Abstract
Background:
Prostate cancer (PCa) is the second leading cancer diagnosed among men. In Spain the incidence of PCa was 70.75 cases per 100,000 males. Advanced PCa has spread outside of the prostate capsule and may involve other parts of the body. The aim of this study was to estimate the lifetime costs of a cohort of advanced PCa patients diagnosed in Spain in 2012.
Methods:
A partitioned economic model was developed in EXCEL incorporating Spanish incidence, mortality, and cost data supplemented with data from the international literature. Progression from Stage III to Stage IV was permitted. Costs were discounted at the standard rate of 3%. Lifetime costs were presented on an individual basis and for the entire cohort of newly diagnosed Stage III and Stage IV PCa patients.
Results:
Lifetime costs for advanced PCa were ∼€19,961 per patient (mean survival of 8.4 years). Using the projected incident cases for 2012 (3047), the total cost for the incident cohort of patients in 2012 would amount to €61 million. These results were more sensitive to changes in the ongoing costs (post-initial 12 months) of Stage III PCa, the rate of progression from Stage III to Stage IV, and the discount rate applied to costs.
Conclusions:
This study provides an estimate of the lifetime costs of advanced PCa in Spain and a framework for further research. The study is limited by the availability of long-term Spanish data and the need to make inferences from international studies. However, until long-term prospective or observational data do become available in Spain, based on the assumptions, the current results indicate that the burden of advanced PCa in Spain is substantial. Any treatments that could potentially reduce the economic burden of the disease should be of interest to healthcare decision makers.
Keywords::
Introduction
Prostate cancer (PCa) is the second leading cancer diagnosed among men (after lung cancer), and the fifth leading cause of cancer death in EuropeCitation1. In a Spanish epidemiological observational study in 25 public hospitals, socio-demographic and clinical variables of all newly diagnosed, histopathologically confirmed PCa cases were collected in 2010, 4087 new cases of PCa were diagnosed for a reference population of 4,933,940 men (21.8% of the Spanish male population)Citation2. The estimated age-standardized PCa incidence was 70.75 cases per 100,000 men. The mean age at diagnosis was 69 years; 11.6% of patients presented with tumor-related symptoms, and 39.5% with Lowe Urinary Tract Symptoms (LUTS). Median PSA was 8 ng/mL. At diagnosis, 89.8% had localized, 6.4% locally advanced, and 3.8% metastatic diseaseCitation2.
According to estimates for 2006, Spain’s incidence rates are relatively low compared with other European Union countries and intermediate when compared with all European countries as a wholeCitation3. In recent decades, while incidence has increased in most developed countriesCitation4 including Spain, in part due to the increased used of prostate-specific antigen (PSA) testing for screening, there has been a simultaneous reduction in PCa mortality rates in developed countriesCitation5. These declines in mortality are similarly evident in 14 out of Spain’s 17 autonomous regions [Comunidades Autónomas]Citation6.
Advanced PCa (Stage III (locally advanced PCa) or IV (metastatic PCa)) is cancer that has spread from the prostate gland to other parts of the body. PCa can spread to any part of the body but it most commonly spreads to the bones and the lymph nodes.
Information about the current or future expected costs of PCa patients and more specifically advanced PCa in Spain is scarce, despite the predictions of higher incidence and lower mortality. A recent paper has looked at the treatment costs of PCa in the first year after diagnosis in a number of European countries including SpainCitation7 and a study carried in the USCitation8 has estimated the lifetime economic burden of PCa, but, to the authors’ knowledge, there are no publications that concentrate on the lifetime costs of advanced PCa in Spain.
Determining the amount of resources that are used for cancer treatment is important to policy-makers and health researchersCitation9. In particular, the knowledge of future costs may facilitate a more efficient allocation of limited public resources. In an age of economic austerity affecting many European countries including Spain, it would appear reasonable to assert that limited resources should be allocated using a rational approach based on the available evidence.
Given that the current data on the costs of advanced PCa are limited, the objective of the present study was to estimate the long-term costs of advanced PCa from the perspective of the healthcare provider in Spain using an incidence cost modeling approach.
Methods
Overview
The study and resultant economic model is based on a synthesis of existing information available in the form of published manuscripts, local studies, and data incorporated from previously published reports. Cost estimates are presented for the year 2012.
Patient population
Since the study was limited to advanced PCa patients it was necessary to restrict the modeling to data associated with Stage III and IV PCa patients. In this study, it was assumed that Stage III refers to locally advanced PCa and Stage IV to metastatic PCa.
Epidemiological data
Incidence and mortality data in Spain were extracted from projections for 2012 from a study by Sánchez et al.Citation10. In that study, relative survival data were derived from six Spanish population-based cancer registries (PBCRs). The information included incident cases which were diagnosed and followed-up from 1985 to 2003Citation10–12.
Sánchez et al.Citation10 applied the Mortality-Incidence Analysis Model (MIAMOD) statistical method to compute incidence estimates. Projected survival behavior beyond the study period was based on dynamic projection assuming that survival would continue to vary at the same rate as it had been during the observation period.
Sub-groups
In order to estimate the number of advanced PCa patients it was necessary to refer to the patient characteristics in a commercial database provided by Information Management Systems, Inc. (IMS). Results from the IMS Oncology Analyser (OA) database are as summarized and reported by Fourcade et al.Citation7.
The total number of patients included in the period September 2004 to June 2006 from the study by Fourcade et al.Citation7 were assigned to one of four stages (I–IV). By using the distribution of patients in Stages III and IV it was possible to assign a percentage of patients in Stages III and IV which could then be applied to the overall incidence value in order to estimate the number of advanced PCa incident cases for the year 2012.
Estimation of yearly treatment stage costs
Estimated yearly costs were based on the mean direct costs of the first year by stage (III and IV) reported by Fourcade et al.Citation7 and up-rated to 2012 from 2006 using the consumer prices index (CPI) from the National Institute of Statistics (INE)Citation13. The relative distribution of costs according to treatment phase (1st 6-month, Average continuing cost/6 months and Average annual cost) was used for ongoing costs as reported by Wilson et al.Citation14 in an economic study of the cumulative cost patterns of PCa treatments.
Costs during the last year of life are important when considering the total cost of PCaCitation15. Unfortunately, few databases other then the Surveillance and Epidemiology and End Results (SEER)–Medicare database have enough longitudinal data to adequately capture end-of-life costs. In one 2008 study, mean annual costs were estimated to be $10,612 in the initial phase and $33,691 in the last year of lifeCitation16. The ratio was applied to first year costs in order to obtain an estimate for the costs associated during the final year of life.
Cost model
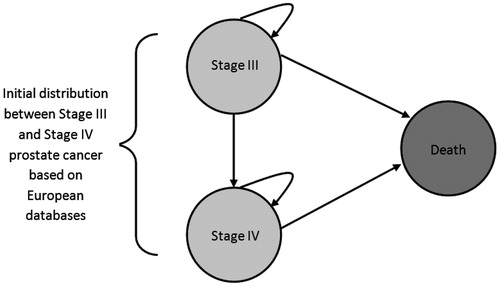
The cost model is represented graphically in . A partitioned survival model was developed in Microsoft EXCEL (version 2007). Patients enter the model upon diagnosis of either Stage III or Stage IV of the disease. The model cycle length is 1 year. In any 1-year period patients in Stage III may remain in Stage III, progress to Stage IV, or die. For Stage IV, patients may either remain in Stage IV or die. The rates of remaining in the same Stage (III or IV), progressing to Stage IV, or dying are dependent on the survival data from the literature. Costs (in 2012 values) are accumulated for every year patients remain in either Stage III or Stage IV, death has no assigned cost. Costs vary according to both the Stage and the length of time in any given stage. Yearly costs were separated into two phases—the initial phase, representing the first 12 months after diagnosis, and the ongoing phase, representing the period after the first 12 months of treatment after diagnosis. This approach is similar to the methodology used by Wilson et al.Citation14 in which first 6-monthly average costs, continuing 6-monthly average costs, and average annual costs were calculated.
Survival analysis for Stage III and Stage IV PCa
Stage III and Stage IV survival data and the progression from Stage III to Stage IV PCa were based on a review of the literature. For Stage III it was assumed that patients’ mortality outcomes would be equivalent to the age-related mortality of the general Spanish populationCitation17. The reasoning behind this approach was that a previous analysis has shown that the relative survival rates for localized/regional PCa were actually above 100% when compared with the general populationCitation18. In other words, by assuming survival of Stage III PCa patients was no worse than the general population we were making a conservative assumption for Stage III survival. For Stage IV survival, we used the data published by Stokes et al.Citation8, who based their analysis on data from the SEER database from the National Cancer Institute (USA). Mean survival times were used to estimate parametric survival curves based on the exponential function. Yearly transition rates were calculated by transforming the values from the exponential function.
Life-time advanced PCa costs
Yearly costs for Stage III and Stage IV were derived from a study that estimated treatment costs of PCa in the first year after diagnosis in five European countries including SpainCitation7. Results were presented for Stages I–IV, of which results for Stage III and Stage IV were used in this study. The analysis was based on resource utilization identified in the IMS Oncology and Disease Analyser databases. Costs were expressed in 2006 values, hence it was necessary to up-rate costs to 2012 values using the consumer price index of the National Institute of Statistics (INE)Citation13. Resources were multiplied by their respective unit costs for every resource item identified in the review of the databases. These costs represent the cost of the initial year of treatment after diagnosis for all patients for each Stage, and all patients, not just those patients who were treated. For ongoing phase costs for each PCa Stage it was necessary to adjust the first-year costs based on the relative cost ratios for annual costs provided by Wilson et al.Citation14. Progression from Stage III to Stage IV was assumed to occur to 50% of surviving Stage III patients after 10 yearsCitation19.
Data analysis
The EXCEL model was populated with the identified epidemiological, resource, and cost data. Total costs were discounted at an annual rate of 3% based on the most recent proposed guidelines for the economic evaluation of health technologies by López Bastida et al.Citation20. A half-cycle correction was applied. In the base case the mean age at diagnosis was 69.0 years based on the IMS OA databaseCitation7. The maximum time horizon for the model was 32 years. Indirect non-medical costs (e.g., productivity losses, etc.) were not included in this analysis given the mean age of patients at diagnosis.
Results are presented in terms of mean lifetime costs per patient, lifetime costs for the entire cohort, and the distribution over time of alive patients is also presented.
As is normal in this type of analysis, sensitivity analysis was undertaken on the values of key variables such as the cost per Stage by phase (±50%), the incidence rate (±50%), and the discount rate (from 0–6%).
Results
Patient characteristics
From Sánchez et al.Citation10, mortality rates were observed to increase slightly until the early 1990s, and decreased progressively thereafter. A steep increase in the European age-standardized incidence rate was estimated to have climbed from 38 to 98 per 100,000 men from 1981 to 2012. An increasing incidence and reduction in mortality over the study period suggests an increase in diagnosis due to increased PSA testing and the increase in survival across the period. It was estimated that PCa would give rise to ∼29,877 incident cases and 5458 deaths in Spain by 2012 ().
Table 1. Key data used in the cost model.
Cost estimates at 1 year for all stages were €3728 (up-rated to 2012 values). Stage III and Stage IV first-year costs were lower: €3267 and 2513, respectively. For all stages the breakdown by type of costs was the following—diagnostic intervention: €362; surgeries: €1318; chemotherapy: €136, radiotherapy: €706, and hormonal therapy: €1206. Hormonal therapy was the most common type of treatment for Stage III and Stage IV—72% and 52%, respectively.
In order to be able to extend the analysis beyond the initial year of diagnosis it was necessary to estimate future yearly costs based on other studies where relative total costs had been estimated. These ratios () were then applied to the Spanish first year annual costs. By applying the cost ratiosCitation14,Citation16, estimated costs for Stage III were: €3267, €1738, and €10,372 for initial, on-going phase, and final year costs, respectively. For Stage IV the corresponding initial, on-going phase, and final year estimated costs were: €2513, €1337, and €7978.
Mean survival time for Stage III patients was 196 months (16.3 years)Citation8 for treated patients, this compares with a mean life expectancy of 15.3 years for a 69 year old Spanish male. For this reason, in the model we assume conservatively that mortality will be equivalent to the age-related mortality of the general population. For Stage IV patients estimated mean survival was 43.7 months (3.7 years). With the competing risk of death, patients in Stage III are also exposed to the possibility of progression to Stage IV. The assumption here is that 50% of surviving Stage III patients will have progressed after 10 yearsCitation19.
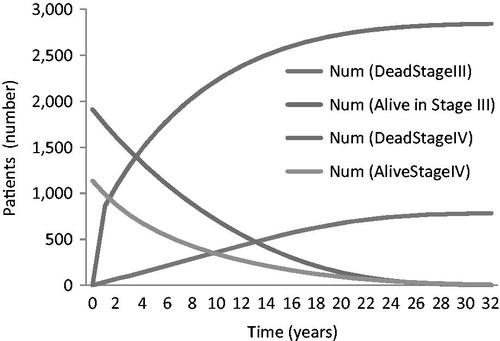
summarizes the results of the base case analysis and illustrates the distribution of patients over time. The mean survival time was 8.4 years.
Table 2. Base case results: mean advanced PCa life-time costs.
Estimated lifetime costs for advanced PCa were just under €20,000 per patient. Using the projected incident cases for 2012, the estimated total cost for the cohort of newly diagnosed patients in 2012 would amount to almost €61 million. It should be noted that in this model patients can progress from Stage III to Stage IV, explaining the reduction in the numbers in Stage III over time (in addition to that attributable to mortality).
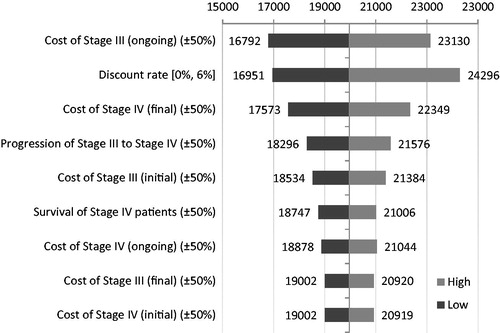
summarizes the sensitivity of the results as the values of key variables were changed.
From the tornado diagram it is apparent that the results are most sensitive to changes in the cost of the ongoing phase of Stage III PCa, the rate of progression from Stage III to Stage IV, the final phase of Stage IV, and the discount rate applied to costs.
The fact that the cost of ongoing Stage III treatment is sensitive to changes is to be expected given that patients in Stage III have longer expected survival and the ongoing cost phase applies to all periods apart from the initial 12 months after diagnosis and the final 12 months. As the rate of progression increases, fewer patients will remain alive as they enter Stage IV, leading to a reduction in overall costs and similarly an increase as the rate of progression is decreased. The discount rate is also a sensitive factor given the long time horizon. In other words, since the model potentially can run for a maximum of 32 years, as the discount rate increases overall costs will fall, with all other variables remaining constant.
What is clear is that, even though in this study we are concentrating on a sub-group of PCa patients, the lifetime costs are significant and are expected to rise as the incident rate increases and mortality continues to fall.
Discussion
In this study we have estimated the lifetime costs of advanced PCa in Spain. Few studies have used the incidence cost methodology applied to PCa, and those that have tend to be carried out from a North American perspectiveCitation8,Citation14. Estimates of the number of incident cases in Spain together with data on the distribution of the stages of the disease and treatment costs of PCa in the first year after diagnosis have been combined with data from other countries in order to construct a model from which lifetime cost estimates have been made. In our base case analysis, we assume an annual incidence of 29,877 based on the 2012 projections by Sánchez et al.Citation10. Other studies would seem to suggest that the incidence is lowerCitation2. However, by choosing to employ the modeling approach, users with their own data (incidence, percentage with Stage III and Stage IV, costs, etc.) are able to rapidly adjust the model to their own local setting.
From a diagnostic and epidemiological perspective, procedures, such as ultrasound-guided biopsy, transurethral resection of the prostate, and the PSA test in particular, were introduced in Spain during the early 1990s. These potentially are mitigating factors when assessing PCa incidence ratesCitation10. Since PCa mainly affects older men, with the aging of the Spanish population it would seem reasonable to expect a significant increase in the number of casesCitation10,Citation21. Accordingly, predictions concerning future PCa incidence rates and costs are crucial for further study. Since there havebeen improvements in PCa survival demonstrated over the past decades, PCa remains one of the leading causes of cancer death among men.
Although the costs in this study were limited to advanced PCa, the total lifetime costs are not insignificant and should not be overlooked by health policy analysts and decision-makers. Since resources for healthcare are limited, any treatments or interventions that might reduce the overall economic burden should be considered in combination with their clinical effectiveness.
In particular, the development and evaluation of novel treatment options for advanced PCa from locally advanced to metastatic castration-resistant PCa post-chemotherapyCitation22 also need to be viewed not just in terms of their clinical benefit on say, overall survival, but their impact on costs to the health service in a context of scarcity.
A number of limitations of our study should be noted. Firstly, we utilized first year costs to extrapolate to a longer period and the ratio of first year to last year for the final year of life. The majority of cost studies in the area of PCa concentrate on the first few months or first year after diagnosis. This is also true in the case of Spain. First year costs for Stages III and IV are available for Spain, even if the data are aggregatedCitation7. Another study does report Spanish costs for the period 2003–2005Citation23, however these costs are limited to up to a maximum of 6 months after the treatment start date. It should be noted that a recently published international systematic review of the direct costs for initial management of prostate cancerCitation24 only included one study that covered Spain, the study by Fourcade at al.Citation7. However, in order to extrapolate to a longer time period, costs for other periods are necessary. We applied relative costs of other time periods from studies in other countries to the Spanish context. Then we undertook a extensive sensitivity analysis on the appropriate variables to evaluate the extent of possible variation from the base case results. Another possible solution would be to extract data from the existing commercial databases (such as the IMS OA and Data Analyser) and this is an approach that can be considered for further studies. It should also be noted, as has been stressed by Stokes et al.Citation8, that the estimates of the life-time costs of a disease should be interpreted with caution since it is an area of research that involves many assumptions. Secondly, we utilized data for patients with Stage III and IV disease only. Due to limited availability of cost data in the literature for Spanish patients with later disease, it was necessary to incorporate cost ratios from the international literature in our model. Thus our lifetime cost assumptions may not completely reflect the total per patient that is utilized for the cost of care of this end stage of PCa progression.
Any model is an approximation of reality and will inevitably require assumptions. However, the developed model does provide a framework that could even be applied to other countries given the availability of the appropriate data.
Conclusions
This study has estimated the lifetime costs of advanced PCa in Spain using a modeling approach. While this approach may not be ideal, it does provide an order of magnitude and a framework for further research. Until improved long-term prospective or observational data do become available, the current results indicate that the burden of advanced PCa is substantial, will in all probability increase due to an increasing number of new cases and reductions in mortality, and hence healthcare decision-makers should be made aware of the burden of the disease to the National Health Service in Spain.
Transparency
Declaration of funding
This study was financed by Astellas Pharma Europe Limited (APEL).
Declaration of financial/other relationships
Warren M. Hart is an employee of EcoStat Consulting UK (ESCUK). ESCUK received funding for the project from APEL. J. Nazir and E. Baskin-Bey E have disclosed that they are employees of APEL.
Acknowledgments
The authors would like to thank Bernardino Miñana and Antonio Alcaraz for reviewing a previous version of the manuscript.
References
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005;16:481-8
- Cozar JM, Miñana BF, Gómez-Veiga F, et al. Prostate cancer incidence and newly-diagnosed patient profile in Spain in 2010. BJU Int 2012;110:E701-6
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92
- Neppl-Huber C, Zappa M, Coebergh JW, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol 2012;23:1325-34
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079-92
- Cayuela A, Rodríguez-Domínguez S, Vigil-Martín E, et al. Cambios recientes en la mortalidad por cáncer de próstata en España: estudio de tendencias en el periodo 1991–2005. Actas Urol Esp 2008;32:184-9
- Fourcade RO, Benedict A, Black LK, et al. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int 2009;105:49-56
- Stokes ME, Ishak J, Proskorovosky I, et al. Lifetime economic burden of prostate cancer. BMC Health Services Res 2011;11:349
- Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with Prostate cancer: estimates from a population-based study. BJU Int 2009;105:338-46
- Sánchez MJ, Payer T, De Angelis R, et al. Cancer incidence and mortality in Spain: estimates and projections for the period 1981-2012. Ann Oncol 2010;21(3 Suppl):ii30-6
- Roazzi P, Capocaccia R, Santaquilani M, et al. Electronic availability of EUROCARE-3 data: a tool for further analysis. Ann Oncol 2003;14:150-5
- Coleman MP, Gatta G, Verdecchia A, et al. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol 2003;14(5 Suppl):128-49
- Instituto Nacional de Estadística. Indice de precios al consume. Madrid. 2012. http://www.ine.es/varipc/index.do. Accessed March 24, 2013
- Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of Prostate cancer treatments. Cancer 2007;109:518-27
- Roehrborn C, Black L. The economic burden of prostate cancer. BJUI 2011;108:806-13
- Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008;100:630-41
- Instituto Nacional de Estadística. Tablas de mortalidad de la población de España 1991--2011. Madrid. 2012. http://www.ine.es/jaxi/tabla.do?path=/t20/p319a/serie/l0/&file=01001.px&type=pcaxis&L=0. Accessed March 14, 2013
- Brenner H, Arndt V. Long-term survival rates of patients with prostate cancer in the prostate-specific antigen screening era: population-based estimates for the year 2000 by period analysis. J Clin Oncol 2005;23:441-7
- Schoenstadt A. Prostate cancer prognosis. Progress for stage III prostate cancer. http://prostate-cancer.emedtv.com/prostate-cancer/prostate-cancer-prognosis-p2.html. Accessed March 14, 2013
- López Bastida J, Oliva J, Antoñanzas F, et al. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit 2010;24:154-70
- Larrañaga N, Galcerán J, Ardanaz E, et al. Prostate cancer incidence trends in Spain before and during the prostate-specific antigen era: impact on mortality. Ann Oncol 2010;21(Suppl 3):iii83-9
- Osanto S, Van Poppel H. Emerging novel therapies for advanced prostate cancer. Therapeut Adv Urol 2012;4:3-12
- Becerra Bachino V, Cots F, Guedea F, et al. Cost comparison of three treatments for localized prostate cancer in Spain: radical prostatectomy, prostate brachytherapy and external 3D conformal radiotherapy. Gac Sanit 2011;25:35-43
- Sanyal C, Aprikian AG, Chevalier S, et al. Direct cost for initial management of prostate cancer: a systematic review. Curr Oncol 2013;20:e522-31