Abstract
Objective:
To provide evidence on recent trends in: (1) market exclusivity periods (MEPs, the time between launch of a brand-name drug and its first generic competitor) for new molecular entities (NMEs); (2) the likelihood and timing of patent challenges under Paragraph IV of the Hatch-Waxman Act; and (3) generic drug penetration.
Methods:
IMS Health National Sales Perspectives data were used to calculate MEPs for the 257 NMEs experiencing initial generic entry between January 1995 and September 2012 and the number of generic competitors for 12 months afterwards, by level of annual sales prior to generic entry and time period. The likelihood and timing of Paragraph IV challenge were calculated using data from Abbreviated New Drug Approval (ANDA) approval letters, the FDA website, and public information searches to identify drugs experiencing Paragraph IV filings, and the first filing date.
Results:
For drugs experiencing initial generic entry in 2011–2012, the MEP was 12.6 years for drugs with sales greater than $100 million (in 2008 dollars) in the year prior to generic entry, 12.9 years overall. After generic entry, the brand rapidly lost sales, with average brand unit share of 16% at 1 year; 11% for NMEs with pre-generic entry sales of at least $250 million (in 2008 dollars). Over 80% of NMEs experiencing 2011–2012 initial generic entry had faced at least one Paragraph IV challenge from a generic manufacturer. These challenges were filed relatively early in the brand-name drug life cycle: within 7 years after brand launch, on average.
Limitations:
Analyses, including Paragraph IV calculations, were restricted to NMEs where generic entry had occurred.
Conclusion:
Pharmaceutical competition continues to evolve; while the average MEP below 13 years for 2011–2012 remains consistent with prior research, Paragraph IV challenges are increasingly frequent and occur earlier, and generic share erosion has intensified.
Introduction
Pharmaceutical competition has evolved considerably since the passage in 1984 of the Hatch-Waxman Act amending the Federal Food, Drug, and Cosmetic Act, with dramatic increases in rates of both generic penetration and patent litigation. Congress’s objective when enacting the legislation was to increase generic competition while balancing the resulting cost savings with sufficient incentives to encourage continued medical innovation through the development of new drugs. The result has been a system where new brand-name drugs generate nearly all of their sales during a market exclusivity period (MEP, the time period between market launch of a brand-name drug and the launch of its first generic), generic manufacturers frequently challenge patents protecting brand-name drugs, and generic drugs tend to rapidly supplant sales of the corresponding brand-name drug following generic entry.
The Hatch-Waxman Act included a number of provisions aimed at facilitating approval of generic drugs by the Food and Drug Administration (FDA) and encouraging generic entry (and other provisions encouraging innovation described later below). One of the primary provisions of the Act established an Abbreviated New Drug Application (ANDA) process, which greatly reduced the cost of completing an FDA application for approval of a generic drug. Prior to the Hatch-Waxman Act, generic manufacturers were required to submit original safety and efficacy data on their products to gain market approval by the FDA. To meet this requirement, the generic manufacturer generally had to duplicate many of the brand-name manufacturer’s trialsCitation1. Under the ANDA process, generic manufacturers need only demonstrate that their products have the same active ingredients as and are ‘bioequivalent’ to their brand-name counterparts. Generic manufacturers also received a research exemption for bioequivalence studies, allowing them to begin research on the innovator’s drug prior to patent expiration, without running afoul of patent law.
The Hatch-Waxman Act also created incentives for generic manufacturers to challenge brand-name patents before they expired. For example, under a so-called Paragraph IV challenge, the generic manufacturer files a Paragraph IV ANDA, notifying the FDA that it claims its generic product does not infringe on a listed patent on the brand-name drug, or that a patent held on the brand-name drug is not valid. If the brand-name drug manufacturer files a patent infringement action against the generic company within 45 days of receiving notice of the Paragraph IV ANDA being filed, the FDA cannot approve the generic company’s ANDA until the company prevails in court or through settlement, or until a 30-month stay expires, whichever comes first. However, as an incentive for generic manufacturers to challenge brand-name patents, the first generic manufacturer (or manufacturers) to file a Paragraph IV challenge and to receive FDA final approval of its application, resulting in entry prior to patent expiration, is granted a 180-day period of exclusivity. During this 180-day period its drug is the only ANDA-approved generic version allowed on the market. Paragraph IV challenges can be made at the dosage form or strength level. The first to file a Paragraph IV challenge is determined by the day of filing, and multiple generic manufacturers can share first-to-file status if they file on the same day. A generic manufacturer’s 180-day exclusivity period applies only to the dosage form or strength level for which that manufacturer was the first to file a Paragraph IV challenge.
The 180-day period of exclusivity generally is very profitable to a generic manufacturer because the generic manufacturer tends to drop price only modestly below the brand price during this period, generic share increases rapidly, and generic sales are enjoyed by a single manufacturer (or a few first-filing manufacturers). This provides substantial incentives for being the first to file a Paragraph IV challenge, or being among the first-filers.
In an effort to balance these provisions aimed at encouraging more generic competition for brand-name drugs, the Hatch-Waxman Act also established new incentives for innovation for brand-name drug manufacturers. For example, innovators can receive an additional period of patent protection through so-called patent term restoration. This provision extends the life of a patent on a drug by up to 5 years, with the aim of compensating for time that the innovator company spent conducting human clinical trials on the drug before it applied to the FDA for approval of the drug through a New Drug Application (NDA) and also for a portion of the time the NDA is under FDA review. Under patent term restoration, the life of the patent cannot be extended by more than 5 years, and the remaining patent term after FDA approval cannot exceed 14 years, including the extension.
In addition to patent term restoration, innovative brand-name drugs are also protected from early ANDA filings through a data exclusivity provision in the Hatch-Waxman Act. Data exclusivity runs concurrently with patent protection and restricts the FDA from receiving a generic application that relies on a brand-name drug’s safety and efficacy data for 5 years following that drug’s approval (unless there is a Paragraph IV challenge, in which case it is 4 years).
Under the Hatch-Waxman Act, therefore, the MEP for new brand-name drugs reflects, among other factors, the combined impact of provisions aimed at facilitating earlier generic entry and other provisions aimed at maintaining incentives for innovation.
The use of generics has increased substantially since the mid-1990s, in part because of increases in the mechanisms available to promote generic use, including incentives in commercial insurance plans and public coverage, such as tiered formularies with lower patient co-payments for generic than for brand-name drugs, and restricting formulary coverage to generics in certain therapeutic categoriesCitation2,Citation3. In addition, a number of state laws allow generic forms to be substituted automatically by pharmacists for brand-name drugs prescribed by physicians, so long as physicians have not specified that the prescription must be ‘dispensed as written’. As a result, generic products’ share of total prescriptions in the US increased from 36% in 1994 to 84% in 2012Citation4.
The impact of Hatch-Waxman on incentives to innovate has received somewhat less attention. A 1998 report by the Congressional Budget Office estimated that generic competition reduces by 12% the net present value of the total stream of future profits expected from the average brand-name drug. In particular, the Congressional Budget Office found that, for brand-name drugs, the negative effects on returns from generic competition probably outweighed the positive effects of patent term restoration, described aboveCitation5.
The objective of this study was to provide evidence on recent trends in three factors that have a potentially substantial influence on the balance of cost savings and incentives for continued innovation in the form of new drugs: (1) MEPs for new brand-name drugs; (2) the likelihood and timing of Paragraph IV patent challenges under the Hatch-Waxman Act, through which generic manufacturers challenge the validity or applicability of patents protecting brand-name drugs; and (3) the rate and extent of generic drug penetration following initial generic entry.
The findings presented extend and expand upon research originally conducted by Grabowski and KyleCitation6 and updated in Grabowski et al.Citation7, which evaluated data on MEPs for all new molecular entities (NMEs) experiencing initial generic entry between 1995 and 2008. Here, we further extend those prior analyses to include data on all NMEs experiencing initial generic entry through September 2012, allowing for evaluation of trends over a long period of time for key features of generic competition. We collected and analyzed new data from 2007 through September 2012, allowing for corroboration of the 2007 and 2008 period considered in prior analyses, while also extending the time period covered through September 2012.
Patients and methods
Data sources
IMS Health National Sales Perspectives data were used for calculating MEPs for drugs experiencing first generic entry between January 2007 and September 2012. This is the same data source relied on for prior analyses, and the data obtained for this study was merged with similar data for the time period 1995–2006. The data set used in the analysis contained information about all 460 drugs experiencing first generic entry during this period, including 257 NMEs, and 203 new formulations of older drugs. New formulations include changes in the form of administration—for example, changing from an injection to a topical application—but not new strengths or new indications. We excluded several products from the analysis based on the following criteria: one product was excluded because generic versions of it were subsequently withdrawn as a result of litigation following initial entry; and seven products were excluded because the original brand FDA approval pre-dated October 1962 and the requirements for safety and efficacy data introduced at that time. Our analysis focused on NMEs and we present data only on the 257 NMEs experiencing initial generic entry between January 1995 and September 2012 because regulations for generic entry differ if the brand-name product is a new formulation.
In addition to providing the information necessary to calculate MEPs, the data also included information on drug characteristics, such as mode of administration and number of generic entrants. All sales data are presented in 2008 dollars, adjusted using the US Department of Labor’s Consumer Price Index for All Urban Consumers as the market deflator.
We supplemented the market exclusivity data with a detailed review of information from the FDA’s website on Paragraph IV ANDA filingsCitation8. We also analyzed ANDA approval letters in the study period and searched other public information including company press releases, court documents, and industry trade reports, to identify all of the drugs in the data set that experienced Paragraph IV ANDA filings, and the date of the first filing for each drug. Our data contained only drugs that experienced generic entry (i.e., drugs that have experienced a Paragraph IV ANDA filing but where generic entry has not yet occurred are not included in our analysis).
For the sub-set of drugs in our sample that experienced first generic entry between 1999 and September 2012, we reviewed IMS Health National Sales Perspectives monthly data on standard units separately for the brand-name drug and for generic versions of the drug. Monthly sales data were not available for drugs experiencing first generic entry prior to 1999. The data were used to calculate the monthly erosion of brand-name drugs’ share of standard units for the 12 months following first generic entry. The extent of brand-name drug share erosion is summarized based on the timing of first generic entry, illustrating the increasing extent of brand-name drug erosion for drugs more recently experiencing first generic entry.
Methods
Consistent with prior research, we defined the MEP as the time between the launch of a brand-name drug and the launch of its first generic competitor. As noted, this definition reflects the often complex interaction among many technical, regulatory, and competitive factors, including: the timing of patent filings, the amount of patent term lost during product development, the duration of regulatory review before FDA approval, the eligibility for patent term restoration under the Hatch-Waxman Act, the likelihood and outcome of generic patent challenges (including the possibility of a stay on generic entry for up to 30 months pending court decisions on patent infringement suits), entry decisions by generic manufacturers, and the duration of FDA review of generics. Any one or a combination of these factors can affect the market exclusivity of a particular drug. The average MEP remains a key determinant of profitability and incentives for innovation.
The average number of generic entrants within 1 year of first generic entry was calculated by level of sales (i.e., less than $100 million, greater than or equal to $100 million and less than $250 million, greater than or equal to $250 million and less than $1 billion, $1 billion or larger), based on sales in the year prior to generic entry and inflation-adjusted to 2008 dollars using the US Department of Labor Consumer Price Index for All Urban Consumers, and by time period of initial generic entry (i.e., 1995–1998, 1999–2003, 2004–2008, 2009–2011).
Paragraph IV filing frequency was calculated as the percentage of NMEs experiencing at least one Paragraph IV filing at any time prior to first generic entry, by year of first generic entry. Paragraph IV timing was calculated as the average time from NME launch to first Paragraph IV filing.
The generic penetration rate was defined as the share of units of the drug that are filled by a generic version of the drug rather than the brand-name drug. Generic penetration rates reflect market factors, an increase over time in the mechanisms available to commercial insurance and public plans to promote generic use, as well as state regulations and laws.
All figures presented are unweighted averages. Figures in parentheses following calculated averages are standard deviations. Figures for Paragraph IV filing frequency and timing (as presented in Exhibit 3) are 3-year moving averages.
Results
Average period of market exclusivity
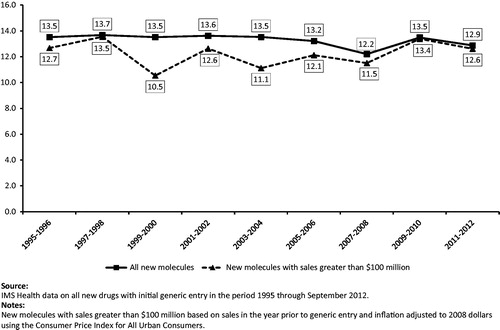
shows the average length of the market exclusivity period for all new drugs, by year of first generic entry, and for those with annual sales greater than $100 million in the 12 months prior to generic entry (in 2008 dollars). Between 1995–2012, the average MEPs for all drugs experiencing first generic entry ranged between 12.2–13.7 years over the period.
Drugs with annual sales greater than $100 million (in 2008 dollars) in the year prior to generic entry represent 54% of all drugs and 97% of sales for all drugs in our data set experiencing generic entry. The average MEP for these higher-revenue drugs was 12.6 years in the most recent period in our study (2011–2012). The average length of exclusivity for all NMEs for 2011–12 was 12.9 years, compared to 13.5 years in the initial period (1995–1996). Figures for each cohort of NMEs, as defined by year of first generic entry, are presented in .
Table 1. Average market exclusivity period by year of first generic entry.
Average MEPs were similar whether we analyzed drugs with annual sales greater than $100 million, $250 million, or $1 billion (in 2008 dollars, data not shown).
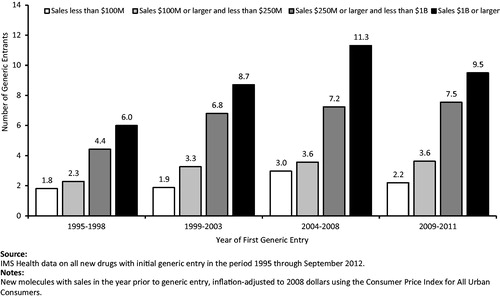
summarizes the number of generic entrants observed for NMEs in the data. The exhibit shows the average number of a brand-name drug’s generic competitors in the market 12 months after the first generic entry, segmented by level of sales and by time period. The number of generic entrants is higher for drugs with larger sales before the first generic entry and for drugs experiencing first generic entry after 1998. For example, one drug with over $1 billion in annual sales prior to generic entry experienced first generic competition in the period 1995–1998 and it faced six generic entrants after 1 year. The corresponding figures for drugs with over $1 billion in annual sales (in 2008 dollars) prior to generic entry were an average of between eight and nine generic entrants for the period 1999–2003, between 11 and 12 for 2004–2008, and between nine and 10 for 2009–2011. Similar trends in the number of generic entrants were experienced for drugs with under $1 billion in sales.
Paragraph IV challenges
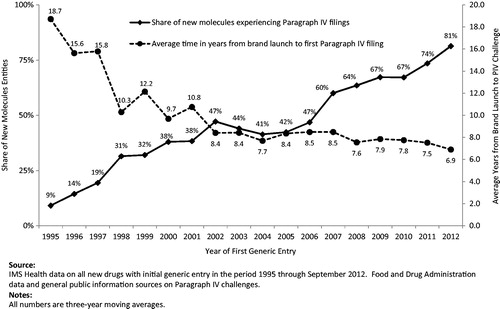
The likelihood of a Paragraph IV challenge being filed has increased substantially in recent years (). Only 9% of drugs experiencing first generic entry in 1995 had experienced a Paragraph IV challenge at any point prior to first generic launch. That share increased steadily to 81% by drugs experiencing first generic entry in 2012. Drugs with sales greater than $100 million the year before first generic entry (in 2008 dollars) faced an even higher probability of a Paragraph IV filing, increasing from 17% in 1995 to 84% in 2012 (data not shown).
Paragraph IV challenges also occur sooner following the launch of a brand-name drug (). For drugs experiencing first generic entry in 1995 and also experiencing a Paragraph IV challenge, the average time between launch and the first Paragraph IV challenge was 18.7 years (6.2). That time fell to 6.9 years (3.4) for drugs experiencing first generic entry in 2012. For new drugs with sales greater than $100 million in the 12 months before first generic entry (in 2008 dollars), the time between brand launch and first Paragraph IV challenge fell from 14.3 years (one drug) in 1995 to 5.9 years (2.7) for 16 drugs in 2012 (data not shown). Paragraph IV challenge activity is even more aggressive for new drugs with sales greater than $250 million (in 2008 dollars). Of these drugs that experienced first generic entry in 2012, 92% also experienced a Paragraph IV challenge (13 of 14 drugs), and the average time from launch to first challenge was 6.3 years (3.0).
The calculations reflected in reflect averages across all new drug introductions associated with first generic entry in a given year. They may vary according to factors such as the drug’s sales prior to generic entry, the nature of the patents protecting the drug, and the ease with which generic manufacturers can imitate the drug to satisfy FDA regulations. In particular, for higher-revenue drugs, generic manufacturers may be less selective when filing challenges, as even a low likelihood of success in litigation can yield a large expected return on the investment necessary to challenge a patent. For instance, researchers have found that court decisions on Paragraph IV challenges filed prior to 2005 involved a disproportionate share of high-revenue drugsCitation9. Others have calculated that, as brand-name drug revenue increases, the probability of success required to justify a patent challenge by a generic manufacturer diminishes to below 1%Citation10. For patent cases litigated to district court decisions between 2000–2009, brand companies prevailed in 52% of themCitation11; for cases decided by district courts between 2009–2012, they prevailed in 54% of themCitation12.
Market share erosion after generic entry
shows the erosion in brand-name drugs’ share for the 12 months following first generic entry, with share defined as the unit share of brands for a given molecule divided by the sum of units for brands and their corresponding generics (brand-name share of total molecule units, which may have increased, decreased or remained relatively constant). Generic erosion has increased dramatically over the past decade. For all NMEs facing first generic entry in 2011–2012, brands retained an average of only 16% (0.13) of units at 1 year. For those NMEs with sales greater than $250 million (in 2008 dollars) prior to first generic entry, generic erosion was even more pronounced; the corresponding figure was only 11% (0.09) of units at 1 year.
Figure 4. Average monthly brand share of standard units of the molecule/form following first generic entry.
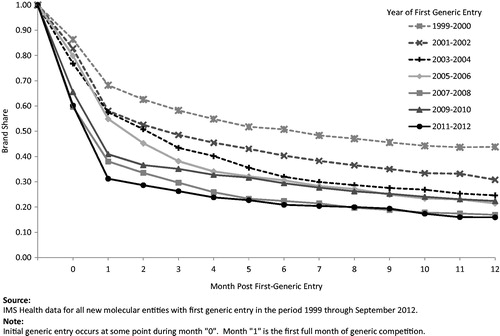
In comparison, drugs experiencing first generic entry in 1999–2000 maintained a share of 44% (0.28) of units at 1 year following first generic entry. Grouping drugs into 2-year periods by the date of first generic entry illustrates the steady increase in the rate and extent of generic penetration over the past decade.
Discussion
Consistent with prior research, MEPs for drugs experiencing initial generic entry in 2011–2012 was 12.6 years for NMEs with sales greater than $100 million (in 2008 dollars) in the year prior to generic entry, and 12.9 years for all NMEs. Further research may reveal variation by type of NME, whether defined by molecule type or other classification.
Generic competition has intensified over the past 10–15 years, and the MEP has become an even more important indicator of the economics of brand-name drugs. The MEP is critical to manufacturers’ ability to earn profits on brand-name drugs to fund future research and development activities, and brand-name drug shares rapidly drop following initial generic entry. For NMEs experiencing initial generic entry in 2011–2012 and with pre-generic entry sales of at least $250 million (in 2008 dollars), average brand unit share had fallen to 11% at 1 year; for all NMEs with initial generic entry in 2011–2012, average brand unit share at 1 year had fallen to 16%.
While the average MEP for brand-name drugs has remained relatively constant over the past 10–15 years, generic manufacturers are challenging the patents protecting brand-name drugs more often and earlier, which may have a downward impact on future MEPs (we calculate MEPs only for those already experiencing generic entry). Over 80% of brand-name drugs experiencing initial generic entry in 2012 had faced at least one Paragraph IV patent challenge from a generic manufacturer, up from only 9% for drugs experiencing initial generic entry in 1995. These challenges are filed relatively early in the brand drug life cycle, on average within 7 years of brand launch.
Conclusions
Pharmaceutical competition continues to evolve since the passage of the Hatch-Waxman Act in 1984. While the average MEP for brand-name drugs, currently 12.6 years for NMEs with pre-generic entry sales of $100 million (in 2008 dollars) and 12.9 years for all drugs, has remained relatively constant, consistent with prior research, other factors have changed. Over the past decade, Paragraph IV challenges have become increasingly frequent and occur earlier, and generic share erosion has intensified.
Transparency
Declaration of funding
The authors received financial support from the Pharmaceutical Research and Manufacturers of America for this research. The analysis presented was designed and executed entirely by the authors, and any errors or misstatements are ours alone.
Declaration of financial/other relationships
Two of the authors (Long and Mortimer) are employees of Analysis Group, Inc., a consulting company that has provided consulting services to biopharmaceutical manufacturers, both brand-name and generic. Henry Grabowski has served as an expert witness in pharmaceutical patent-related litigation on behalf of both plaintiffs and defendants. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Anna Kaltenboeck of Analysis Group, Inc. for research and technical assistance in the preparation of this article.
References
- Grabowski H, Vernon J. Longer patents for lower imitation barriers: the 1984 drug act. Amer Econ Rev 1986;76:195-8
- Berndt E. Pharmaceuticals in U.S. health care: determinants of quantity and price. J Econ Perspect 2002;16:45-66
- Science and Data Policy, Assistant Secretary for Planning and Evaluation. Expanding the Use of Generic Drugs. Washington, DC. 2010. Available at: http://aspe.hhs.gov/sp/reports/2010/genericdrugs/ib.pdf. Accessed May 15, 2013
- IMS Institute for Healthcare Informatics. Declining Medicine Use and Costs: For Better or Worse? Parsippany, NJ. 2013. Available at: http://www.imshealth.com/portal/site/imshealth/menuitem.762a961826aad98f53c753c71ad8c22a/?vgnextoid=5b21ee0a8e631410VgnVCM10000076192ca2RCRD. Accessed May 15, 2013
- The Congress of the United States, Congressional Budget Office. How increased competition from generic drugs has affected prices and returns in the pharmaceutical industry. Washington, DC. 1998. Available at: http://www.cbo.gov/sites/default/files/cbofiles/ftpdocs/6xx/doc655/pharm.pdf. Accessed May 15, 2013
- Grabowski H, Kyle M. Generic competition and market exclusivity periods in pharmaceuticals. Manage Decis Econ 2007;28:491-502
- Grabowski H, Kyle M, Mortimer R, et al. Evolving brand-name and generic drug competition may warrant a revision of the Hatch-Waxman Act. Health Affairs 2011;30:2157-66
- U.S. Food and Drug Administration. Abbreviated New Drug Application and Generics. 2013:1-42. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM293268.pdf. Accessed May 15, 2013
- Panattoni LE. The effect of Paragraph IV decisions and generic entry before patent expiration on brand pharmaceutical firms. J Health Econ 2011;30:126-45
- Smith K, Gleklen K. Generic drugmakers will challenge patents even when they have a 97% chance of losing: The FTC Report that K-Dur Ignored. Boston, MA: CPI Antitrust Chronicle, 2012. www.competitionpolicyinternational.com/generic-drugmakers-will-challenge-patents-even-when-they-have-a-97-chance-of-losing-the-ftc-report-that-k-dur-ignored/. Accessed October 3, 2013
- Greene A, Steadman DD. Analyzing Litigation Success Rates. Pharmaceuticals. 2010;(212):1-24. http://www.aipla.org/committees/committee_pages/FDA/CommitteeDocuments/MeetingMaterials/2010SpringMeeting/AnalyzingLitigationSuccessRates.pdf. Accessed May 15, 2013
- Glass G. Legal defenses and outcomes in Paragraph IV Patent Litigation. J Generic Med 2013;10:4-13