Abstract
Purpose:
People with insulin-treated diabetes often face strict regimens with inflexible dose timing, frequent injections, and frequent self-measured blood glucose (SMBG) testing. The objective of this study was to estimate the health-related quality-of-life (HRQoL) impact of these aspects using time trade-off (TTO) methods.
Methods:
HRQoL was examined via a TTO survey in the UK, Canada, and Sweden with separate analyses of 2465 respondents from the general population, 274 people with type 1 diabetes, and 417 people with type 2 diabetes. Respondents evaluated health states with diabetes, SMBG testing, and basal injections that were once-daily time flexible, once-daily at a fixed time, and twice-daily at a fixed time in a basal or basal–bolus regimen.
Results:
Time-flexible basal injections were associated with 0.016 and 0.013 higher utility vs a fixed time of injection for basal-only and basal–bolus regimens, respectively, as evaluated by the general population. The diabetes respondents confirmed the basal-only results with 0.015 higher utility, but the difference in utility was non-significant for basal–bolus. Once-daily injections had higher utility compared with twice-daily injections for basal (0.039 and 0.042) and basal–bolus (0.022 and 0.021) regimens, as evaluated by the general population and people with diabetes, respectively. Increased frequency of SMBG negatively affected health utility.
Limitations:
This study has the limitation that it measures hypothetical health states rather than the HRQoL of people with these health states; furthermore, it could be suggested that the web-based nature of this survey is biased towards literate respondents with internet access and IT competence.
Conclusions:
Flexible dosing and fewer injections have a positive HRQoL impact, which potentially may enhance therapy adherence and could contribute to improved long-term outcomes. The impact of flexibility is greater in people treated with basal-only insulin regimens, and diminishes if bolus injections are part of the treatment regimen.
Keywords::
Introduction
Insulin therapy remains the most efficacious glucose-lowering therapy available for patients with diabetesCitation1. However, patients view the rigidity of glucose-lowering treatment as a concernCitation2 and many are reluctant to initiate insulin therapy as it is perceived to be inflexibleCitation3,Citation4. Insulin therapy can be particularly cumbersome with the requirement for basal insulin dosing at the same time every day, and for some people twice a day. Fixed dosing regimens may have a negative impact on health-related quality-of-life (HRQoL) and potential non-adherence. Moreover, for patients with type 1 diabetes and those with type 2 diabetes requiring treatment intensification, frequent injections of fast-acting bolus (meal-time) insulin are needed in a basal–bolus regimen. The potential burden of insulin therapy on HRQoL is associated with greater injection frequencyCitation2. Several studies have investigated barriers to insulin adherence, as reviewed by Davies et al.Citation5, with patients finding it difficult to incorporate insulin injections into daily life, due to changing daily routines, thus making therapy adherence a challengeCitation5–7. Indeed, a recent study showed that one third of patients reported at least one occurrence of insulin omission or non-adherence during a 1-month period. The most common reasons for this were: too busy, travelling, skipped meals, stress/emotional problems, or public embarrassmentCitation7.
Poor glycemic control and diabetic complications are positively associated with missing insulin dosesCitation8,Citation9, while decreased medication adherence has been associated with increased healthcare service use and poor health-related outcomes in patients with type 2 diabetesCitation10.
Insulin treatment of diabetes often involves drawing blood from a finger prick to conduct self-measured blood glucose (SMBG) testing at various intervals throughout the day, which is regarded as burdensome by patients and an interruption to daily activity; ultimately, this can have a negative impact on HRQoLCitation2. Treatment guidelines recommend that people with diabetes using multiple daily insulin injections or insulin pumps perform SMBG testing before meals at the very least, which equates to three or more measurements per dayCitation11. People using less frequent insulin injection therapy or non-insulin glucose-lowering therapy are also recommended to perform SMBG testing to help guide treatment decisions and patient self-managementCitation11,Citation12. Furthermore, blood glucose test strips represent a significant direct cost to healthcare systems, accounting for 24–41% of the total pharmacy-related costs in insulin-treated patients with diabetes in the US, Canada, and GermanyCitation13–15.
Different insulin analogs vary in terms of the number of injections, flexibility, and the necessity of SMBG testing. This should be taken into account when evaluating the cost-effectiveness of one insulin treatment over another. Insulin regimens that are more flexible may be easier for patients to adhere to and could improve HRQoL. HRQoL can be quantified using health utility values, which are on a scale from 0–1, where 0 = death and 1 = perfect health. Boye et al.Citation16 previously estimated HRQoL values for different features of diabetes treatment using standard gamble (SG) methods, but the study did not specifically include insulin-treated patients.
In this study, the time trade-off (TTO) method was used to examine the HRQoL impact of various features of insulin treatment. It was conducted in three countries and included a sample of the general population as well as people with diabetes. The TTO method estimates the health utility of a certain disease state and is a well-recognized and extensively used tool in health-economic assessmentsCitation17–19. Utility values are obtained by asking respondents to ‘trade off’ a portion of their remaining life-span for an improved health state. Methods such as TTO are recognized by the Health Technology Assessment (HTA) agencies and are used to calculate quality-adjusted life years (QALYs) and in processes to estimate the cost-effectiveness of particular treatmentsCitation20–24. The aim of this study was to assess the impact on HRQoL of flexible timing of basal insulin doses, the number of basal insulin doses, and the frequency of SMBG testing, using a TTO utility approach in a population.
Patients and methods
Study design
The TTO methodology has been well described elsewhere and the methodology for this study follows that described by Evans et al.Citation24. Briefly, each respondent is asked to make a choice or be indifferent about two health states: a defined health state for the remainder of their lifetime or perfect health for a shorter lifetime. The shorter lifetime is then changed and the question repeated until the point of indifference is reached (where both options are equally acceptable)Citation19. For example, option A: you have diabetes and give yourself one injection at the same time every day and will live for 40 years, or option B: you will live in full health for 36 years. If the respondent chooses option B they are willing to trade 4 years to have full health, and the utility is 0.9 (36/40 years). To identify the point of indifference (i.e., where two health states are equally acceptable) respondents were asked the same questions repeatedly, varying the number of years to trade off for the improved health state. This procedure followed standard bisection methodology, using a starting point of utility of 0.6 and after four questions reducing the utility value to an interval of 0.05. Particular attention was given to the distribution tails. Respondents who either chose not to trade lifetime at a utility value of 0.95, or who were willing to trade a very high proportion of their remaining lifetime (0.875) to be restored to full health, were carefully screened and received additional questions. Responses were excluded if the respondents refused to trade on ethical or religious grounds or if they did not understand the question. However, those who believed the health state manageable or who stated a desire to live as long as possible due to obligations (e.g., caregivers) were retained. Negative utilities were not allowed in this study. To ensure the trade-off values were as accurate and relevant as possible, the time horizons used in the survey were based on each respondent’s projected life expectancy. These were calculated using World Health Organization life tables accounting for respondent age and genderCitation25.
Survey description
Data were collected via an internet-based survey, using existing email panels of respondents. All respondents had previously agreed to participate in online surveys, were >18 years old and participated at their discretion, remaining anonymous throughout the study. The patients with diabetes were also sampled from online panels as the panel providers have panels with disease characteristics such as diabetes from which the respondents can be sampled from.
Depending on the country of origin, remuneration (nominal value €1–2) for participating was offered via ‘points’ for online shopping or entry in a draw. In order to improve the quality of the answers and prevent unconsidered responses, a delay feature was introduced to pages containing a large amount of text such that respondents were unable to click the ‘Next’ button until 10 s had passed.
The survey was conducted in the UK, Sweden, and Canada and was performed in Swedish, English, and French. Canadian respondents could choose which version of the questionnaire to answer (French or English). The questionnaire was back-translated to ensure the accuracy of each translation. Respondents were asked to provide demographic information (age, sex, employment, household, regional details). Those with diabetes were asked to disclose details on their disease duration and medication.
There remains a debate in the literature, and there is also a difference in opinion between HTA bodies, as to whether patient or public preferences carry the most influence when determining the value attached to a particular health stateCitation26–28. In order to determine whether responses differed between the general population and those respondents who may have personal experience of using insulin, a second population with type 1 diabetes or type 2 diabetes were also identified from the available panels. Therefore, the study included three samples: general population (including some respondents with diabetes), a sample of respondents with type 1 diabetes (including those with type 1 diabetes who were also included in the general population and answered the basal–bolus questionnaire), and a sample of respondents with type 2 diabetes (including those with type 2 diabetes also included in the general population) (). A similar study design was used by Evans et al.Citation24.
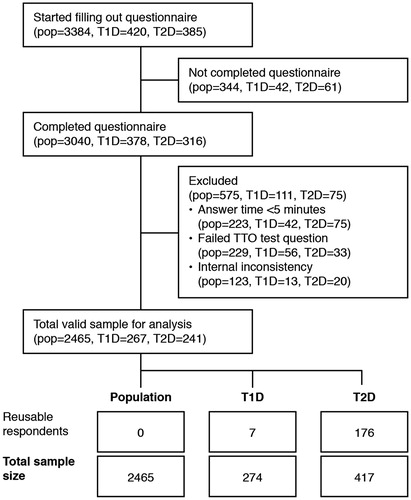
Figure 1. Patient consort flow diagram. pop, general population; TTO, time trade-off; T1D, type 1 diabetes; T2D, type 2 diabetes. There were a number of ‘reusable respondents’ who were included in the general population but also declared that they had diabetes. Of the 31 respondents in the general population with type 1 diabetes; seven were also included in the type 1 diabetes analysis because they had answered the basal–bolus questionnaire and the six daily SMBG questionnaire. Additionally, all 176 respondents in the general population cohort with type 2 diabetes were also included in the relevant analyses (basal-only or basal–bolus) of the diabetes populations.
To validate the functionality of the questionnaire, a pilot survey of 200 respondents was conducted in Canada in English. This pilot survey showed that a warm-up question was needed in order to make respondents familiar with the TTO methodology. It was observed that respondents were answering differently to the first TTO question compared to the following ones, making the results of the first TTO exercise unreliable. Accordingly, a warm-up TTO exercise was added to the questionnaire and to further improve the reliability of the results the order of the subsequent three TTO exercises about injections and flexibility were randomly asked. To test the revised questionnaire a second pilot survey with another 200 respondents was performed (also in Canada); results were as expected, so the main survey was initiated.
Two versions of the questionnaire were used (examples of the health states assessed are depicted in ):
Basal-only: in this version respondents were to imagine having baseline diabetes and administer basal insulin injections according to the description in the health state.
Basal–bolus: in this version the respondents were asked to imagine having baseline diabetes and administer basal insulin injections according to the description in the health state, whilst also taking a bolus insulin injection with every meal.
Table 1. Description of health states.
To avoid fatigue, each respondent answered one version of the questionnaire (basal-only or basal–bolus). Those in the population with type 1 diabetes all answered the basal–bolus questionnaire (as this would be the most relevant for them). Half of those in the general population and the population with type 2 diabetes answered the basal-only questionnaire and the other half answered the basal–bolus questionnaire.
Health states
The health states used are shown in . All respondents were shown the baseline diabetes health state which was used to familiarize participants with the TTO method. The baseline diabetes health state was developed based on patient questionnaires described in Evans et al.Citation24. This description of the health state with bolus insulin injections was only shown in the bolus version of the questionnaire. Three different health states were evaluated to assess the impact of flexibility and the number of daily basal insulin injections:
Once-daily flexible injection,
Once-daily fixed injection, and
Two fixed injections.
The disutility of injections and flexibility were calculated by subtracting the utility values of these different health states. To assess the impact of SMBG testing, respondents were randomly allocated to evaluate one of the following health states: once-weekly, once-daily, three times daily, six times daily SMBG tests.
Exclusion criteria
The exclusion criteria were applied sequentially, with respondents failing the test question excluded first. The test question asked respondents to choose between living for a longer time in full health or living with diabetes for a shorter time. Those who prefer to live with diabetes for a shorter time are screened out because they probably have not read or understood the question properly. Second, respondents completing the general population version of the questionnaire in less than 5 min or the diabetes version in less than 7.5 min were excluded. Third, respondents could be excluded if there was an inconsistency in their responses. It was required that a valid utility value exist for all three health states concerning flexibility and injections for each respondent to be included in the analysis ().
Ethical approval
This study was performed in accordance with the code of conduct of the European Pharmaceutical Market Research Association. In Canada, ethical approval was gained from Institutional Review Board Services (Aurora, Ontario, Canada). In the UK and Sweden, an ethical application was not necessary for this type of survey, as it was not a clinical trial, and did not collect human or biological samples or identifiable personal information.
Statistical analysis
All statistical analyses were performed using SAS® version 9.2 statistical software. A utility value was assigned to each health state based on each individual response; the mean utility value across respondents was then calculated for each health state.
For SMBG measurements, an ordinary least squares (OLS) linear regression model was estimated with individual level data to capture the utility value of one less SMBG test. The model estimated utility as a function of annual number of SMBG tests with a binary variable for regimen. A number of tests were conducted and a non-parametric bootstrapping method was used to make sure the distributional assumptions were not violated. As the data were not normally distributed, bootstrapping was used to calculate standard errors and confidence intervals (CI)Citation29. As recommended, the estimation of each utility was repeated 10,000 times by drawing the original number of observations from the dataset with replacement 10,000 times. The CI and standard deviation (SD) were then taken from these distributions.
Results
Respondent demographics
The patient consort flow is summarized in , including details of those who were excluded. A total of 3384, 420, and 385 respondents in the general, type 1 diabetes, and type 2 diabetes populations, respectively, started the questionnaire. Data from 2465 respondents (73%) from the general population, 274 respondents from the population with type 1 diabetes, and 417 respondents from the population with type 2 diabetes were used for the analysis (). In the general population, 31 (1.3%) and 176 (7.1%) respondents disclosed that they had type 1 or type 2 diabetes, respectively. The numbers of respondents used for the diabetes population analysis also includes some respondents included in the general population as the diabetes data was used in both samples; hence the higher number of respondents with type 2 analyzed than started the questionnaire. Of the 31 respondents in the general population with type 1 diabetes; seven were also included in the type 1 diabetes analysis because they had answered the basal–bolus questionnaire and the six daily SMBG questionnaire. Additionally, all 176 respondents in the general population cohort with type 2 diabetes were also included in the relevant analyses (basal-only or basal–bolus) of the diabetes populations. The median answer time was 13 min for the general population, 18 min for those with type 1 diabetes, and 17 min for those with type 2 diabetes.
The demographic characteristics of the final sample of respondents used for statistical analysis are summarized in . Approximately equal numbers of respondents came from the three participating countries: Canada, Sweden, and the UK. The characteristics of the general population and diabetes populations were similar, except that the respondents with type 1 diabetes had a younger average age (mean 43.15 years) than the general population (mean 47.51 years), whilst those in the type 2 diabetes population were on average older (58.59 years) than the general population. The mean (±SD) disease duration for respondents with type 1 diabetes (n = 274) was 16.6 (±14.4) years and for those with type 2 diabetes (n = 417) was 8.7 (±7.9) years.
Table 2. Background characteristics of the final sample.
TTO results
In the general population, once-daily, time-flexible basal insulin injections were associated with 0.016 [95% CI: 0.011; 0.022] and 0.013 [95% CI: 0.007; 0.020] higher utility vs a once-daily fixed time of injection for basal-only and basal–bolus regimens, respectively (). The diabetes respondents confirmed the basal-only results (0.015 [95% CI: 0.004; 0.027]), but elicited a smaller utility for time-flexibility in a basal–bolus regimen (0.004 [95% CI: −0.006; 0.04]) ().
Table 3. Utility differences from the time trade-off survey.
Once-daily injections (both fixed and flexible timing) had significantly higher utility compared with twice-daily, as evaluated by all populations (). The greatest increase in utility was estimated by the diabetes population for a basal-only regimen, where a once-daily flexible dose had a 0.057 [95% CI: 0.040; 0.076] higher utility compared with two fixed-time injections. The effect of flexible dosing was less pronounced for all groups investigated when the flexible basal insulin dose was part of basal–bolus therapy (where timing of bolus doses was fixed) ().
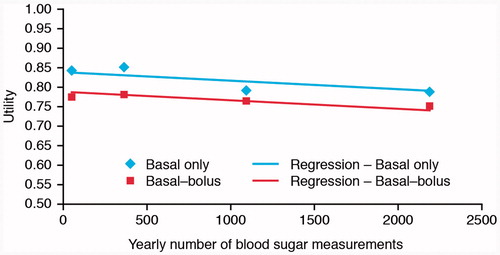
Frequency of SMBG testing decreased health utility, with the annual number of tests significantly correlated with reduced utility in basal–bolus regimens (, ). One additional SMBG test per annum had a negative impact of 0.000,022,1 in annual utility [95% CI: 0.000,011; 0.000,033,4].
Table 4. Regression of utility on number of self-monitored blood glucose tests.
Discussion
This is the first study to use the TTO survey, an established health-economic methodology, to show that a large cohort of people considered that the ability to dose basal insulin flexibly had an influence on health utility in diabetes. This study supports the notion that flexible dosing of basal insulin and dosing once rather than twice daily would increase health utility in people with diabetes, particularly in relation to basal-only therapy. Therefore, the ability to dose basal insulin flexibly (by changing the time of administration from day-to-day according to patient requirements) could positively impact on HRQoL, thereby enabling patients to more easily incorporate therapy into their existing lifestyles, and potentially contributing to greater therapy adherence and long-term outcome benefits.
The results from the basal-only questionnaire were consistent for the general population and the population with diabetes. However, the population with diabetes did not value flexibility to the same degree as the general population when part of a basal–bolus regimen. When the basal injections are part of a regimen with meal-time bolus injections, the flexibility does not mean as much as when the basal injection is the only injection per day. The difference in this result may reflect the better understanding of the treatments by the respondents in the population with diabetes who have experience of fixed-time dosing regimens. Another study has shown that increasing flexibility of bolus insulin doses with insulin lispro vs human insulin can also improve QoLCitation30. Therefore, it should be recognized that, although the benefits of flexible dosing would be greater for patients using basal-only therapy, increased flexibility would be advantageous also to patients receiving basal–bolus therapy and could potentially make therapy adherence easier. Furthermore, the estimated HRQoL increment associated with flexible basal insulin dosing observed in this study may under-estimate the effects on health utility in specific sub-groups where such an attribute may be particularly important, e.g., people with varying shift working patterns. A large-scale survey investigating the issue of psychological insulin resistance in people with type 2 diabetes found that 45.2% cited ‘restrictiveness’ as a reason to avoid insulin therapyCitation4. Adherence to therapy is vital for maintaining good glycemic controlCitation8,Citation9, with simulations using patient data suggesting that missing an average of 2.1 basal insulin injections per week would lead to an increase in glycosylated hemoglobin (HbA1c) of 0.3–0.4%Citation31.
Boye et al.Citation16 investigated the impact of flexible insulin dosing on health utility in 151 patients with type 2 diabetes using standard gamble interviews. The average added utility associated with flexible vs inflexible dosing was 0.006, which, whilst significant, was lower than the utility values observed in the current studyCitation16.
This study showed that an increased frequency of blood glucose monitoring decreased health utility. The SMBG-associated disutility appears to be quite low (relative to that associated with injection frequency), but many tests are taken each year, so the increment has to be small to make sense when they are aggregated. The Digem study showed an annual utility of −0.029 (for a scheme with SMBG tests approaching five per week) for patients with type 2 diabetes. If constant utility per SMBG test is assumed, the utility for an annual SMBG test can be calculated by dividing the −0.029 with the number of tests per year (5*365.25/7). This amounts to a small decrease in utility of −0.000,111 per testCitation32, being greater than that found in the present study (equivalent to −0.0011 for one additional test per week). Additionally, more frequent SMBG testing is associated with higher healthcare costs and reduced patient quality-of-lifeCitation33, without significantly contributing to improved HbA1cCitation29.
Whilst several studies have used cost-utility models to examine the value of treatments for diabetesCitation34–36, there is a lack of data specifically addressing the effects of insulin-related attributes, such as injection frequency, on HRQoL, which become increasingly important during the course of therapy intensification in people with type 2 diabetes. This study has an advantage in that it looked specifically at the characteristics of insulin therapy, namely injection frequency and timing, but also the frequency of SMBG testing. Furthermore, this study used a large cohort of respondents from the general population and with type 1 and type 2 diabetes. The similar response from the general population and the population with diabetes increases the face validity of the results. Despite the small increase in utility estimated in this study, dose flexibility and reducing the number of SMBG test strips are important factors to consider in cost-utility models, due to the large number of patients they affect and the frequency at which they are utilizedCitation13.
TTO is a preference-based instrument that is relatively easy to use and understand, and was, therefore, considered to be more appropriate for this study than the widely used standard gamble instrument, which can be difficult for the respondents to understandCitation26. The use of an age-dependent, life-expectancy adaptation of the TTO questionnaire may have increased the relevance of the questions in this study, compared with the fixed 10- or 30-year trade-offs used in other studies. The TTO approach that specifically estimates the influence of basal-insulin dose flexibility on an individual may be more sensitive and more applicable than generic measures of QoL, such as the Short Form (36) Health Survey (SF-36)® or EQ-5D™ that lack a treatment- and disease-specific dimension. This study has the limitation that it measures hypothetical health states rather than the HRQoL of people with these health states; however, the preferences of the general population are often recommended as the most relevantCitation17,Citation27,Citation28. The repetitive nature of TTO questionnaires may give rise to respondent fatigue. To avoid this, a limited number of health states were evaluated by each respondent, and for each health state only four iterative questions were given plus two follow-up questions to clarify extreme answers. It could be suggested that the web-based nature of this survey is biased towards literate respondents with internet access and IT competence. However, the literacy rates and proportion of internet users is very high in the three countries investigated here. Due to the web-based nature of the survey no help was available to respondents when answering the questions, which could lead to inconsistencies and misunderstandings in the responses. The drop-out rate of >10% is quite high; however, this is typical of many of the web-based surveys that are commonly used in health-economic assessmentsCitation37–39.
Conclusion
This large TTO-based survey of a general population and a population with diabetes showed that utility values derived for flexible dosing of basal insulin were higher than for once- and twice-daily fixed-time basal insulin injections. This effect was most pronounced in the context of basal-only therapy, which is used by a majority of patients with type 2 diabetes. Respondents to this survey also perceived SMBG testing to be a burden, with an increase in the frequency of testing associated with reduced health utility.
Transparency
Declaration of funding
This study was sponsored by Novo Nordisk.
Declaration of financial/other relationships
ME has been an advisory panel member and received speaker honoraria for Novo Nordisk, Sanofi, Bristol Myers Squibb, Novartis, and MSD. KK has received an NHS R&D study grant and served on advisory boards for Novo Nordisk, Eli Lilly, Merck, Sharp & Dohme, Bristol-Myers Squibb, Roche, Novartis, Boehringer Ingelheim-Lilly, Astra Zeneca, Takeda, Janssen, and Sanofi. MB and HHJ have received consultancy fees from Novo Nordisk. BC and JG are employees at Novo Nordisk.
The sponsor was involved in the study design, data collection, data review, and data analysis. All authors had full access to the data and were involved in data interpretation and manuscript writing. As stated in the ethics applications and approvals, the raw data are stored at Incentive, with access restricted to HHJ and MB, who take responsibility for the integrity of the data and the accuracy of the data analysis. ME had the final decision to submit the manuscript for publication and takes responsibility for its content.
Acknowledgments
The authors take full responsibility for the content of this manuscript. Adele Norman and Mark Nelson at Watermeadow Medical (supported by Novo Nordisk) provided medical writing and editing support.
References
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Vijan S, Hayward RA, Ronis DL, et al. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005;20:479-82
- Jenkins N, Hallowell N, Farmer AJ, et al. Participants' experiences of intensifying insulin therapy during the Treating to Target in Type 2 Diabetes (4-T) trial: qualitative interview study. Diabet Med 2011;28:543-8
- Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005;28:2543-5
- Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med 2013;30:512-24
- Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes 2010;4(1 Suppl):S11-18
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682-9
- Donnelly LA, Morris AD, Evans JM. Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM 2007;100:345-50
- Morris AD, Boyle DI, McMahon AD, et al. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and Research in Tayside Scotland. Medicines Monitoring Unit. Lancet 1997;350:1505-10
- Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521-30
- American Diabetes Association. Standards of medical care in diabetes - 2012. Diabetes Care 2012;35:S11-63
- International Diabetes Federation. Guidelines for management of postmeal glucose. 2012. http://www.idf.org/2011-guideline-management-postmeal-glucose-diabetes. Accessed August 8, 2012
- Yeaw J, Lee WC, Wolden ML, et al. Cost of self-monitoring of blood glucose in Canada among patients on an insulin regimen for diabetes. Diabetes Ther 2012;3:7
- Yeaw J, Lee WC, Aagren M, et al. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm 2012;18:21-32
- Liebl A, Breitscheidel L, Nicolay C, et al. Direct costs and health-related resource utilisation in the 6 months after insulin initiation in German patients with type 2 diabetes mellitus in 2006: INSTIGATE study. Curr Med Res Opin 2008;24:2349-58
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ 2011;12:219-30
- Dolan P. Modelling valuations for health states: the effect of duration. Health Policy 1996;38:189-203
- Levy AR, Christensen TL, Johnson JA. Utility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes 2008;6:73
- Torrance GW, Furlong W, Feeny D. Health utility estimation. Expert Rev Pharmacoecon Outcomes Res 2002;2:99-108
- National Institute for Health and Clinical Excellence. National Institute for Health and Clinical Excellence: Guide to the methods of technology appraisal. 2013. http://www.nice.org.uk/media/D45/1E/GuideToMethodsTechnologyAppraisal2013.pdf. Accessed Oct 01 2013
- Scottish Medicines Consortium. Guidance to Manufacturers for Completion of New Product Assessment Form (NPAF). 2013. http://www.scottishmedicines.org.uk/Submission_Process/Submission_Guidance_and_Templates_for_Industry/Templates-Guidance-for-Submission/Templates-Guidance-for-Submission. Accessed August 25, 2013
- CADTH. HTA Guidelines for the Economic Evaluation of Health Technologies: Canada, 3rd edn. 2006. http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed August 25, 2013
- TLV (the Dental and Pharmaceutical Benefits Agency in Sweden). 2003. Läkemedelsförmånsnämndens allmänna råd. Läkemedelsförmånsnämndens allmänna råd om ekonomiska utvärderingar. http://www.tlv.se/Upload/Lagar_och_foreskrifter/LAG-lfnar-2003-2.pdf. Accessed August 25, 2013
- Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11:90
- World Health Organization. WHO life tables. 2013. http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/. Accessed Oct 01 2013
- Dolan P. The measurement of health-related quality of life for use in resource allocation decisions in health care. In: Culyer AJ, Newhouse JP, eds. Handbook of Health Economics. Amsterdam: Elsevier Science BV, 2000. p 1738-40
- Gold MR, Siegal JE, Rutten GE, et al. Identifying and valuing outcomes. In: Gold MR, Siegal JE, Russell LB, et al, eds. Cost-effectiveness in health and medicine. Oxford: Oxford University Press, 1996. p 105-6
- Hurley J. An overview of the normative economics of the health sector. In: Gold MR, Siegal JE, Russell LB, et al, eds. Cost-effectiveness in health and medicine. Oxford: Oxford University Press, 1996
- Atkinson SE, Wilson PW. Comparing mean efficiency and productivity scores from small samples: a bootstrap methodology. J Prod Anal 1995;6:137-52
- Kotsanos JG, Vignati L, Huster W, et al. Health-related quality-of-life results from multinational clinical trials of insulin lispro. Assessing benefits of a new diabetes therapy. Diabetes Care 1997;20:948-58
- Randlöv J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin A1c in people with diabetes? A simulation study. J Diabetes Sci Technol 2008;2:229-35
- Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess 2009;13:iii-xi, 1
- Simon J, Gray A, Clarke P, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ 2008;336:1177-80
- Clarke PM, Gray AM, Briggs A, et al. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72). Diabetologia 2005;48:868-77
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(1 Suppl):S5-26
- Ekman A, Dickman PW, Klint A, et al. Feasibility of using web-based questionnaires in large population-based epidemiological studies. Eur J Epidemiol 2006;21:103-11
- Lund E, Gram IT. Response rate according to title and length of questionnaire. Scand J Soc Med 1998;26:154-60
- van Gelder MM, Bretveld RW, Roeleveld N. Web-based questionnaires: the future in epidemiology? Am J Epidemiol 2010;172:1292-8