Abstract
Objectives:
To evaluate resource use and associated costs in patients with a diagnosis of heart failure with preserved ejection fraction (HF-PEF) in Sweden.
Methods:
This retrospective study identified real-world patients with an ICD-10 diagnosis code for heart failure (I50) for the period between July 1, 2005 and December 31, 2006 from electronic medical records of primary care centers in Uppsala County Council, and in the Swedish patient registry data. Patients were categorized as having HF-PEF (left ventricle ejection fraction [LVEF] > 50%) during the index period. The study assessed medication utilization, outpatient visits, hospitalizations, and associated healthcare costs, as well as the incidence rates and time to all-cause and heart failure mortality following the index period.
Results:
The study included 137 HF-PEF patients with a mean age of 77.1 (SD = 9.1) years. Over 50% of HF-PEF patients were female and hypertensive. Nearly all patients received ≥1 medication post-index. Patients had an average of 1.5 heart failure related hospitalizations per follow-up year. The average annual per patient cost for the management of a HF-PEF patient was found in Sweden to be Swedish Krona (SEK) 108,246 (EURO [EUR] 11,344). Hospitalizations contributed to more than 80% of the total cost. All-cause mortality over the 18-month study period was 25.5%, and more than 50% of these deaths occurred within 1 year of index.
Limitations:
Due to the limitations of registry data, it is not possible to confirm the HF diagnosis, and therefore the accuracy of registry records must be assumed. Other factors such as short follow-up time, the study-mandated LVEF assessment, and a lack of drug duration data may also have an impact on the study results.
Conclusions:
All-cause mortality was high in the HF-PEF population, with more than half of patients dying within 1 year of study follow-up. Study results also indicate that 60% of HF-PEF patients have ≥1 hospitalization during follow-up. Hospitalizations, especially heart failure related admissions, represent a substantial proportion of the total healthcare burden of patients with HF-PEF in Sweden.
Introduction
Cardiovascular Disease (CVD) is the primary cause of mortality in Europe, accounting for over 4.3 million deaths annually, according to a 2008 reportCitation1. CVD’s main manifestations include stroke and Heart failure (HF), with the latter accounting for approximately half of all CVD-related deathsCitation1. HF poses a significant healthcare burden in Sweden, with a population prevalence of 2% as of 2001Citation2. Generally, the prevalence of HF is found to be higher in older populations, and this is supported by epidemiological data findings across Europe, where the prevalence is as high as 10–20% among the age group of 70–80 yearsCitation3. The costs associated with heart failure is high, with an estimated annual treatment cost in Sweden of Swedish Krona (SEK) 5–6.7 billion (EURO [EUR] 0.55–0.73 billion) in 2005. Adjusted to 2010 values, this equates to SEK 5.4–7.2 billion (EUR 0.57–0.76 billion)Citation4. Due to the rapid increase of the median age of the Swedish population and the high costs incurred in the management of HF, the associated treatment and management of this disease will have a significant impact on healthcare expenditureCitation2,Citation4,Citation5.
The heart failure population consists of individuals with reduced left ventricular systolic function; and near normal or normal LVEFCitation3,Citation6. Heart failure in patients with normal or ‘near-normal’ systolic function (i.e., patients with LVEF above 35–50%) is known as preserved ejection fraction heart failure (HF-PEF)Citation3,Citation6,Citation7, and as many as 47% of patients have this form of HFCitation8. Diagnosis of HF-PEF can be more difficult to ascertain than that of heart failure with reduced ejection fraction (HF-REF), as the diagnosis relies primarily on excluding possible non-cardiac causes for symptoms such as anemia, chronic lung disease, and others associated with HF. As a result of these differences in diagnosis, the HF-PEF population is not as well defined as that of HF-REFCitation7. However, current data suggest that patients with HF-PEF are more likely to be of older age, female, and to have atrial fibrillation and a history of hypertensionCitation6.
Therapies that benefit heart failure patients with reduced ejection fraction (REF) have not shown benefit in patients with HF-PEFCitation9. As there are no guideline-supported pharmacologic treatment options for HF-PEFCitation10, the use of non-guideline and HF-REF therapies may not provide effective treatment benefit and could lead to over-use of healthcare resources for HF-PEF patients.
Studies published in the past have estimated the cost of heart failure in SwedenCitation6,Citation9; however, none have assessed outcomes and costs of HF-PEF in the Swedish healthcare system. The aim of this study is to estimate resource utilization, outcomes, and cost in patients diagnosed with HF-PEF in Sweden.
Materials and methods
Data source
The study used real-world, patient level data from the electronic medical records (EMR) of 31 primary care centers in Uppsala County, Sweden, that was extracted using the Pygargus Customized eXtraction Program (CXP), as was previously described in other publicationsCitation11–15. Uppsala County represents ∼4% of the total population of Sweden. Patient demographics were obtained from primary care data and extracted from the EMR. In addition to the primary care data, information was sourced from a Swedish patient registry, a Swedish prescription registry, and a Swedish cause of death registry to obtain information on outpatient/inpatient visits, medication use, and mortality. Medication data was available after July 2005. A local echocardiography registry from the Department of Physiology of Uppsala University Hospital was used to source the LVEF data of patients with heart failure. Data from the different registries were then linked to primary care data resulting in a complete collection of patient histories in a real-world setting.
Study design and patients
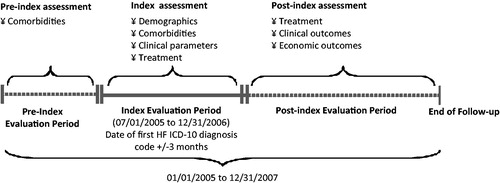
Patients with a diagnosis of heart failure (ICD-10: I50) and at least 18 years of age were identified between July 1, 2005 and December 31, 2006 using primary care and patient registry data (). Patients were classified as HF-PEF patients if they had a documented LVEF >50% within 3 months before or after the index date. The majority of diagnoses were identified in the Swedish Patient Registry with a smaller percentage identified in the hospital setting. All patients were followed until the end of the study on December 31, 2007, or death. Patients were excluded if their age and gender information were missing.
Baseline and outcome variables
Baseline characteristics evaluated for the study were age, gender, comorbidities, and medications. All comorbidities were evaluated both at pre-index and index periods (). Since there are no medications specifically utilized to treat HF-PEF, the most common medications used to treat the comorbidities associated with heart failure were identified a priori (e.g., angiotensin-converting-enzyme [ACE] inhibitors, angiotensin receptor blocker [ARB]s, alpha blockers, beta blockers, calcium channel blockers, cardiac glycosides, cardiac stimulants, diuretics, statins, and vasodilators; ).
Table 1. Pre-index and index comorbidity.
Table 2. Medications at index and post-index (through study follow-up).
Outcome variables were assessed over the post-index evaluation period or until death. Six patients died during their index hospitalization and, hence, could not be assessed for post-index outcomes. The primary outcomes of interest captured for this study were mortality (all cause and HF-related) and resource utilization including medication use, number of visits, the proportion of patients requiring a hospitalization, and the subsequent length of stay. Cardiovascular-related hospitalizations included any hospitalization related to unstable angina, myocardial infarction (MI), ventricular or atrial dysrhythmia, stroke, or heart failure. Subsequent hospitalizations were captured up to the first five hospitalizations post-index. It should be noted that, due to the limited sample size, overall mortality was low (see Results for further details), and, therefore, it was difficult to meaningfully examine mortality (overall and HF-related) beyond the fifth hospitalization.
Cost calculations
Average cost per patient per follow-up year was calculated, and did not include indirect costs related to days of work lost. The costs included in this estimate were hospitalization costs (cardiovascular and heart failure related), primary care visit costs, outpatient clinic visit costs, and medication costs. Unit costs for hospitalizations were based on Nordic diagnosis-related group (NordDRG) codes obtained from the literatureCitation16, while primary care and outpatient clinic costs were based on data obtained from the Swedish Southern healthcare region. The unit costs of each resource were then multiplied by the mean per patient number of hospitalizations, primary care visits, and outpatient clinic visits, respectively. Medication per prescription cost was obtained directly from the Swedish Prescription Registry and reported as mean cost per drug category. All costs were adjusted to 2010 values using the Swedish consumer price index. SEK costs were converted to EUR values based on the 2010 average conversion rate of 1 SEK = 0.1048 EUR.
Statistical analysis
Descriptive statistics were compiled for patient characteristics, comorbidities (pre-index and index), medication use (index and post-index), as well as post-index hospitalizations, primary care, and outpatient clinic visits. Frequencies and distributions were examined for categorical and continuous variables, respectively. Kaplan-Meier analyses were performed to calculate the median time from index to first hospitalization, first to second hospitalization, and similarly up to the fifth hospitalization. Multivariate regression analyses were performed to evaluate predictors of the rate of cardiovascular and HF related hospitalizations per year of follow-up. A zero inflated negative binomial (ZINB) model was selected for the analysis as it corrects for over-dispersion due to the high frequency of zero hospitalizations observed in the data. The predictors assessed in the final model include age, gender, inpatient or outpatient status at index, and number of pre-index comorbidities categorized per quartile. The final model included clinical relevance and utilized Akaike Information Criteria (AIC) model fit statistics.
Results
A total of 137 eligible patients were identified based on the pre-defined inclusion/exclusion criteria. The proportion of females suffering from HF-PEF (59.8%) was higher than males (40.2%). The mean patient age was 77.1 (SD = 9.1) years. More than 80% of patients had follow-up data extending over 1 year or longer. A large proportion of patients (34.4%) were obese (). The pre-index and index comorbidities were similar. Hypertension was the most common comorbid diagnosis in HF-PEF patients. Other frequently diagnosed comorbidities include atrial fibrillation, diabetes, previous MI, and coronary artery disease (CAD; ).
Table 3. Patient characteristics at index event.
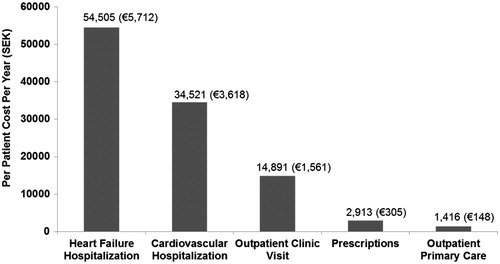
The overall direct medical costs were estimated for all identified patients with HF-PEF (). The total annualized per patient costs amounted to SEK 108,246 (EUR 11,344), of which the hospitalization costs were SEK 89,026 (EUR 9330), or 82%. Of this amount, hospitalization only related to heart failure accounted for SEK 54,505 (EUR 5712), 61% of total cost. The annualized costs incurred for prescription drugs were SEK 2913 (EUR 305), constituting only 3% of total costs. Outpatient care amounted to a substantial portion of the total cost at SEK 16,307 (EUR 1709) or 15%, of which outpatient primary care accounted for SEK 1416 (EUR 148) or 8.6%.
Figure 2. The average cost per follow-up year per HF-PEF patient for key cost categories. SEK to EUR conversions are based on conversion rate of 1SEK = 0.1048 EUR.
Pharmacotherapy
Approximately 88% of patients received one or more of the pre-specified medications at diagnosis date (index; ) At index, diuretics were the most commonly prescribed (65%), followed by beta blockers (52.5%) and ACE inhibitors (37.9%). The high-ceiling loop diuretics were prescribed most frequently in their class. Calcium channel blockers (29.9%) were prescribed more often than vasodilators (26.2%); 24.8% of patients were prescribed statins, and 13.1 % ARBs. Cardiac glycosides were prescribed in only 15.3% of patients.
During the post-index period, records indicated that most (96.2%) patients received one or more of the identified medications (). Similar to observations at index, diuretics remained the most commonly prescribed (84.7%), followed by beta blockers (71.8%) and ACE inhibitors (55.7%). In contrast to the index time point, vasodilators (42.7%) were prescribed more often than calcium channel blockers (35.9%) during the post-index period. There were increases in the proportion of patients prescribed statins, cardiac glycosides, and ARBs, increasing from 24.8, 15.3, and 13.1% to 32%, 20.6%, and 25.9%, respectively. Annual mean medication costs during follow-up () were comparable for ARBs, at SEK 644 (EUR 67), diuretics at SEK 625 (EUR 65), and beta-blockers at SEK 592 (EUR 62). The prescription drugs identified in this study only accounted for a minor fraction of the total healthcare costs at ∼3% (); however, other medications could have been received by these patients. The mean annual per patient prescription drug cost was SEK 2913 (EUR 305) in our patient cohort.
Table 4. Hospitalization and outpatient resource utilization costs (2010 SEK) per year of follow-up.
Outpatient visits
The mean number of outpatient clinic visits (including primary care visits) was 11.9 per year, and amounted to a total cost of SEK 16,307 (EUR 1709) or 15% of the total annual per patient healthcare costs (). Patients had an average of 0.4 cardiac care clinic visits per follow-up year (), with a mean annualized cost of SEK 1315 (EUR 138) per patient. The maximum average number of clinic visits recorded was for renal care at 2.4 visits per year, and the mean cost incurred was SEK 4939 (EUR 517). Visits were also recorded for internal medicine clinics with an average of 1.5 per year of follow-up and an average cost of SEK 4940 (EUR 517). Patients had an average of 0.8 primary care doctor visits per year of follow-up (), with an annual cost of SEK 1196 (EUR 125). Primary care nurse visits occurred at a rate of 0.1 per annum of follow-up, with an average cost of SEK 106 (EUR 11). Utilization of other services such as neurology, respiratory, or gastroenterology clinics was infrequent.
Hospitalizations
Hospitalization accounted for the largest proportion of total healthcare resource utilization among HF-PEF patients and cardiovascular hospitalizations accounted for 82% of the total healthcare resource utilization costs per patient per year. These patients had an average of 1.5 heart failure-related hospitalizations per annum and 0.6 hospitalizations for cardiovascular conditions other than heart failure (patients with zero hospitalizations were included in the calculation of the average hospitalization rate; ). The mean annual cost of heart failure related hospitalization was SEK 54,505 (EUR 5712), and for cardiovascular hospitalization not related to heart failure SEK 34,521 (EUR 3617). Kaplan-Meier analysis was performed to calculate time to first hospitalization and the interval between subsequent hospitalizations. The median time from admission to first cardiovascular hospitalization was shorter than the time from admission to first heart failure related hospitalization (304 vs 592 days, respectively), and the time between subsequent hospitalizations presented a decreasing trend for both CVD related and HF related hospitalizations (see Appendix A). In contrast, the time between the fourth and fifth hospitalization was longer than the time intervals separating the second to third and third to fourth hospitalizations, respectively.
Results from the ZINB regression analysis indicate that there was no significant difference between the annual post-index cardiovascular or heart failure related hospitalizations for patients who were treated as inpatients or outpatients at index (relative rates 0.89 [p = 0.701] and 1.38 [p = 0.436], respectively). The presence of pre-index comorbidities was a significant predictor of post-index hospitalization in patients with a cardiovascular-related index hospitalization (relative rate 3.03; p = 0.013). In HF-PEF patients, age ≥65 was a significant predictor of post-index hospitalization (relative rate 3.60; p = 0.047).
Although first and second cardiovascular-related hospitalization rates were not significantly different for patients who were treated as inpatient vs outpatient at index, the rates were significantly different for the third hospitalization in multivariate analysis, being higher for inpatients vs outpatient (hazard ratio [HR] 0.38; p = 0.049). For heart failure related hospitalization, patients treated as inpatients at index were almost twice as likely to be re-hospitalized compared to patients who were outpatients at index (HR = 1.96; p = 0.024).
Mortality
Over the 18-month study period, the overall all-cause mortality rate among patients was 25.5%, with 13.9% dying within a year of the index date (see Appendix B) and 10.2% of deaths occurring during the 2nd year after the index date (10.2%). In contrast, the overall heart failure related mortality rate was extremely low at 1.5% (n = 2), with both all cause and heart failure deaths occurring during the first 2 years post-index date (see Appendix B).
Discussion
The purpose of this study was to assess resource utilization and costs related to the treatment of HF-PEF in Sweden using a robust data set that closely represents a real-world patient population. As such, study findings indicate that resource utilization for HF-PEF patients is high, driven primarily by hospitalizations, with heart failure-related hospitalization accounting for 50% and cardiovascular-related hospitalization accounting for 32% of total resource utilization. While there is a lack of currently-available data on HF-PEF-specific resource utilization, our study findings are consistent with published resource utilization and cost studies for overall heart failureCitation2,Citation4,Citation17,Citation18, showing higher hospitalization costs for HF-REF. Of note, the results of this HF-PEF-focused study show similarities to a recently accepted publication on the burden of patients with HF-REFCitation19. Specifically, both studies found hospitalizations to be the major driver of cost. However, this HF-PEF study demonstrates a larger difference in the percentage cost for hospitalization compared to medication. For instance, a study of HF costs in Sweden performed by Agvall et al.Citation4 reports that medication accounts for 18% of HF-related costs while hospitalization accounts for 47%. In an earlier study also conducted in a Swedish population, Rydén-Bergsten and AnderssonCitation18 report a lower proportion for medication (11%), with a higher proportion for institutional care (65–75%). This variation may be explained by differences in the selection of medications to be explored within the studies, unit costs used, study designs, as well as patient populations. For example, Agvall et al. used an average per day cost for hospital ward and intensive care unit; while our study used heart failure and cardiovascular specific hospitalization costs (based on diagnosis-related group [DRG] codes, which includes inpatient medications), and this may have resulted in higher hospitalization unit costs for our study. Similarly, Rydén-Bergsten and Andersson. also used department level average per bed day hospitalization cost, which may be lower as compared to our DRG-specific costs. Moreover, contrary to our study, the Agvall et al. study included heart failure patients diagnosed only in primary care centers. These patients are more stable than those that are hospitalized (included in our study), and thus have fewer hospitalizations, and may be managed primarily with medications. Despite these differences, both analyses demonstrate the ultimate cost-driver among patients with heart failure is hospitalizations.
Overall, the results of this analysis indicate considerably higher total per patient annual costs than what has been previously reported in the literature. The reported per patient annual total cost of SEK 108,247 (EUR 11,344) is significantly higher than that reported by Agvall et al.Citation4 and others, even when adjusting to 2010 values (SEK 40,489 [EUR 4243]). This greater cost is likely due to the higher proportion of hospitalizations observed in our analysis. The difference may be attributable to the fact that Agvall et al. included only primary care patients, whereas our study included patients diagnosed in both inpatient as well as outpatient settings. This conclusion is supported by our multivariate analysis that indicates that patients who were inpatients at index were nearly 2-times more likely to experience heart failure re-hospitalization as compared to patients who were outpatients at index. Heart failure patients diagnosed in an outpatient setting are known to have better prognosis than those hospitalized with heart failure [5].
Notably, the results of our study indicate an overall all-cause mortality of 25% (over a minimum 1 year of follow-up), with more than half of these deaths (14%) occurring during the first year after index, findings which are consistent with those reported in other studiesCitation20–23. Due to coding and potential misclassifications of the cause of death, it is not certain whether some of the all-cause deaths were in fact due to HF-PEF. However, a recent study by Lund et al.Citation24 conducted using the Swedish Heart Failure registry reported a 1-year survival of 77% (95% CI = 75–78%). These mortality data correspond closely with our finding of a 25% 1-year mortality rate, despite the Lund study’s significantly larger sample size (16,216 HF-PEF patients)Citation24.
Limitations
There were limitations to the study analysis. Notably, as this was a retrospective study performed using registry data, it is impossible to confirm the diagnoses of HF with the available data. Therefore, the study assumes the accuracy of registry records in the absence of specifics on diagnostic tests (e.g., b-type natriuretic peptide [BNP] or the ratio of early transmitral flow velocity to early mitral annular velocity [E/E]). Additionally, the average follow-up time observed in this study was relatively short at 1.4 years, with nearly 14% of patients having less than a year of follow-up. The short follow-up period for these patients could potentially increase the maximum values for the calculated annual incidence rate ranges (i.e., hospitalizations/year and outpatient visits/year). Also, due to limitations in the data source, available patient data did not extend beyond the year 2007. Given potential changes in demographics, reimbursement, and clinical practice have likely taken place over time, the study results should be interpreted with caution.
The study population was on average older than the overall HF population, as well as predominantly female; however, this is typical of the HF-PEF population in generalCitation25. While the age of the population and the gender imbalance could potentially impact the study results, there were no statistically significant differences in cost between males and females (p = 0.8721), and age above and below 77 (p = 0.0583).
In order to exclude systolic heart failure (HF-REF) the study inclusion criteria mandated an LVEF assessment within the index period. This placed a significant but unavoidable restriction on the recruitment rate and, therefore, also on the follow-up period. The LVEF assessment requirement also impacted the study sample size, leading to the exclusion of a large number of patients for whom LVEF data were not available. Further, data on duration of drug prescriptions filled, or drug units (package size and dosages) were not available. It was, therefore, not possible to calculate medication possession ratios or adherence, both of which impact the effectiveness of treatment. In future analyses, it would be informative to examine the impact of patient compliance on hospitalization and mortality outcomes.
Because of a lack of data on the severity of the disease (New York Heart Association [NYHA] functional classification), we could not assess the impact of severity on resource use and, hence, the overall costs. In addition, primary cause of death was included in the study analysis, and, therefore, overall heart failure mortality could have been under-estimated. While a multivariate analysis was performed, missing data on many baseline variables like laboratory tests limited the number of variables that could be included, negatively impacting the scope and robustness of the multivariate results.
Conclusion
The results of this study indicate that 60% of patients with heart failure with preserved ejection fraction have at least one cardiovascular or heart failure hospitalization during their follow-up, and on average are hospitalized ∼1.5 times per year for cardiovascular or heart failure events. High all-cause mortality rates were observed in the HF-PEF population and, additionally, about one in two deaths occurred during the first year after index date. Overall, the cost for heart failure and cardiovascular hospitalization is a substantial proportion of the total cost of therapy. An effective treatment regimen including structured and well-organized care that reduces hospitalization as well as mortality could result in a substantial decrease in patient suffering and cost savings for the healthcare system. In the future, further research in a broader population of HF-PEF patients is warranted in order to fully elucidate the differences and similarities in resource utilization and outcomes between these patients and those with the more common diagnosis of HF-REF, in both Sweden and internationally.
Transparency
Declaration of funding
This work was supported by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
J. Stålhammar received compensation for his participation in the research. L. Stern, S. Sherman, and R. Parikh are employed by The LA-SER Group, which received funding for the research. R. Linder is employed by Pygargus AB, which received funding for the research. R. Ariely was formerly employed by Novartis Pharmaceuticals Corporation, which provided funding for the research. C. Deschaseaux is employed by Novartis Pharmaceuticals Corporation, which provided funding for the research. G. Wikström received compensation for his participation in the research. JME Peer Reviewers on this manuscript have no relevant or other financial relationships to disclose. J. Stålhammar, L. Stern, S. Sherman, and R. Parikh, R. Ariely, C. Deschaseaux, and G. Wikström made contributions to the study concept/design, data analysis/interpretation, and to the drafting, critical revision, and approval of the article. All authors approve the manuscript and believe that it represents honest work and adheres to International Committee of Medical Journal Editors (ICMJE) requirements.
Acknowledgments
The authors thank Dan Sandberg for providing data from the local echocardiography registry kept by the Department of Physiology, Uppsala University Hospital; and Dr Lars Benson, Luthagens Specialistläkar mottagning, Husläkargruppen in Uppsala and all public Primary Care Centers within Uppsala County Council for contributing with primary care data. Editorial assistance was provided by Jacob M. Willet, MPH, who received compensation from The LA-SER Group for the work.
References
- Allender S, Scarborough P, Peto V, et al. European Cardiovascular Disease Statistics: 2008 Edition. British Heart Foundation Health Promotion Research Group, Department of Public Health and Health Economics Research Centre. University of Oxford, London, England, 2008
- Cline CMJ, Boman K, Holst M, et al.; for the Swedish Society of Cardiology Working Group for Heart Failure. The management of heart failure in Sweden. Eur J Heart Fail 2002;4:373-6
- Schwinger RHG. Pathophysiology of heart failure. Clin Res Cardiol Suppl 2010;5:16-20
- Agvall B, Borgquist L, Foldevi M, et al. Cost of heart failure in Swedish primary healthcare. Scand J Prim Health Care 2005;23:227-32
- Mejhert M, Persson H, Edner M, et al. Epidemiology of heart failure in Sweden—a national survey. Eur J Heart Fail 2001;3:97-103. PubMed PMID:11163742
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260-9
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803-69
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9
- From AM, Borlaug BA. Heart failure with preserved ejection fraction: pathophysiology and emerging therapies. Cardiovasc Ther 2010;29:e6-21
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787-847
- Ringborg A, Yin DD, Martinell M, et al. The impact of acute myocardial infarction and stroke on health care costs in patients with type 2 diabetes in Sweden. Eur J Cardiovasc Prev Rehabil 2009;16:576-82
- Kjeldsen SE, Stalhammar J, Hasvold P, et al. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens 2010;24:263-73
- Carlsson A, Borgström F, Stålhammar J, et al. Cost of care for patients treated with lipid-lowering drugs. Pharmacoeconomics 2004;22(3 Suppl):25-35
- Ringborg A, Martinell M, Stålhammar J, et al. Resource use and costs of type 2 diabetes in Sweden – estimates from population-based register data. Int J Clin Pract 2008;62:708-16
- Kjeldsen SE, Stålhammar J, Hasvold P, et al. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Human Hypertension 2010;24:263-73
- Henriksson M, Russell D, Bodegard J, et al. Health-care costs of losartan and candesartan in the primary treatment of hypertension. J Human Hypertension 2010;25:130-6
- Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2001;3:97-103
- Rydén-Bergsten T, Andersson F. The healthcare costs of heart failure in Sweden. J Intern Med 1999;246:275-84
- Stalhammar J, Stern L, Linder R, et al. Resource utilization and cost of heart failure associated with reduced ejection fraction in Swedish patients. J Med Econ 2012;15:938-46
- Jonsson A, Edner M, Alehagen U, et al. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail 2010;12:25-31
- Kawashiro N, Kasanuki H, Ogawa H, et al. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circ J 2008;72:2015-20
- Hobbs FD, Roalfe AK, Davis RC, et al. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J 2007;28:1128-34
- Mehta PA, Dubrey SW, McIntyre HF, et al. Mode of death in patients with newly diagnosed heart failure in the general population. Eur J Heart Fail 2008;10:1108-16
- Lund LH, Benson L, Dahlstrom U, et al. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA: the Journal of the American Medical Association 2012;308:2108-17
- Campbell RT, Jhund PS, Castagno D, et al. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? Am Coll Cardiol 2012;60:2349-56
Appendix
Appendix A Time to subsequent post-index hospitalizations—HF-PEF patients (n = 131).
Appendix B All cause and heart failure related mortality.