adults) and treatment group (treatment-naïve and previously treated). The proportion of patients who discontinued their index medication during the first year following the initiation of the treatment was relatively high across all treatment groups. LDX prescribed patients were associated with a better persistence profile compared to patients initiated on each frequently prescribed ADHD medication, except for the comparisons with OROS MPH and ATX patients in treatment-naïve children and adolescents and with AMPH LA patients in adults.
Transparency
Declaration of funding
This analysis was supported by Shire Development, LLC. Shire develops and markets psychiatric ADHD medications including those for the treatment of ADHD.
Declaration of financial/other relationships
Juliana Setyawan, Paul Hodgkins, and M. Haim Erder are employees of Shire Development, LLC and own stock/stock options. Annie Guérin, Geneviève Gauthier, Martin Cloutier, and Eric Q. Wu are employees of Analysis Group Inc., which has received consultancy fees from Shire Development, LLC for this project. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Supplementary material available online Supplementary Appendix Tables 1--5
Supplementary Material
Download PDF (46 KB)Acknowledgments
Editorial assistance in formatting, proofreading, copy editing, and fact checking was provided by Caudex Medical Ltd, funded by Shire AG, Switzerland. Amina Elsner from Shire AG, Switzerland also reviewed and edited the manuscript for scientific accuracy. Although Shire was involved in the design, collection, analysis, interpretation, and fact checking of information, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Journal of Medical Economics was made by the authors independently.
References
- Verma R, Balhara YP, Mathur S. Management of attention-deficit hyperactivity disorder. J Pediatr Neurosci 2011;6:13-18
- Danckaerts M, Sonuga-Barke EJ, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry 2010;19:83-105
- Geissler J, Lesch KP. A lifetime of attention-deficit/hyperactivity disorder: diagnostic challenges, treatment and neurobiological mechanisms. Expert Rev Neurother 2011;11:1467-84
- National Institute for Health and Clinical Excellence. Attention deficit hyperactivity disorder – Diagnosis and management of ADHD in children, young people and adults – NICE clinical guideline 72. 2009. Available at: http://www.nice.org.uk/nicemedia/live/12061/42059/42059.pdf
- MTA Cooperative Group. Multimodal treatment study of children with ADHD. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 1999;56:1073-86
- Spencer, TJ. ADHD treatment across the life cycle. J Clin Psychiatry 2004;65:22-6
- Group Health. Attention Deficit Hyperactivity Disorder (ADHD): Adults – Diagnosis and Treatment Guideline. 2011. Available at: https://www.ghc.org/all-sites/guidelines/adhd-adult.pdf
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in ADHD: effects of treatment and non-treatment. BMC Med 2012;10:99
- Cascade E, Kalali AH, Weisler RH, et al. Seasonality and the changing adult/child prescription ratios in ADHD Therapy. Psychiatry (Edgmont) 2008;5:23-5
- Canadian ADHD Practice Guidelines (CAP Guidelines). 3rd edn. 2010. Available at: http://www.caddra.ca/cms4/pdfs/caddraGuidelines2011.pdf
- Murray-Close D, Hoza B, Hinshaw S, et al. Developmental processes in peer problems of children with ADHD in the MTA Study: developmental cascades and vicious cycles. Dev Psychopathol 2010;22:785-802
- McCarthy S, Asherson P, Coghill D, et al. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry 2009;194:273-7
- Sobanski E, Sabljic D, Alm B, et al. Driving performance in adults with ADHD: results from a randomized, waiting list controlled trial with atomoxetine. Eur Psychiatry 2013;28:379-85.
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry 2006;15:105-25
- Barner J, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin 2011;27(2 Suppl):13-22
- Christensen L, Sasané R, Hodgkins P, et al. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin 2010;26:977-89
- Wong IC, Asherson P, Bilbow A, et al. Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY)—a pharmacoepidemiological and qualitative study. Health Technol Assess 2009;13:iii-iv, ix–xi, 1–120
- Perring C. Medicating children: the case of ritalin. Bioethics 1997;11:228-40
- Brinkman W, Epstein J. Treatment planning for children with attention-deficit/hyperactivity disorder: treatment utilization and family preferences. Patient Prefer Adherence 2011;5:45-56
- Van den Ban E, Souverein PC, Hanna Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. ADHD Atten Def Hyp Disord 2010;2:213-20
- van de Loo-Neus G, Rommelse N, Buitelaar J. To stop or not to stop? How long should medication treatment of attention-deficit hyperactivity disorder be extended? Eur Neuropsychopharmacol 2011;21:584-99
- Kaplan G, Newcorn J. Pharmacotherapy for child and adolescent attention-deficit hyperactivity disorder. Pediatr Clin N Am 2011;58:99-120
- Setyawan J, Hodgkins P, Guerin A, et al. Comparing treatment adherence of lisdexamfetamine and other medications for the treatment of attention deficit/hyperactivity disorder: a retrospective analysis: a retrospective analysis J Med Econ 2013;16:962--75
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Elixhauser A, Steiner C, Kruzikas D. Comorbidity software documentation. HCUP Methods Series Report # 2004-1. ONLINE February 6, 2004. US Agency for Healthcare Research and Quality; 2004. http://www.hcupus.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. [Accessed 12 Sep 2013]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edition [DSM-IV]. APA; 1994. Washington, doi:10.1176/appi.books.9780890423349.7060
- Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med 2005;159:572-8
- Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother 2008;42:24-31
- Centers for Medicare & Medicaid Services; Medicaid by Population. Medicaid website. http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Population/By-Population.html. Accessed March 2013
- Bones RK, Pérez K, Rodríguez-Sanz M, et al. Prevalence of mental health problems and their association with socioeconomic, work and health variables: findings from the Spain National Health Survey. Psicothema 2010;22:389-95
- Davis E, Sawyer MG, Lo SK, et al. Socioeconomic risk factors for mental health problems in 4-5-year-old children: Australian population study. Acad Pediatr 2010;10:41-7
- Hudson CG. Socioeconomic status and mental illness: tests of the social causation and selection hypotheses. Am J Orthopsychiatry 2005;75:3-18
- Ibrahim AK, Kelly SJ, Glazebrook C. Socioeconomic status and the risk of depression among UK higher education students. Soc Psychiatry Psychiatr Epidemiol 2013;48:1491-501