Abstract
Background:
Venous thromboembolism (VTE), comprised of deep vein thrombosis (DVT) and pulmonary embolism (PE), is commonly treated with a low-molecular-weight heparin such as enoxaparin plus a vitamin K antagonist (VKA) to prevent recurrence. Administration of enoxaparin + VKA is hampered by complexities of laboratory monitoring and frequent dose adjustments. Rivaroxaban, an orally administered anticoagulant, has been compared with enoxaparin + VKA in the EINSTEIN trials. The objective was to evaluate the cost-effectiveness of rivaroxaban compared with enoxaparin + VKA as anticoagulation treatment for acute, symptomatic, objectively-confirmed DVT or PE.
Methods:
A Markov model was built to evaluate the costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios associated with rivaroxaban compared to enoxaparin + VKA in adult patients treated for acute DVT or PE. All patients entered the model in the ‘on-treatment’ state upon commencement of oral rivaroxaban or enoxaparin + VKA for 3, 6, or 12 months. Transition probabilities were obtained from the EINSTEIN trials during treatment and published literature after treatment. A 3-month cycle length, US payer perspective ($2012), 5-year time horizon and a 3% annual discount rate were used.
Results:
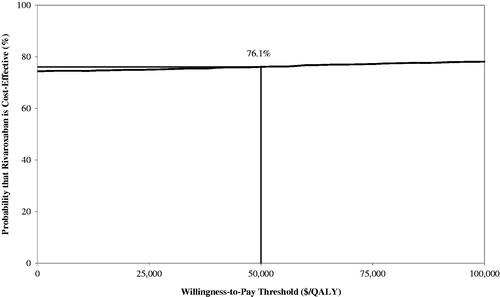
Treatment with rivaroxaban cost $2,448 per-patient less and was associated with 0.0058 more QALYs compared with enoxaparin + VKA, making it a dominant economic strategy. Upon one-way sensitivity analysis, the model’s results were sensitive to the reduction in index VTE hospitalization length-of-stay associated with rivaroxaban compared with enoxaparin + VKA. At a willingness-to-pay threshold of $50,000/QALY, probabilistic sensitivity analysis showed rivaroxaban to be cost-effective compared with enoxaparin + VKA approximately 76% of the time.
Limitations:
The model did not account for the benefits associated with an oral and minimally invasive administration of rivaroxaban. ‘Real-world’ applicability is limited because data from the EINSTEIN trials were used in the model. Also, resource utilization and costs were based on the US healthcare system.
Conclusion:
Rivaroxaban is a cost-effective option for anticoagulation treatment of acute VTE patients.
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) constitute venous thromboembolism (VTE), a health problem with approximately 900,000 annual incidence in the USCitation1. To minimize the risk of recurrence, VTE is frequently treated with a low-molecular-weight heparin (LMWH) (e.g., enoxaparin), followed by administration of a vitamin K antagonist (VKA) (e.g., warfarin). The enoxaparin + VKA regimen, however, requires laboratory monitoring, frequent dose-adjustment of the VKA, and may be complicated by drug and food interactions.
Rivaroxaban is an orally-administered anticoagulant that does not require laboratory monitoring, has no known food interactions, and presents fewer drug interactions compared with VKAsCitation2–4. The EINSTEIN DVT and PE trials compared rivaroxaban with enoxaparin + VKA in acute DVT and PE patients and found that rivaroxaban was non-inferior to enoxaparin + VKACitation5–7. The economic practicality of rivaroxaban in patients with VTE has not been considered. The aim of the current study was to evaluate the cost-effectiveness of rivaroxaban vs enoxaparin + VKA when used as anticoagulation treatment for patients with acute VTE.
Patients and methods
Markov state transition model
A Markov model was built to evaluate the cost-effectiveness of rivaroxaban compared with enoxaparin + VKA in adult patients with acute, symptomatic, objectively confirmed DVT or PE. The model base-case assumed 67% and 33% of patients suffered an index DVT and PE event, respectively, reflecting the distribution in clinical practiceCitation8. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) was followed in reporting the cost-effectiveness analysis of rivaroxaban compared with enoxaparin + VKACitation9.
Direct medical costs from a US payer’s perspective were considered, with future costs and effectiveness discounted at 3% per year. A 3-month model-cycle length with a 5-year time horizon was used for the base-case analysis. The primary effectiveness outcomes were quality-adjusted life-years (QALYs) with life-years (LYs), hospitalized days, VTE recurrence, and bleeds evaluated as secondary measures.
The Markov model was comprised of nine health states (). Patients progressed between the modeled health states according to transition probabilities derived from the EINSTEIN trialsCitation5,Citation6 and published literature (). ‘On-treatment’ included patients who experienced an index VTE and received 3, 6, or 12 months of anticoagulation treatment with rivaroxaban or enoxaparin + VKA. ‘Off-treatment’ indicated that patients no longer received rivaroxaban or enoxaparin + VKA. ‘Recurrent DVT’ and ‘recurrent PE’ included recurrent DVT and PE patients. ‘Major bleeds’ were categorized as intracranial (IC) and extracranial (EC), and non-major bleeds were categorized as ‘clinically-relevant non-major’ (CRNM). ‘Post-IC bleed’ referred to patients who previously experienced an IC bleed. ‘Death’ was a terminal state. Patients could die due to events captured in the model (e.g., PE or major bleed) or due to all-cause mortality. PE and major bleeds (IC and EC) were assumed to be associated with excess mortality. Patients in all states except death could develop mild/moderate or severe post-thrombotic syndrome (PTS).
Figure 1. VTE Markov model structure. Each rectangle represents a health state. Patients enter the model in the ‘On-treatment’ state. After each cycle, patients may move from one health state to the next, as indicated by the arrows, based on the transition probabilities from the EINSTEIN trials. DVT, Deep vein thrombosis; PE, Pulmonary embolism; CRNM, Clinically relevant non-major bleed; VTE, Venous thromboembolism.
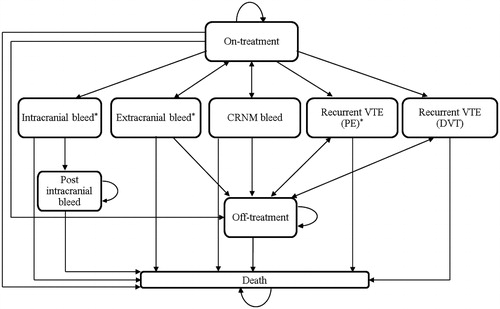
Table 1. Base-case model inputs and ranges used in sensitivity analyses.
Patient population
The modeled population was adults (i.e., ages were 56 and 58 years for DVT and PE patients at baseline, respectively) with confirmed DVT or PE. Patients entered the model in the ‘on-treatment’ state upon administration with either oral rivaroxaban alone (15 mg twice daily for 21 days, followed by 20 mg once daily starting on day 22) or subcutaneous enoxaparin for 8 days (1.0 mg per kg body weight twice daily, assuming a 80 kg patient) plus adjusted-dose VKA with a target international normalized ratio (INR) of 2.0–3.0, initiated concurrently with enoxaparin. The model assumed proportions of patients receiving 3, 6, and 12 months of anticoagulation treatment equal to those assigned in the EINSTEIN DVT (11.9%, 62.8%, and 25.3%, respectively) and PE (5.2%, 57.4%, and 37.4%, respectively) trialsCitation5,Citation6. Probabilities of discontinuing treatment due to bleeds and non-compliance were accounted for in ‘on-treatment’ periods and were derived from the EINSTEIN trialsCitation5,Citation6.
Transition probabilities
Since event risks change over time, a transition matrix, specifying different transition probabilities, was defined for time periods of 0–3 months (cycle 1), 3–6 months (cycle 2), 6–12 months (cycle 3 and 4), and 12+ months (cycles 5+). The probabilities reflected the highest risk of recurrent VTE (rVTE) during the first 3-month period, followed by a rapid decline thereafter. Transition probabilities for recurrent DVT and PE events were dependent on their respective index events. Model inputs related to transition probabilities are described in .
Baseline event rates with enoxaparin + VKA treatment
For the ‘on-treatment’ period, baseline event rates were derived from the enoxaparin + VKA-treated patients in the EINSTEIN trialsCitation5,Citation6. Probabilities were derived by dividing the number of events that occurred within each time period by the respective number at risk at the beginning of each period. The probabilities of events with rivaroxaban were computed from the hazard ratio (HR) or relative risk (RR) of rivaroxaban compared with enoxaparin + VKA.
Recurrent VTE (post-treatment)
The model assumed that ‘off-treatment’ patients were at risk of recurrent VTE that was independent of treatment used, but conditional upon the time from last anticoagulation treatment. The post-treatment risk of recurrence was obtained from the Prandoni et al.Citation10 study.
Post-thrombotic syndrome (PTS)
In the Markov model for the index DVT population, the impact of mild/moderate and severe PTS was modeled by applying the relevant costs and consequences to all states. For the index PE population, only patients who developed recurrent DVT were at risk of developing PTS. To account for greater incidence of PTS in the first year post-index-DVT, the probability of PTS diagnosis was differentiated for up to 1 year and beyond. Time spent with PTS was assumed to represent a percentage of the patient-time within post-DVT states. The cumulative incidences of PTS were obtained from Prandoni et al.Citation11.
Mortality
Event-related mortality was included for recurrent PE and major bleed events. No case fatality was assumed to be associated with recurrent DVT and CRNM bleeds. Mortality rates were assumed to be independent of treatment. For PE, the derived ‘overall PE mortality’ from pooled results across treatment arms in both EINSTEIN trials was used. For major bleeds, a review of mortality associated with bleeds in patients on anticoagulation therapy was usedCitation12. The model also accounted for all-cause mortality. Probabilities were based on the Centers for Disease Control and Prevention (CDC) US Life Tables, 2007, and the mean age at index VTE in the EINSTEIN DVT and PE trials (56 and 58 years, respectively)Citation5,Citation6,Citation13.
Utilities
To estimate quality-adjusted life-years (QALYs), each model state was associated with a utility weight obtained from the published literature (). The half-cycle correction was not applied because patients were initially treated for DVT or PE at the beginning of the cycle and immediately transitioned to subsequent health states before the end of the cycle. The duration of disutility was assumed to be 1 month for acute states such as VTE events and major bleeds and lifetime for chronic states such as post-IC bleed and PTS. The anchor point in modeling utility was the US population norm by Luo et al.Citation14 using the EQ-5D survey. Adjustments were made based on the published literatureCitation15–18 to obtain utility weights for each health state. The resulting utility weights can be found in the supplemental material section. The resulting utility weights can be found in the supplemental material section. The model conservatively assumed that the administration of enoxaparin + VKA was not associated with disutility. This assumption was tested in sensitivity analysis by varying the disutility associated with warfarin and enoxaparin administration to the range specified by Marchetti et al.Citation19.
Resource utilization rates
For index VTE, the proportions of patients hospitalized and the median length of stay (LOS) by type of VTE and anticoagulation treatment received were obtained from the EINSTEIN trialsCitation5–7. Non-hospitalized patients were assumed to have been managed in the outpatient setting. Enoxaparin + VKA-treated patients incurred costs for medications, enoxaparin administration, and office visits with INR monitoring. The number of office visits with INR monitoring was assumed to be eight during the first 3 months and three for subsequent cycles. Rivaroxaban-treated patients incurred costs for medications and office visits.
For recurrent VTE, 50% of recurrent DVT events and 90% of recurrent PE events were managed in an inpatient setting, with the remaining managed in an outpatient settingCitation5–7. Hospitalized events were assumed to incur the direct medical costs of an inpatient stay with principal diagnosis codes for either DVT or PECitation20. Outpatient events were assumed to require the same resources as outpatient index events. Each recurrent VTE event was assumed to require 12 months of enoxaparin + VKA, including medications, enoxaparin administration, office visits with INR monitoring, and the insertion and removal of a retrievable inferior vena cava (IVC) filter performed in an outpatient setting among 76% of the patient populationCitation21. Resource utilization inputs are described in .
Table 2. Model inputs for resource utilization and unit costs and ranges used in sensitivity analyses.
Unit costs
Expected costs and effectiveness were calculated based on time spent in each health state over the modeled time horizon by treatment cohort. The unit cost of rivaroxaban was obtained from AnalySource22, while the unit costs of enoxaparin and warfarin were obtained from Redbook23. For the base-case, all patients were assumed to take generic enoxaparin and warfarin. The unit costs of other resources were identified by using applicable Current Procedural Terminology (CPT) and International Statistical Classification of Diseases, 9th Revision (ICD-9) codes and inflated to 2012 US dollarsCitation24. Costs incurred in each health state are described in .
Sensitivity analyses
The impact of uncertainty around model inputs was assessed using deterministic (one-way) and probabilistic sensitivity analyses (PSA). One-way sensitivity analysis was conducted by changing the base-case to lower and upper bound values found in and . PSA was performed by taking a random sample from the assumed input parameter distributions, calculating QALYs and $/QALY, and repeating the process 1000 times. The resulting 1,000 model-outcomes were plotted on a cost-effectiveness plane and assessed using a willingness-to-pay threshold of $50,000/QALY.
For the PSA, independent beta distributions were assumed for probabilities, proportions, and utility weights, with alpha and beta simulated such that the base-case value was the point estimate, and a certain percentage (50% for interquartile range and 95% otherwise) of the random samples fell within the lower and upper range. Log-normal distributions were assumed for HRs and RRs associated with rivaroxaban defined by the mean and SE observed in the EINSTEIN trialsCitation5–7,Citation25,Citation26. Gamma distributions were assumed for resource utilization parameters and unit costs.
Results
Over the 5-year time horizon, the discounted total direct medical cost associated with rivaroxaban was lower than that associated with enoxaparin + VKA by $2,448 per patient ($13,806 vs $16,253). As presented in , similar findings were observed for the 1-year time horizon model.
Table 3. Direct medical costs per patient for all DVT and PE patients.
For all effectiveness measures, rivaroxaban was associated with better effectiveness than enoxaparin + VKA. Over the 5-year time horizon, the discounted QALYs gained per patient on rivaroxaban were 3.8486, while the discounted QALYs gained per patient on enoxaparin + VKA were 3.8427, suggesting that rivaroxaban was associated with 0.0058 more QALYs gained than enoxaparin + VKA. Rivaroxaban was also associated with an increase in life-years (4.4989 vs 4.4928), VTE-free life-years (4.4316 vs 4.4242), and bleed-free life-years (4.4771 vs 4.4702), and a reduction in the number of hospitalized days (4.9266 vs 6.4321), recurrent VTE events (0.2694 vs 0.2746), and bleeding events (0.0874 vs 0.0906) compared with enoxaparin + VKA.
The resulting incremental cost-effectiveness ratios (ICERs) for all effectiveness measures presented in , including those pertaining to QALYs, LYs, and other outcomes, were dominant for rivaroxaban compared with enoxaparin + VKA.
Table 4. Incremental cost-effectiveness ratios.
Sensitivity analysis
One-way sensitivity analysis showed that the base-case model result of rivaroxaban being dominant over enoxaparin + VKA was robust. The only variation that influenced dominance was LOS for the index VTE when LOS was longer for rivaroxaban compared with enoxaparin + VKA. When rivaroxaban index LOS was longer by 1 day, the direct medical cost associated with rivaroxaban was higher than enoxaparin + VKA by $1,118, with 0.0058 more QALYs gained than enoxaparin + VKA (ICER = $192,071/QALY). When no difference in LOS between rivaroxaban and enoxaparin + VKA was assumed, the direct medical cost associated with rivaroxaban was lower by $79, therefore dominant over enoxaparin + VKA. In the PSA model, rivaroxaban was found to be cost-effective compared with enoxaparin + VKA approximately 76% of the time ( and ).
Figure 2. Incremental cost-effectiveness plane. The graph presents the results of the probability sensitivity analysis of rivaroxaban vs enoxaparin + VKA over a 5-year time horizon. The data points represent the 1,000 simulated incremental cost on the y-axis and incremental QALY on the x-axis. The area below the willingness-to-pay line represents rivaroxaban being cost-effective at the threshold level.
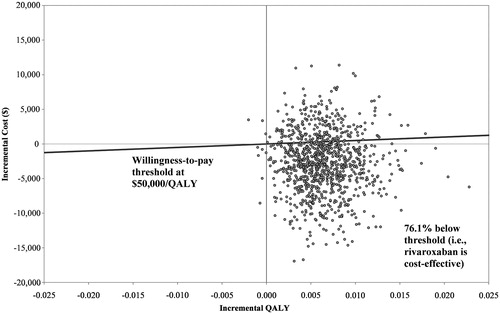
Figure 3. Cost-effectiveness acceptability curve. The curve plots the probability that rivaroxaban is cost-effective compared with enoxaparin + VKA against the willingness-to-pay threshold range. The acceptability curve depicts the proportion of the joint probability density is below the willingness-to-pay threshold.
Discussion
The Markov model demonstrated that rivaroxaban was associated with lower direct medical costs and a marginally higher effectiveness than enoxaparin + VKA (0.0058 QALYs). Because the difference in QALYs was marginal, reflecting equivalence in efficacy, a cost minimization analysis was warranted. The cost minimization analysis showed that the incremental cost between rivaroxaban and enoxaparin + VKA was $2,448 less for a patient treated with rivaroxaban. When efficacy (e.g., recurrence of PE and DVT) and safety (e.g., major bleeds) rates were varied in the one-way sensitivity analyses, patients treated with rivaroxaban were observed to have a lower cost and more QALYs gained than patients treated with enoxaparin + VKA. In addition, the model showed that rivaroxaban also averted outcomes such as the number of hospitalized days related to VTE and bleed events.
Hospital LOS contributes to the clinical and economic burden of VTE; hence, selection of an effective treatment regimen that reduces LOS is an important factor for clinicians and healthcare systems. A key driver of the model’s results was the hospital LOS associated with the index VTE. The median hospital LOS was significantly shorter among rivaroxaban-treated patients than the enoxaparin + VKA-treated patients by 3 days (p < 0.0001) in the EINSTEIN-DVT trial and by 1 day (p < 0.0001) in the EINSTEIN-PE trialCitation7. This difference in hospital LOS observed in the EINSTEIN trials between patients treated with rivaroxaban alone and enoxaparin +VKA is also supported by the literatureCitation27–29. For example, Perez-de-Llano et al.Citation28 open-label trial evaluated tinzaparin in symptomatic patients with acute PE as compared with standard therapy over a 6-month follow-up period, while Beckman et al.Citation29 randomized PE patients to receive enoxaparin or unfractionated heparin for 3 months. The median difference in LOS of 1 day observed in the EINSTEIN PE trial was comparable with Perez-de-Llano et al.Citation28, reporting a median difference in LOS of 2 days in PE patients, and Beckman et al.Citation29, reporting a LOS reduction of 2 days without the administration of warfarin. Of note, rivaroxaban was still found cost-effective in the model when assuming no difference in the index hospital LOS between rivaroxaban and enoxaparin + VKA. Furthermore, in the PSAs when rivaroxaban-treated patients were allowed to have a longer index VTE hospital LOS than enoxaparin + VKA-treated patients, the probability of rivaroxaban being cost-effective was 76% over a 5-year time horizon.
In addition to reducing index LOS, reduction in hospitalization may be observed, specifically, in DVT patients by administering regimens that do not require bridging with heparin to warfarin. The number of hospital admissions is minimized, as outpatient treatment is available for rivaroxaban because of the oral administration. This economic benefit of fewer hospitalizations is supported by a recent randomized non-inferiority trial at 19 emergency departments in Switzerland, France, Belgium, and the USCitation30. Aujesky et al.Citation30 reported that acute PE patients treated with enoxaparin followed by oral anticoagulation in the outpatient setting had a 3.4 day reduction in mean LOS compared with patients treated in the inpatient setting for VTE. In addition, patients were not required to wait for therapeutic INR monitoring.
Limitations
Several limitations to the study should be noted. First, data pertaining to the risks of events among patients ‘on and off treatment’ were taken from the EINSTEIN trials and published observational studies. Applying risks from two different study designs in the model may bias the risk towards a null effect. Secondly, the method of administration (i.e., oral vs subcutaneous injection) may affect patients’ utility. The additional benefits associated with an oral and minimally invasive administration that rivaroxaban provides were not accounted for in the model. Disutility associated with the administration of enoxaparin and warfarin was only included in the one-way sensitivity analysis, but did not fully capture the potential convenience and reduction in burden for both patients and clinicians. Thirdly, since data from the EINSTEIN trials were used in the model and patients in clinical trial settings received continuous care and close monitoring, the ‘real-world’ applicability may be limited. For example, the time-in-the-therapeutic INR range in the real-world setting might be different than that observed in EINSTEIN trials (57% and 58–63% time-in-the-therapeutic INR range, respectivelyCitation31). Furthermore, chronic thromboembolic pulmonary hypertension (CTPH), a serious complication of PE, was not accounted for in the model, which may impact resource utilization and cost given that the cumulative incidence rate was 3.8%Citation32. Another limitation is that costs were based on the US healthcare system, which may over-estimate the cost of care because efficacy and resource utilization were taken from the EINSTEIN trials which were conducted in an international setting. Finally, the model was based on the CDC US Life Tables, 2007, which was available at the time the model was developed. Since then, CDC US Life Tables, 2008 was published. Despite these limitations, direct medical costs from the cost-effectiveness model were consistent with the reported literature for VTE treatmentCitation33,Citation34, and sensitivity analyses did not affect the dominance of rivaroxaban over enoxaparin + VKA for most scenarios.
Conclusions
The updated 2012 American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines recommend initial parenteral anticoagulant therapy (Grade 1B) or anticoagulation with rivaroxaban for acute DVT or PECitation35. The ACCP recommendations along with the findings of this study (i.e., rivaroxaban was cost-effective relative to enoxaparin + VKA) provide additional support to decision-makers and clinicians for the considerations of integrating rivaroxaban in the treatment of patients with VTE.
Transparency
Declaration of funding
Financial support for this study was provided by Janssen Scientific Affairs, LLC.
Declaration of financial/other relationships
PL, S-TW, KNT, DYZ, and LH are employees of Analysis Group, Inc., a consulting company that has received research grants from Janssen Scientific Affairs, LLC. BKB and SHM are employees of Janssen Scientific Affairs, LLC. CIC and EAN have received research funding from Janssen Scientific Affairs, LLC.
Supplementary material available online
Supplementary Material
Download PDF (27.1 KB)Acknowledgments
No assistance in the preparation of this article is to be declared.
Notes
References
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008;28:370-2
- Eriksson BI, Kakkar AK, Turpie AG, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg 2009;91:636-44
- Kubitza D, Becka M, Voith B, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. J Clin Pharmacol Therapeut 2005;78:412-21
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673-80
- Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510
- Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97
- van Bellen B, Prins M, Bamber L, et al. Reduction in initial length of stay with rivaroxaban single-drug regimen versus LMWH-VKA standard of care: findings from the EINSTEIN trial program. Atlanta, GA: American Society of Hematology [Poster presentation], 2012. https://ash.confex.com/ash/2012/webprogram/Paper51519.html. Accessed December 10, 2012
- Killeen M, Hughes M. Venous thromboembolism. Cardium Study: a pharmacor service. 2012. Burlington. MA: Decision Resources. http://decisionresources.com/Products-and-Services/Report?r=pcorcv0412. The report was received on February 11, 2012.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 2013;16:e1-5
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1626 patients. Haematologica 2007;92:199-205
- Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997;82:423-8
- Linkins L, O'Donnell M, Julian JA, et al. Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost 2010;8:2201-7
- Arias E. United States Life Tables, 2007. September 28, 2011. National Vital Statistics Reports; No. 59, No. 9. 9. Hyattsville, MD: National Center for Health Statistics. 2011. http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_09.pdf. Accessed July 31, 2012
- Luo N, Johnson JA, Shaw JW, et al. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. J Med Care 2005;43:1078-86
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc 1997;4:49-56
- Locadia M, Bossuyt PM, Stalmeier PF, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients' health state valuations and treatment preferences. J Thromb Haemost 2004;92:1336-41
- van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. J Lancet Neurol 2010;9:167-76
- Xie J, Wu EQ, Zheng ZJ, et al. Impact of stroke on health-related quality of life in the noninstitutionalized population in the United States. Stroke J Cereb Circ 2006;37:2567-72
- Marchetti M, Pistorio A, Barone M, et al. Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med 2001;111:130-9
- National statistics - principal diagnosis only. Outcomes by ICD-9-CM diagnosis codes. Rockville, MD: Agency for Healthcare Research and Quality. 2012. http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed August 31, 2012
- Kaufman JA, Kinney TB, Streiff MB, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interventional Radiology 2006;17:449-59
- AnalySource. AnalySource online: the online resource for drug pricing and deal information [online]. Available from http://www.analysource.com. Accessed January 3, 2013
- Red Book. Truven Health Analytics: Average Wholesale Price (AWP) [online]. Available from http://sites.truvenhealth.com/redbook/index.html. Accessed October 18, 2012
- Consumer Price Index, All Urban Consumers, Medical care -- Not Seasonally Adjusted. Washington, DC: United States Bureau of Labor Statistics. 2012. http://data.bls.gov/cgi-bin/surveymost?cu. Accessed August 12, 2012
- The EINSTEIN PE Investigators. Oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic pulmonary embolism. The EINSTEIN PE Study. Bayer HealthCare and Janssen Research & Development, 2012
- The EINSTEIN DVT Investigators. Oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic deep-vein thrombosis: The EINSTEIN DVT Study. Bayer HealthCare & Ortho McNeil Pharmaceuticals, 2010
- Das SK, Cohen AT, Edmondson RA, et al. Low-molecular-weight heparin versus warfarin for prevention of recurrent venous thromboembolism: a randomized trial. World J Surg 1996;20:521-6; discussion 526-7
- Perez-de-Llano LA, Leiro-Fernandez V, Golpe R, et al. Comparison of tinzaparin and acenocoumarol for the secondary prevention of venous thromboembolism: a multicentre, randomized study. Blood Coagul Fibrinolysis 2010;21:744-9
- Beckman JA, Dunn K, Sasahara AA, et al. Enoxaparin monotherapy without oral anticoagulation to treat acute symptomatic pulmonary embolism. Thromb Haemost 2003;89:953-8
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41-8
- Cios DA, Baker WL, Sander SD, et al. Evaluating the impact of study-level factors on warfarin control in U.S.-based primary studies: a meta-analysis. Am J Health Syst Pharm 2009;66:916-25
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64
- Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm 2007;13:475-86
- Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm 2005;11:663-73
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis. 9th edn. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(2 Suppl):e419S-94S
- American Medical Association. CodeManager Online: Average 2011 Medicare's relative value payment amounts for CPT codes. 99201--99205, 99211--99215, 93970--93971, 71275, 71010, 71015, 71020--71023, 71030, 71034, 71035, 93005, 93010, 37191, 37193, 30901. Atlanta, GA: American Medical Association. 2011. https://commerce.ama-assn.org/ocm/index.jsp. Accessed August 31, 2012
- Professional Services Fee Schedule - Medicine Fees (2011) for CPT code 99601. Olympia, WA: Washington State Department of Labor & Industries. 2011. http://www.lni.wa.gov/ClaimsIns/Providers/Billing/FeeSched/2011/default.asp. Accessed September 4, 2012
- Clinical Diagnostic Laboratory Fee Schedule for the CPT code 85610. Baltimore, MD: Centers for Medicare & Medicaid Services. 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed September 4, 2012
- National Health Care Expenses in the U.S. Civilian Non-institutionalized Population, 2009. Statistical Brief # 355. Rockville, MD: Agency for Healthcare Research and Quality. 2012. http://meps.ahrq.gov/mepsweb/data_files/publications/st355/stat355.pdf. Accessed September 4, 2012
- Lee WC, Christensen MC, Joshi AV, et al. Long-term cost of stroke subtypes among Medicare beneficiaries. Cerebrovasc Dis 2007;23:57-65
- Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6:59-74
