Abstract
Background:
Systemic Candida infections (SCI) occur predominantly in intensive care unit patients and are a common cause of morbidity and mortality. Recently, changes in Candida epidemiology with an increasing prevalence of SCI caused by Candida non-albicans species have been reported. Resistance to fluconazole and azoles in general is not uncommon for non-albicans species. Despite guidelines recommending initial treatment with broad-spectrum antifungals such as echinocandins with subsequent switch to fluconazole if isolates are sensitive (de-escalation strategy), fluconazole is still the preferred first-line antifungal (escalation) in many clinical practice settings. After diagnosis of the pathogen, the initial therapy with fluconazole is switched to a broad-spectrum antifungal if a non-albicans is identified.
Methods:
The cost-effectiveness of initial treatment with micafungin (de-escalation) vs fluconazole (escalation) in patients with SCI was estimated using decision analysis based on clinical and microbiological data from pertinent studies. The model horizon was 42 days, and was extrapolated to cover a lifetime horizon. All costs were analyzed from the UK NHS perspective. Several assumptions were taken to address uncertainties; the limitations of these assumptions are discussed in the article.
Results:
In patients with fluconazole-resistant isolates, initial treatment with micafungin avoids 30% more deaths and successfully treats 23% more patients than initial treatment with fluconazole, with cost savings of £1621 per treated patient. In the overall SCI population, de-escalation results in 1.2% fewer deaths at a marginal cost of £740 per patient. Over a lifetime horizon, the incremental cost-effectiveness of de-escalation vs escalation was £15,522 per life-year and £25,673 per QALY.
Conclusions:
De-escalation from micafungin may improve clinical outcomes and overall survival, particularly among patients with fluconazole-resistant Candida strains. De-escalation from initial treatment with micafungin is a cost-effective alternative to escalation from a UK NHS perspective, with a differential cost per QALY below the ‘willingness-to-pay’ threshold of £30,000.
Introduction
Systemic Candida infections (SCI) frequently occur as a complication of underlying diseases and treatments which severely impair the immune system of patients and make them vulnerable to opportunistic and nosocomial infections. Candida is one of the 10 leading causes of bloodstream infections in developed countriesCitation1. In the UK, the incidence of SCI ranges between 3.05 per 100,000 population in England, Wales and Northern IrelandCitation2 and 4.8 per 100,000 habitants in ScotlandCitation3. SCI predominantly affects intensive care unit (ICU) patients. In this patient group, SCI is a common cause of morbidity and mortalityCitation4 and is associated with prolonged hospitalization and substantial care-related costsCitation5,Citation6.
Given the high mortality of SCI, its prevention in patients at high risk of infection is recommended by most guidelinesCitation7–9. Fluconazole has become the most frequently used anti-fungal in the prophylaxis of SCI. Its widespread use has reduced the incidence of invasive infections caused by Candida albicans, but this has coincided with an increase in those caused by Candida non-albicans, particularly C. glabrata and C. kruseiCitation10,Citation11, two species frequently resistant to fluconazoleCitation12,Citation13. Fluconazole-resistant non-albicans infections tend to be associated with higher mortalityCitation14 and are often perceived as a challenge to treatCitation12.
The changes in Candida profile have prompted the introduction of international and national guidelinesCitation7–9 which recommend an echinocandin for the initial management of invasive candidiasis. When the susceptibility results become available, the echinocandin should be replaced by fluconazole if the pathogen is susceptible to this anti-fungal; otherwise the patient is maintained on the echinocandin. This is known as a ‘de-escalation strategy’, and aims to provide adequate treatment to all patients whilst reducing the development of anti-fungal resistanceCitation15.
Fluconazole, however, is still the preferred first-line agent for SCI in many clinical practice settingsCitation16 because of its efficacy against most Candida speciesCitation17–19 and its low acquisition costs. This applies particularly to non-neutropenic patients. Fluconazole is replaced by a broader spectrum anti-fungal if the infecting strain is found to be fluconazole-resistant (escalation approach), but this risks that a patient may receive inappropriate treatment for a number of days before susceptibility results are available.
Prompt treatment initiation is indeed critical in SCI. A delay in the initiation of appropriate treatment is associated with increased mortality, prolonged hospitalization, and substantial related costsCitation20–23. Overall mortality increases from 15.4% to 41.4% when therapy is delayed from Day 1 to Day 3 of blood samplingCitation20. Similarly, delayed appropriate treatment initiation has been reported to prolong hospitalization by an average of 7.7 additional daysCitation6. However, identification of the optimal time point to commence anti-fungal therapy remains challenging and varies across hospitals. Given the difficulty with differential clinical diagnosis between albicans and non-albicans infections, prompt initiation of treatment with a broad spectrum anti-fungal which would cover both pathogen groups may be critical to ensure a beneficial outcome for the patientCitation22,Citation24.
Reboli et al.Citation25 observed high minimal inhibitory concentration (MIC) values for C. parapsilosis against the echinocandin anidulafungin. However, high MIC values for anidulafungin did not result in significantly lower successful microbiological or global response when compared to fluconazoleCitation25. Although the clinical relevance of this finding is not yet fully elucidated, a recent meta-analysisCitation26 on the comparative efficacy of echinochandins to non-echinochandins for the treatment of C. parapsilosis infections has failed to show any differences between echinochandins and non-echinocandins in the treatment of C. parapsilosis-related SCI.
The increasing proportion of SCI caused by fluconazole-resistant isolates, the recommendations of current guidelines and the difficulty in differentiating non-albicans from albicans infections on the basis of the clinical manifestations, highlight the need for the use of a broader spectrum anti-fungal as first-line treatment of SCI. The cost-effectiveness of de-escalation vs escalation therapeutic approach in SCI has not yet been evaluated. The main objective of this study was to assess the cost-effectiveness of these two approaches in ICU non-neutropenic patients with SCI from the United Kingdom National Health Service (UK NHS) perspective and using micafungin as the investigatory echinocandin.
Patients and methods
Model structure
The cost-effectiveness of de-escalation compared to the traditional escalation strategy was estimated using a decision analytic model. The health economic model was based on primary clinical and microbiological end-points from pertinent published studies and the standard duration of treatment in clinical practice in the UKCitation27. The model did not need ethical approval. The model assumed that treatment was initiated in all patients as soon as the laboratory tests were taken and that Candida identification takes place within 24 h of clinical suspicion and susceptibility tests are made available within 3 days of treatment. Three days were chosen based on average time for susceptibility tests to be conducted in the UKCitation28. In order to simplify the model, it was assumed that systemic Candida was identified for all patients. The model also assumes that all patients are non-neutropenic.
Again, for simplicity in the model, all patients were treated either with micafungin (100 mg/day; de-escalation strategy) or fluconazole (400 mg/day; escalation strategy) until the susceptibility results were available. At that time (end of the initial 3 day treatment period), patients were either alive or dead. Patients alive in the de-escalating strategy were switched to fluconazole if the isolated Candida spp. was sensitive to this anti-fungal, or remained on micafungin if it was fluconazole-resistant (MIC values of ≥64 µg/mlCitation29).
In the escalation approach, patients alive remained on fluconazole if the isolate was fluconazole-sensitive and switched to micafungin if it was fluconazole-resistant. If the susceptibility results revealed that Candida isolates were fluconazole dose-dependent (MIC values of 16 or 32 µg/mlCitation29), then the patient was treated with fluconazole 800 mg/day irrespective of treatment strategy (). In line with its label, the dose of fluconazole on day 1 was taken to be 800 mg for all patients in the escalation approach and 400 mg thereafterCitation30. No loading dose is required for micafungin; this anti-fungal was therefore assumed to be dosed as 100 mg/day from beginning of treatmentCitation30 Costs for fluconazole and micafungin were those published in the British National Formulary (BNF)-59Citation30 (BNF-59)Citation31.
Figure 1. Schematic diagram of escalation and de-escalation strategies in patients with systemic Candida infections. In the escalation strategy all patients initiate treatment with fluconazole (FLU) 400 mg (with 800 mg loading dose). When susceptibility results are available on day 3, patients switch to micafungin (MYC) if Candida is fluconazole-resistant, have the fluconazole dose increased to 800 mg if the Candida is dose-dependent (S-DD), or remain on the same dose of fluconazole if the Candida isolates are sensitive to this anti-fungal. In the de-escalation strategy all patients initiate treatment with micafungin and switch to fluconazole 400 mg if Candida is susceptible or to fluconazole 800 mg if the Candida is dose-dependent. In our model we consider that treatment is initiated as soon as the laboratory tests are taken.
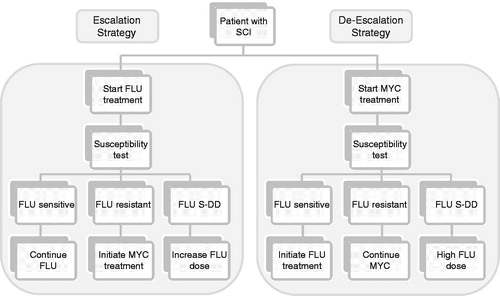
The overall treatment duration for patients alive was 14 days with appropriate treatmentCitation27. Once susceptibility results were available (i.e., day 3), patients were treated for an additional 11 days if they had received appropriate initial treatment or for 14 days if they had not. Treatment success or failure was assessed at the end of the second treatment period (day 14 or 17). Success was defined as clearance of Candida from the bloodstream and resolution of symptoms attributable to candidaemia. If the outcome was failure, then patients were either switched to micafungin 100 mg/day if they were on fluconazole, or as recommended in the label, to micafungin 200 mg/day if they were already on micafungin (100 mg/day).
The outcomes considered in the model encompassed cost per successfully treated patient and cost per patient alive over a horizon of 42 days. Forty-two days post-initiation of treatment was chosen as the horizon since this was the time point when mortality was assessed in the phase III micafungin studiesCitation32–35. The model was extrapolated to cover a lifetime horizon using the average life expectancy for an ICU survivor and the corresponding utilities. The outcomes were given as incremental cost per life year and incremental cost per QALY.
Model population and parameters
The clinical and microbiological parameters used in the model included early (after 3 days of treatment) and late (at end of second treatment period) mortality, and clinical success at the end of the second treatment period (at Day 14 or 17). The parameters used in the analysis as well as the sources used are provided in and .
Table 1. Clinical and treatment-related model input parameters.
Table 2. Candida epidemiology and fluconazole-susceptibility in the base case and scenarios.
Early mortality data was obtained solely from the study of Slavin et al.Citation36, where the authors provide mortality at day 5 separately for patients treated with appropriate or inappropriate treatment. Accordingly, the early mortality for patients initially treated inappropriately was 14.49% in our model, and 9.84% for all other patients. For patients with fluconazole-sensitive isolates, late mortality was 21.26% after subtracting the early mortality rateCitation36. For all other patients, late mortality was 50.85%Citation36 in the escalation strategy and 19.85% (i.e., weighted average mortality of patients treated with micafungin in Kubiak et al.Citation34 and the two large international phase III micafungin studiesCitation32,Citation33).
The clinical success rates considered in the model for patients with fluconazole-sensitive or dose-dependent isolates were the weighted average values of these two parameters in four studies conducted with fluconazoleCitation17–19,Citation37 (i.e., 65.57%), while those for patients with fluconazole-resistant isolates were the weighted average values observed in the micafungin studiesCitation32–34,Citation38 (i.e., 76.91%).
Candida epidemiology data for the UK were obtained from the Health Protection Agency (HPA) and corresponded to the most recent available dataCitation39. The Candida profile was that published for England; Wales and Northern Ireland in 2008. Fluconazole-susceptibility data corresponded to the weighted average data for 2006–2007 in the UKCitation40. As seen in , in the UK ∼50% (51.74%) of Candida isolates from blood cultures were albicans. The second most common isolate was C. glabrata (20.32%) followed by C. parapsilosis (10.71%). This Candida profile remained fairly similar between 2005–2008 and did not differ from that reported for ScotlandCitation3. In line with the published literature for other developed countries, fluconazole-resistance in the UK affects primarily non-albicans species (7.72% vs 0.57% of albicans). The two species with the highest fluconazole-resistance rates in the UK were C. glabrata and C. krusei with 11.56% and 23.08% of fluconazole-resistant strains, respectively. In addition, 10.9% of all strains were dose-dependent.
Health resource utilization
Health resource consumption by patients with SCI was used to calculate the cost per health state. The data considered in the model covered first and second-line anti-fungals (fluconazole, micafungin), excess hospitalization due to inappropriate treatment, and average direct medical costs for lifetime follow-up of SCI survivors. The economic analysis was performed from a UK NHS perspective, and all costs considered in the model were for 2009 ().
Table 3. Costs, health resource utilization, and utilities used in the model.
The costs for day spent in ICU or in a general ward were obtained from the NHS reference costs for 2008–2009. The ICU daily costs were set as £1149 based on costs reported for the NHS Trusts Critical Care ServicesCitation41 and the general ward costs per day as £463.08. Only excess hospitalization incurred by patients with inappropriate treatment rather than the actual overall length of hospitalization per patient was considered. The excess hospitalization data included in the model is based on the study of Zilberberg et al.Citation6. The authors document an excess hospitalization in a general ward of 7.7 days for patients with initial inappropriate treatment after adjustment for all confounders. No excess ICU stay was observed for these patients. No additional costs were considered in the model.
Life expectancy, risk of death, and QALY adjustment
The mean age and underlying diseases of the model population were derived from the two large international micafungin studiesCitation32,Citation33. The base case patient was 54.7 years old and had primarily cancer (47.0%), diabetes (9.6%), or a gastro-intestinal disease (8.6%)Citation32,Citation33. The probability of having any other underlying disease was less than 5% for any disease. The average life expectancy of the base case patient was that derived using the DEALE methodCitation42 from the study of Cuthbertson et al.Citation43, i.e., 42% survival at 5 years, which corresponds to a life expectancy of 7.1 years. For the one-way sensitivity analysis, the weighted average life expectancy ranged between 3.95Citation43 and 17.5 years. The latter was calculated taking into consideration the proportion of patients with the different underlying diseasesCitation32,Citation33 and the life expectancy reported for each disease.
The model included two health state utilities. The first utility weight (0.5) was applied to all patients during the period of treatment for SCI. This utility has been reported for acute severe sepsis without treatment complicationsCitation44. The second utility (0.605) was applied to all SCI survivors and was the weighted average utility for the main underlying diseases (cancerCitation45, diabetesCitation46, gastrointestinal disordersCitation47) reported in the micafungin studiesCitation32,Citation33.
Very scant information is available regarding long-term costs of SCI survivors. Given that ∼50% of patients with SCI in our model were oncology patients, we took a conservative approach whereby we considered the annual healthcare costs per survivor in the base case scenario to be £5716, i.e., the estimated average annual costs for cancer patientsCitation48–51. In the one-way sensitivity analysis, the follow-up costs were varied from £1335Citation48 to £10,420Citation51. All projected costs and effects were discounted at 3.5% per year (0–5% in the sensitivity analysis).
Sensitivity analyses and special scenarios
Several one-way sensitivity analyses were performed to test the strength of the conclusions of the analysis. The parameters included in the sensitivity analyses were: Candida epidemiology and fluconazole-susceptibility profile, hospitalization duration and costs, mortality rates, clinical success outcomes, life expectancy, weight utilities, and annual follow-up costs ().
In addition to the one-way sensitivity analysis, the impact of the two main underlying diseases (cancer and diabetes) in the differential costs between de-escalation and escalation was evaluated in three scenarios. In the diabetes scenario all patients were assumed to have diabetes type 2. The utility weight for these patients was 0.81Citation4Citation6 and the life expectancy 26.55 years based on the UK Prospective Diabetes Study outcomesCitation52. The annual follow-up costs for these patients were the costs from the CODEIRE study inflated to 2009 costs (i.e., £2404)Citation53.
In the cancer scenario all patients had cancer and life expectancy was based on the 5-year survival rates published by the Office of National Statistics for the 21 most common cancers in the UKCitation54. The derived life expectancy (6.32 years) corresponded to survival for patients of 50–59 years of age who had been diagnosed with cancer between 2003–2007 and followed-up until 2008. The utility weight for cancer was 0.62 and corresponded to that reported for cancer patients on chemotherapyCitation45. A third scenario (worst case) was modeled where all patients had advanced cancer. Life expectancy was 1.38 years based on the mean survival reported for patients with advanced breastCitation55, prostateCitation56, or colorectal cancerCitation57. The utility weight for these patients was 0.52Citation5Citation8 and the follow-up costs £29,340, i.e., the average cost for the most expensive chemotherapeutic drugs.
Results
Based on our model, in the UK the escalation strategy with fluconazole infers that nearly 15% of patients receive an inappropriate first-line treatment because either a higher dose is required; this is the case for dose-dependent isolates (10.9% of all patients), or because an azole is ineffective (fluconazole-resistant isolates in 3.9% of patients). For inappropriately-treated patients, particularly those with fluconazole-resistant Candida, the de-escalation approach is associated with better clinical outcomes ().
Figure 2. Incremental effects and costs of de-escalation according to the fluconazole-susceptibility profile. The incremental proportion of successfully-treated patients and of patients alive in de-escalation strategy compared with an escalation approach are provided in (a) and (b), respectively, for patients with fluconazole-sensitive, dose dependent (S-DD), and resistant isolates. The incremental costs per treated patient associated with de-escalation are provided in (c).
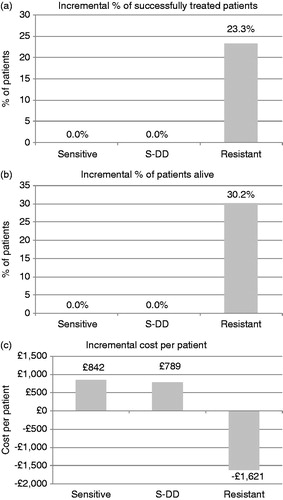
In patients with fluconazole-resistant isolates, the de-escalation strategy results in 23.25% more patients successfully treated and 30.23% less deaths than initial treatment with fluconazole. This corresponds to a relative improvement of 72% in the rate of both clinical success and survival over the escalation strategy. The clinical advantage of the de-escalation strategy is cost saving from the NHS perspective. Over a timeframe of 42 days, the de-escalation strategy saves the NHS £1621 per treated patient with fluconazole-resistant isolates. All costs incurred in the de-escalation strategy correspond to medication only (£2747), whereas, in the escalation, these correspond to medication (£1319) and excess hospitalization (£3049). In the latter strategy, costs are driven primarily by hospitalization. It should be noted that the model assessed only excess costs associated with inappropriate treatment and it did not incorporate any healthcare costs common to both strategies, such as Candida diagnosis, susceptibility tests, or duration of stay in ICU.
For patients with dose-dependent or fluconazole-sensitive isolates the de-escalation advantage in terms of clinical success and survival is less pronounced than for patients with fluconazole-resistant episodes (). For the overall cohort, irrespective of Candida strain and fluconazole-susceptibility profile, de-escalation translates into 0.90% more patients successfully treated, i.e., a relative improvement by 2.0%. It also prevents 1.17% more deaths (relative improvement of 1.7% in survival) at an excess cost of £740 per patient treated. All costs in the de-escalation strategy are due to medication (£2878 per treated patient), while in the escalation strategy they correspond to medication (£2019) and excess hospitalization (£118 per treated patient).
Table 4. Cost-effectiveness of de-escalation and escalation over a horizon of 42 days and lifetime and with clinical success as outcome.
Over a lifetime horizon, de-escalation yields 0.08 more life years and 0.05 more QALYs than the escalation strategy. The overall life years and QALY gained in the de-escalation strategy are 4.66 and 2.81, respectively, and in the escalation strategy 4.59 and 2.76. Overall, de-escalation costs £1170 more per treated patient than escalation. Compared to escalation and with a lifetime horizon, initial treatment with micafungin costs £15,522 more per life year and £25,673 more per QALY; the latter falling within the ICER threshold (£20,000–£30,000) recommended by NICECitation59.
Sensitivity analyses and special scenarios
The univariate analyses performed with the different input parameters indicate that the incremental costs per QALY of de-escalation are highly sensitive to the fluconazole-susceptibility profile, late mortality in fluconazole-resistant Candida infections, the clinical success rate of fluconazole in sensitive isolates, excess hospitalization either in a general ward or in ICU, life expectancy, the underlying diseases, and the follow-up costs (). The incremental costs are insensitive to the Candida epidemiology, the early excess mortality in patients inappropriately treated, clinical success rate of fluconazole in dose-dependent isolates or of micafungin in all patients, hospitalization costs, utility weight during SCI, or discounting.
Figure 3. One-way sensitivity analyses. Tornado diagram. The solid vertical line represents the base case total incremental lifetime costs per QALY for survivors treated in the de-escalation approach (£25,673). The horizontal bars represent the range of the incremental costs when the corresponding single input is varied across its designated range with all other input parameters held constant. DE-ESC, de-escalation approach; ESC, escalation approach; FLU, fluconazole; GW, general ward; ICU, intensive care unit; MYC, micafungin; SCI, systemic Candida infection; RES C, Fluconazole-resistant Candida; S-DD, dose-dependent susceptibility; SENS C, Fluconazole-sensitive Candida.

In the univariate sensitivity analysis, increasing the proportion of patients with fluconazole-resistant isolates decreases the incremental cost per QALY gained. The ICER ranges between £22,656 (5.17% of isolates being resistant) and £39,143 (1.98% of all isolates). When the late mortality rate for patients with fluconazole-resistant isolates in the escalation strategy is increased to 63%, the ICER decreases (£21,572), whereas if it is increased in the de-escalation therapy (to 31%), the ICER increases (£33,740). The differential costs per QALY gained are also sensitive to the clinical success of fluconazole against sensitive Candida (£22,041–£29,168), but not to the clinical efficacy of micafungin (£25,559–£25,782).
The incremental costs per QALY are also sensitive to the excess hospitalization in the general ward or ICU. The longer the excess hospitalization is for inadequately-treated patients, the lower the ICER is (range for ICU: £19,239–£25,673; general ward: £23,720–£28,064). Varying the cost per hospitalized day in a general ward has very little impact in the ICER (range: £26,134–£27,289).
While the incremental costs per QALY are highly sensitive to the patient’s underlying disease and corresponding utility weight (ICER between £17,861 and £31,045), the utility weight associated with SCI has very little impact on the ICER (£25,687 and £25,659). In both instances, the ICER decreases with increasing values for the utilities. The differential costs per QALY are also sensitive to life expectancy and follow-up costs, although while the incremental costs decreases when life expectancy increases, they increase with increasing follow-up costs. When life expectancy is considered as 17.51 years (weighted average according to the underlying diseases), the differential costs decrease to £18,445 per QALY (base case: £25,673). When the follow-up costs are doubled (£10,420), the ICER increases to £33,434.
The probabilistic sensitivity analysis based on 10,000 simulations is provided in . The ICER based on this probabilistic sensitivity was £25,673 (95% CI = £4380–£3,525,450) per QALY (). In the cost-acceptability curve, the probability for the de-escalation strategy to be more cost-effective than the escalation approach was 44% for a willingness-to-pay value of £30,000Citation60.
Figure 4. Probabilistic sensitivity analysis of the cost-effectiveness of the de-escalation vs the escalation strategies. Results shown are based on 10,000 Monte Carlo simulations. The continuous line corresponds to a willingness-to-pay (WTP) threshold of £30,000.
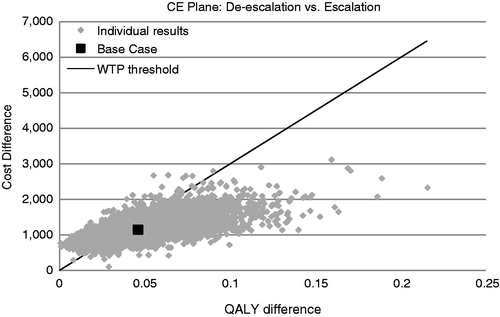
Table 5. Probabilistic sensitivity analyses outcomes based on 10,000 simulations.
In patients with diabetes type 2, the de-escalation strategy results in 0.17 more QALYs at an incremental cost of £7333 per QALY, well below the £30,000 UK willingness-to-pay threshold (). The differential costs when all patients have cancer at different stages are similar to those of the base case (ICER of £26,787 per QALY). In the worst case scenario, where all patients are considered to have advanced cancer with a life expectancy of 1.38, the de-escalation strategy yields only 0.01 additional QALY at a cost of £143,155 per QALY.
Table 6. Cost-effectiveness of de-escalation and escalation in patients with diabetes or cancer.
Discussion
The ESCMID and IDSA guidelines recommend using an echinocandin as first-line treatment of invasive candidiasis and de-escalate to fluconazole if appropriate once the susceptibility results become available. Despite these recommendations which are based on the changes on Candida epidemiology, fluconazole is still the preferred initial therapy by many physicians partly because of its low acquisition cost. Our study compares the cost-effectiveness of a de-escalation anti-fungal strategy with an echinocandin in the treatment of SCI to the traditional escalation strategy with fluconazole. Our economic evaluation shows that de-escalation from micafungin is a cost-effective strategy in the treatment of SCI from the UK NHS perspective; it improves both survival and the proportion of successfully-treated patients with an ICER below the £30,000 per QALY threshold.
The clinical and economic advantages of de-escalation are more evident in patients with fluconazole-resistant isolates where it is associated with the prevention of 30% more deaths, the successful treatment of 23% more patients than escalation, and cost savings of £1621 per treated patient. In the overall population with SCI, de-escalation would avoid 1.2% more deaths and result in 0.9% more successfully-treated patients with a marginal cost of £740 more per patient treated over a 42-day timeframe. When projected over a lifetime, de-escalation results in an additional 0.05 QALYs at an incremental cost of £25,673 per QALY.
The model is sensitive to the overall fluconazole-resistance profile of Candida and documents that cost-effectiveness of de-escalation increases in those settings where fluconazole-resistance is more prevalent. In our base case, the overall prevalence of (intrinsic and acquired) fluconazole-resistant isolates corresponded to that reported for the UK in 2006–2007. This prevalence was low (3.9%), but may increase in certain countries or clinical practice settings.
Decisions to use de-escalation should be taken by clinicians after weighing the risk of fluconazole-resistance in their centers and, hence, the likely cost-effectiveness of de-escalation. Given the risk of increased mortality and prolonged hospitalization associated with inappropriate treatmentCitation20,Citation22,Citation23, use of de-escalation should be considered particularly for patients who have previously received prophylaxis or treatment with an azole as such patients are recognized to have an increased risk of azole-resistant Candida infectionsCitation22.
One of the weaknesses of economic models is that they often give a simplified representation of actual clinical practice. In this respect, our model assumed that all ICU patients initially treated with fluconazole or micafungin will develop SCI. This does not accurately reflect clinical practice. In a recent publication, Prowle et al.Citation61 estimated that 15.5% of all bloodstream infections in Australian ICU patients were caused by Candida. This is in line with the candidaemia prevalence of 14% reported by Zilberberg et al.Citation5 based on data for Europe, Canada, and China. We are aware that our assumption may benefit the de-escalation strategy. However, Zilberberg et al.Citation5 showed that the de-escalation strategy with micafungin was cost-effective when used as an empiric treatment of suspected ICU-acquired candidaemia over fluconazole. The authors showed that, with a candidaemia prevalence of 14% (range = 5–48%), empiric treatment with micafungin results in four fewer deaths per 1000 treated patients with suspected candidaemia at a marginal incremental cost/death averted of $61,446 and an incremental cost-effectiveness of $34,734 (95% CI = $26,312–$49,209) per QALY compared with empiric treatment with fluconazole. Although treatment duration differed in both studies (10 days vs 14 days in our model), this is unlikely to have any impact on the final outcomes; our sensitivity analyses showed the ICER is only marginally affected by the overall treatment duration.
The duration of appropriate treatment in our model was set as 14 days for all patients except those who failed to show a response at the end of this treatment period. Current treatment guidelines recommend continuing treatment 14 days after resolution of signs and symptoms attributable to infection and clearance of Candida species from the bloodstream. However, in clinical practice patients may be exposed for a shorter period of timeCitation27. As already mentioned, our model was insensitive to the overall treatment duration and, thus, the cost-effectiveness findings would still apply to those centers where treatment duration is longer.
The model was also simplified by assuming that Candida culture isolation takes place within 24 h of a blood culture being taken and that susceptibility results are available in 72 h. In clinical practice these timelines will vary across hospitals. A recent UK audit highlighted that only 38% of the participating laboratories performed anti-fungal susceptibility testing locallyCitation62; thus, delaying the results of these tests. This infers a delay also in switching to the appropriate treatment with its corresponding increase in early mortality for patients treated with an inappropriate anti-fungal. This is associated with higher incremental costs for the de-escalation strategy because of less patients surviving to switch to micafungin. In these cases, using a broad spectrum anti-fungal as first-line treatment may be the best strategy to contain the increased mortality associated with delayed treatment initiation.
Several input parameters were derived from the literature and, therefore, several assumptions had to be made. For example due the heterogeneity of the population in the two phase III international micafungin studies, a simplistic approach was taken whereby average life expectancy and overall utility values have been used. In addition, the approach for long-term outcomes was to use the published life expectancy for UK ICU survivorsCitation43 which may be different from a population of SCI patients. Finally, none of the studies with micafungin or fluconazole provide any follow-up costs for SCI survivors. Thus, these were estimated as the average follow-up costs for cancer patients and then applied to the whole population.
In our model Candida parapsilosis isolates were considered primarily micafungin-sensitive based on the results of the micafungin pivotal studies, where overall clinical success of this anti-fungal against Candida parapsilosis was 83% vs a clinical success of 88% against Candida non albicans and 90% against Candida albicansCitation63. The IDSA guidelines do not recommend the use of echinocandins when this strain is suspected based on the lower in vitro susceptibility of this species to echinocandinsCitation8. However, both IDSACitation8 and ESCMIDCitation7 highlight the uncertain clinical relevance of high MIC values of Candida parapsilosis given that high MIC values are not associated with lower clinical efficacy. As already mentioned, the meta-analysis of Kale-Pradhan et al.Citation26 concluded that echinochandins are as effective as comparator drugs in the treatment of candidiasis and candidaemia due to Candida parapsilosis. Lower MIC values of C. parapsilosis for micafungin compared to the other echinocandins have been reported in SwedenCitation64, but need to be confirmed in other studies.
Although further research is required to investigate the large uncertainty of the use of de-escalation in SCI, the marked improved survival and cost-savings among patients with fluconazole-resistant Candida should encourage clinicians to consider this strategy and, especially, when the risks of azole resistance are high.
Conclusion
Current treatment guidelines recommend de-escalation with an echinocandin as first-line treatment because this strategy may improve clinical outcomes and overall survival, particularly among patients with fluconazole-resistant Candida strains. Our study shows that de-escalation from initial treatment with micafungin is a cost-effective alternative to the traditional escalation approach from a UK NHS perspective, with a differential cost per QALY below the recognized ‘willingness-to-pay’ threshold of £30,000.
Transparency
Declaration of funding
The research described in this article, the drafting of this manuscript, and its publication were funded by Astellas Pharma Europe Ltd. The work of Quintiles Consulting was also funded by Astellas Pharma Europe Ltd.
Declaration of interest
RGM, MC, and AE are consultants to Astellas Pharma Europe Ltd. OO and IAOO are employees of Astellas Pharma Europe Ltd. PM and RZ are former employees of Astellas. All authors have participated in the design, data analysis, and drafting of this article. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
| Abbreviations | ||
| DEALE, | = | Declining Exponential Approximation of Life Expectancy |
| FLU, | = | fluconazole |
| HPA, | = | Health Protection Agency |
| ICU, | = | intensive care unit |
| ICER, | = | incremental cost-effectiveness ratio |
| IDSA, | = | Infectious Diseases Society of America |
| MYC, | = | micafungin |
| QALY, | = | quality adjusted life year |
| SCI, | = | systemic Candida infections |
| SD, | = | standard deviation |
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Hassan I, Powell G, Sidhu M, et al. Excess mortality, length of stay and cost attributable to candidaemia. J Infect 2009;59:360–5
- HPA. Candidaemia in England, Wales and Northern Ireland: 2009. Health Protection Report [serial online] 2008 [date cited]; 4(37): HCAI. Available at: http://www.hpa.org.uk/Publications/HealthProtection/HealthProtectionReport/HealthProtectionReportHPR/. Accessed 2 Feb 2011
- Odds FC, Hanson MF, Davidson AD, et al. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol 2007;56:1066–75
- Karabinis A, Hill C, Leclercq B, et al. Risk factors for candidemia in cancer patients: a case-control study. J Clin Microbiol 1988;26:429–32
- Zilberberg MD, Kothari S, Shorr AF. Cost-effectiveness of micafungin as an alternative to fluconazole empiric treatment of suspected ICU-acquired candidemia among patients with sepsis: a model simulation. Crit Care 2009;13:R94. doi: 10.1186/cc7924. Epub 2009 Jun 19
- Zilberberg MD, Kollef MH, Arnold H, et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis 2010;10:150
- Ullmann AJ, Cornely OA, Donnelly JP, et al. ESCMID guideline for the diagnosis and management of candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect 2012;18:1–8
- Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503–35
- Prentice AG, Glasmacher A, Hobson RP, et al. Guidelines on the management of invasive fungal infection during therapy for haematological malignancy. British Committee for Standards in Haematology, 2008 . Available at: http://www.bcshguidelines.com/documents/fungal_infection_bcsh_2008.pdf
- Snydman DR. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest 2003;123:500–3
- Nguyen MH, Peacock JE, Jr Morris AJ, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med 1996;100:617–23
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007;20:133–63
- Antoniadou A, Torres HA, Lewis RE, et al. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine (Baltimore) 2003;82:309–21
- Baddley JW, Patel M, Bhavnani SM, et al. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob Agents Chemother 2008;52:3022–8
- Kollef MH. Appropriate empirical antibacterial therapy for nosocomial infections: getting it right the first time. Drugs 2003;63:2157–68
- Charlier C, Hart E, Lefort A, et al. Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? J Antimicrob Chemother 2006;57:384–410
- Phillips P, Shafran S, Garber G, et al. Multicenter randomized trial of fluconazole versus amphotericin B for treatment of candidemia in non-neutropenic patients. Canadian Candidemia Study Group. Eur J Clin Microbiol Infect Dis 1997;16:337–45
- Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med 1994;331:1325–30
- Anaissie EJ, Darouiche RO, Abi-Said D, et al. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis 1996;23:964–72
- Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidaemia: a multi-institutional study. Clin Infect Dis 2006;43:25–31
- Kumar A, Ellis P, Arabi Y, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136:1237–48
- Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005;49:3640–5
- Labelle AJ, Micek ST, Roubinian N, et al. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 2008;36:2967–2
- Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000;118:146–55
- Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 2007;356:2472–82
- Kale-Pradhan PB, Morgan G, Wilhelm SM, et al. Comparative efficacy of echinocandins and nonechinocandins for the treatment of Candida parapsilosis Infections: a meta-analysis. Pharmacotherapy 2010;30:1207–13
- Chalmers CM, Bal AM. Management of fungal infections in the intensive care unit: a survey of UK practice. Br J Anaesth 2011;106:827–31
- Review of time needed for Candida identification and susceptibility test in several hospitals in the UK. Unpublished research conducted by the authors. In-house data.
- Pfaller MA, Diekema DJ, Sheehan DJ. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev 2006;19:435–47
- Summary of product characteristics for Mycamine. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000734/WC500031075.pdf. Accessed 5 Dec 2010.
- British National Formulary 59. British Medical Association and the Royal Pharmaceutical Society of Great Britain, 2010. Available at: http://www.bnf.org/bnf/index.htm
- Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidaemia and other forms of invasive candidiasis. Clin Infect Dis 2007;45:883–93
- Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007;369:1519–27
- Kubiak DW, Bryar JM, McDonnell AM, et al. Evaluation of caspofungin or micafungin as empiric antifungal therapy in adult patients with persistent febrile neutropenia: a retrospective, observational, sequential cohort analysis. Clin Ther 2010;32:637–48
- Horn DL, Ostrosky-Zeichner L, Morris MI, et al. Factors related to survival and treatment success in invasive candidiasis or candidaemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis 2010;29:223–9
- Slavin MA, Sorrell TC, Marriott D, et al. Candidaemia in adult cancer patients: risks for fluconazole-resistant isolates and death. J Antimicrob Chemother 2010;65:1042–51
- Sipsas NV, Lewis RE, Tarrand J, et al. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009;115:4745–52
- Marfo K, Guo Y. Pharmacoeconomic analysis of Micafungin (Mycamine) 100 mg and 150 mg daily in the treatment of candidemia. Pharm Ther 2009;34:196–9.
- HPA. Candidaemia in England, Wales and Northern Ireland: 2007. Health Protection Report [serial online] 2009 [date cited]; 3(37): HCAI. Available at: http://www.hpa.org.uk/Publications/HealthProtection/HealthProtectionReport/HealthProtectionReportHPR/. Accessed 2 Feb 2011
- Unpublished data, personal communication with the HPA
- Weighted average of codes XC01Z, XC02Z, XC03Z, XC04Z, XC05Z, XC06Z and XC07Z, 0 to 6 organs supported. NHS Trusts Critical Care Services - Adult: Critical Care Unit. NHS costs 2008--2009. Available at: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591
- Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med 1982;73:883–8
- Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14:R6 . doi: 10.1186/cc8848. Epub 2010 Jan 20
- Fowler RA, Garber AM, Hill-Popper M, et al. Cost-effectiveness of recombinant human activated protein C and the influence of severity of illness in the treatment of patients with severe sepsis. J Crit Care 2003;18:181–91; discussion 191–4
- Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health 2008;11:172–9
- Tunis S, Baran R, Charles M. Pioglitazone versus rosiglitazone treatment in patients with type 2 diabetes and dyslipidemia: cost-effectiveness in the US. Curr Med Res Opin 2008;24:3085–96.
- Yun HR, Bae S-C, Yun HR. Cost-effectiveness analysis of NSAIDs, NSAIDs with concomitant therapy to prevent gastrointestinal toxicity, and COX-2 specific inhibitors in the treatment of rheumatoid arthritis. Rheumatol Int 2005;25:9–14
- Morris S, Cox B, Bosanquet N. Cost of skin cancer in England. Eur J Health Econ 2009;10:267–73
- Fourcade RO, Benedict A, Black LK, et al. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int 2010;105:49–56
- Lacau St Guily J, Borget I, Vainchtock A, et al. Head and neck cancers in France: an analysis of the hospital medical information system (PMSI) database. Head Neck Oncol 2010;2:22 . doi: 10.1186/1758-3284-2-22
- Thomas RJ, Williams M, Marshall C, et al. The total hospital and community UK costs of managing patients with relapsed breast cancer. Br J Cancer 2009;100:598–600
- Clarke PM, Gray AM, Briggs A, et al. UK Prospective Diabetes Study (UKDPS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747–59
- Nolan JJ, O'Halloran D, McKenna TJ, et al. The cost of treating type 2 diabetes (CODEIRE). Ir Med J 2006;99:307–10
- Walters S, Rachet B, Westlake S, et al. Cancer survival, England, patients diagnosed 2001--2006 and followed up to 2007: one-year and five-year survival for 21 common cancers, by sex and age. Available at: http://www.ons.gov.uk/ons/search/index.html?newquery=cancer+survival%2C+england%2C+patients+diagnosed+2001-2006. Accessed 1 Oct 2010
- O'Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncoglogist 2005;10(3 Suppl):20–9
- Mulcahy N. New chemotherapy improves survival in metastatic prostate cancer 2010 Genitourinary Cancers Symposium (GUCS), Abstract 9. San Francisco, CA. Available at: http://www.medscape.com/viewarticle/717928. Accessed 1 Oct 2010.
- Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010;28:453–9
- Sher DJ. Cost-effectiveness of CT and PET-CT for determining the need for adjuvant neck dissection in locally advanced head and neck cancer. Ann Oncol 2009;21:1072–7
- NHS NICE. Guide to the methods of technology appraisal. 2008. National Institute for Health and Clinical Excellence. Available at: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. Accessed 5 Oct 2010
- Cost-acceptability curve, data on-file.
- Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care 2011;15:R100 . doi: 10.1186/cc10114. Epub 2011 Mar 21
- Schelenz S, Barnes RA, Kibbler CC, et al. Standards of care for patients with invasive fungal infections within the United Kingdom: a national audit. J Infect 2009;58:145–53
- Electronic Medicines Compendium. Available at: http://www.medicines.org.uk/emc/medicine/20997/SPC/Mycamine+50mg+and+100mg+powder+for+solution+for+infusion/. Accessed 1 Oct 2010
- Axner-Elings M, Botero-Kleiven S, Jensen RH, et al. Echinocandin susceptibility testing of Candida isolates collected during a 1-year period in Sweden. J Clin Microbiol 2011;49:2516–21