Abstract
Objective:
To determine the cost-effectiveness of bioengineered hyaluronic acid (BioHA, 1% sodium hyaluronate) intra-articular injections in treating osteoarthritis knee pain in poor responders to conventional care (CC) including non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics.
Methods:
Two decision analytic models compared BioHA treatment with either continuation of patient’s baseline CC with no assumption of disease progression (Model 1), or CC including escalating care costs due to disease progression (NSAIDs and analgesics, corticosteroid injections, and surgery; Model 2). Analyses were based on patients who received two courses of 3-weekly intra-articular BioHA (26-week FLEXX Trial + 26-week Extension Study). BioHA group costs included fees for physician assessment and injection regimen, plus half of CC costs. Cost-effectiveness ratios were expressed as averages and incremental costs per QALY. One-way sensitivity analyses used the 95% confidence interval (CI) of QALYs gained in BioHA-treated patients, and ±20% of BioHA treatment and CC costs. Probabilistic sensitivity analyses were performed for Model 2.
Results:
For 214 BioHA patients, the average utility gain was 0.163 QALYs (95% CI = −0.162 to 0.488) over 52 weeks. Model 1 treatment costs were $3469 and $4562 for the BioHA and CC groups, respectively; sensitivity analyses showed BioHA to be the dominant treatment strategy, except when at the lower end of the 95% CI. Model 2 annual treatment costs per QALY gained were $1446 and $516 for the BioHA and CC groups, respectively. Using CC as baseline strategy, the incremental cost-effectiveness ratio (ICER) of BioHA was $38,741/QALY gained, and was sensitive to response rates in either the BioHA or CC groups.
Conclusion:
BioHA is less costly and more effective than CC with NSAIDs and analgesics, and is the dominant treatment strategy. Compared with escalating CC, the $38,741/QALY ICER of BioHA remains within the $50,000 per QALY willingness-to-pay threshold to adopt a new technology.
Introduction
Osteoarthritis (OA), the most common form of arthritis, affects millions of adults worldwideCitation1,Citation2. OA is characterized by the breakdown of joint cartilage, resulting in joint swelling and inflammation, with associated pain and loss of movementCitation3. The World Health Organization estimates that at least 10% of adults aged ≥60 years experience OA-related health issuesCitation4. In addition, OA is the fifth leading cause of disability among US adultsCitation5.
Due to heterogeneity in research methods, OA prevalence rates are difficult to determineCitation4. Studies have used radiographic, clinical, or symptomatic definitions to identify OA patientsCitation6. Based on available data, the lifetime risk of developing symptomatic knee OA has been estimated at ∼40% in men and ∼47% in women, with obese persons at higher riskCitation7. While the current prevalence of OA is unknown, it is clear that rates are increasing. In the US, estimated OA prevalence increased from 21 million in 1990 to 27 million in 2005Citation1, and by 2030 an estimated 67 million adults are projected to be diagnosed with arthritisCitation8. Because OA is associated with older age, an increasing elderly population also signals future significant rises in prevalenceCitation4.
As a leading cause of disability in men and womenCitation9, OA significantly impacts patient health-related quality-of-life (HRQoL), causing fatigue, decreased sleep quality, and impaired mental health, social function, and work productivity. As shown in a 2007 Centers for Disease Control (CDC) Morbidity and Mortality Weekly Report (MMWR), OA-related annual costs to US society in terms of medical care and lost wages exceeded $128 billion in 2003 (equivalent to $197 billion in adjusted 2013 US dollars)Citation10,Citation11. Medical expenditures accounted for $80.8 billion in costs, while indirect costs were $47 billionCitation10. The CDC MMWR report noted that, nationally, direct costs associated with OA increased by 24% from 1997–2003, and these costs are expected to increase as OA prevalence continues to riseCitation10. As the leading cause of joint replacement surgery, $42.3 billion was spent on OA-related surgeries in 2009 aloneCitation6. Also in 2009, OA was responsible for an estimated 921,000 hospitalizations, with a mean cost per stay of $45,443 Citation6. Moreover, a 2011 evaluation of national inpatient hospital costs ranked OA treatment as the second most expensive condition treated in US hospitalsCitation12.
Injection with intra-articular hyaluronic acid (HA), also known as viscosupplementation, is indicated for the symptomatic relief from pain in patients with OA of the knee after the failure of conservative treatment. Results from a 2006 Cochrane Review found viscosupplementation to be an effective treatment for OA of the knee, with beneficial effects on pain, function, and Patient Global Assessment. Hyaluronic acid treatment was associated with a moderate-to-large effect size, depending on preparation used, outcome indicators, and evaluation time points. For example, viscosupplementation provides the largest benefit on weight-bearing pain at 5–13 weeks post-injectionCitation13. A 2012 update to the American College of Rheumatology (ACR) guidelines on management of OA of the hand, hip, and knee recommends the use of intra-articular HA injections for patients deemed poor responders to conventional therapy. Conventional therapy includes non-pharmacologic therapies (e.g., exercise, weight loss, physical therapy, walking aids, and support devices, etc.) and pharmacologic therapies (e.g., acetaminophen, non-steroidal anti-inflammatory drugs [NSAIDs], topical NSAIDs, tramadol, and corticosteroid injections)Citation14.
Sodium hyaluronate 1% (EUFLEXXA) is a highly purified, BioHA produced from Streptococcus zooepidemicus using a fermentation, recovery, and purification process, which creates high molecular weight HA without chemical cross-linking. In a 3-month registration randomized controlled trial (RCT) that evaluated OA patients with moderate-to-severe pain who failed to respond or responded poorly to conventional therapy, BioHA was shown to have comparable efficacy to hylan G-F 20, an avian-derived, cross-linked, HA-based preparation (CL-HA)Citation15. The 26-week FLEXX Trial assessed the safety and efficacy of BioHA in a randomized, placebo-controlled study of 588 patients who were non-responders or poor responders to prior conventional therapy. Results showed a decrease in 100 mm visual analog scale (VAS) scores of 25.7 mm and 18.5 mm for the BioHA and intra-articular saline groups, respectively, with a least-squares means difference of −6.6 mm (p = 0.002). This corresponded to a 53% median reduction in pain score from baseline for the BioHA group, compared with a 38% reduction for the intra-articular saline group (p = 0.002)Citation16. The FLEXX Trial also found that the effect of BioHA injections was durable in that the reduction in pain was sustained for up to 26 weeksCitation16. These results led to the additional 26-week open-label FLEXX Trial Extension Study which evaluated the safety of a repeated series of 3-weekly BioHA injections, and demonstrated that repeat injections of BioHA were safe, well tolerated, and not associated with an increase in adverse events (AEs) such as synovial effusionsCitation17.
Additionally, a recent study of FLEXX Trial patients examined the effects of BioHA on HRQoL. This analysis found that patients treated with BioHA had significant improvements in physical functioning, bodily pain, general health perceptions, and the physical component summary score, as measured by the 36-item Short-Form Health SurveyCitation18.
Cost-effectiveness analysis is a tool for comparing the costs and outcomes of specific treatment programsCitation19. Often, outcomes are presented in terms of a measurement of patient preference or utility, the quality-adjusted life year (QALY) being the most commonCitation20. The calculation of QALYs is based on utility values, which are quantifications of the desirability of health statesCitation19. Cost-effectiveness analysis then takes costs from real-world data and uses them to rank the programs being compared, frequently in terms of an incremental cost-effectiveness ratio, which divides the difference in cost by the difference in outcomeCitation19. Although real-world cost parameters are variable, cost-effectiveness analysis must use fixed data points and estimates. However, cost-effectiveness analysis typically includes sensitivity analysis as well, in which the values used are adjusted to account for uncertainties or imprecision in the base caseCitation19.
Only a few studies have been published in North America and Europe on the cost-effectiveness of CL-HA (Synvisc) in OA of the kneeCitation21–23. In 2001, Waddell et al.Citation23 simulated viscosupplementation treatment in a managed care setting. The authors found that, over a 3-year period, the addition of CL-HA to the standard treatment pathway yielded savings of $4706 per OA patient treated, or ∼$1569 per year in 1998 US dollars. A 2002 study by Torrance et al.Citation21, based on a 1997 RCT of CL-HA, compared appropriate care (defined as following ACR guidelines) plus CL-HA, vs appropriate care without CL-HA. This study showed an incremental cost-effectiveness ratio (ICER) of $10,000 (in 1999 Canadian dollars) per QALY gained for CL-HA plus appropriate care. In a 2003 study, Kahan et al.Citation22 compared CL-HA to conventional care and found that CL-HA was more effective with no additional cost. Similarly, a French observational study (n = 296) found that costs associated with HA injections were offset by reductions in medical and non-medical costsCitation24. Results from a few studies conducted outside of North America and Europe have also mostly found intra-articular HA treatment to be cost-effective with associated increased short-term costs and increased QALYsCitation25,Citation26. Results from a study in Thailand (n = 146) found that intra-articular HA injections increased short-term treatment costs, but were associated with the delay or cancellation of surgical treatment and a consequent savings of 63.3%Citation25. One Taiwan-based study by Yen et al.Citation26 modeled a comparison of naproxen, celecoxib, and intra-articular HA treatment. Results from this study show that intra-articular HA had an ICER of $42,000 per QALY, and was, thus, not a cost-effective therapy for the Taiwanese healthcare system.
The objective of the present study is to assess the use of BioHA in adult patients with moderate-to-severe knee pain due to OA, who either failed to respond or responded poorly to conventional therapy, using data from the FLEXX Trial and Extension Study as the basis for treatment data in 2 cost-effectiveness analyses. The first analysis (Model 1) was developed to determine, from the payers’ perspective, if BioHA treatment makes economic as well as clinical sense. Model 1 evaluated costs associated with the continuation of therapies that patients were using prior to study enrollment, and compared those to the cost of BioHA treatment in the same patient population. In this model, the patient population was derived from the FLEXX Trial and Extension Study with the conventional care arm modeled from baseline data. A threshold of $50,000 per QALY was applied to investigate whether BioHA could be adopted as a cost-effective technologyCitation27. To further test the cost-effectiveness of BioHA treatment for OA, a secondary analysis (Model 2) was run using BioHA response data from the FLEXX trial. In this analysis, the modeled conventional care program was based on the response rate from the conventional care arm of an RCT of CL-HA, in which appropriate care was based on ACR guidelines, but did not include intra-articular injectionsCitation28.
Patients and methods
Study design
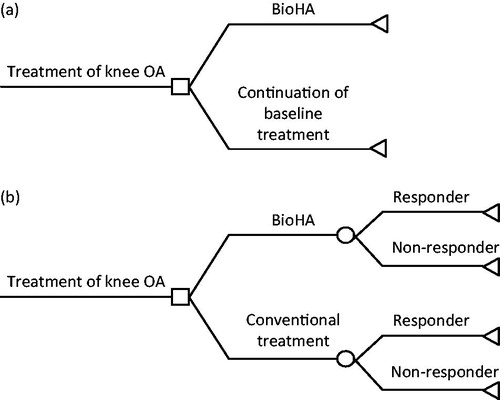
The Model 1 study used a decision analytic model to compare BioHA treatment with continuation of patients’ existing conventional OA care (). This model assumed patients would have been maintained on their pre-study conventional treatment had they not enrolled in the FLEXX Trial and Extension Study. This approach allowed for a model that estimated the incremental outcome if patients remained on their conventional, baseline treatment vs if they received BioHA.
Figure 1. (a) Model 1: Cost-effectiveness model of BioHA vs continuation of baseline treatment. (b) Model 2: Cost-effectiveness model of BioHA vs conventional care, responders and non-responders. BioHA, bioengineered hyaluronic acid; OA, osteoarthritis.
Model 1 assumed that the conventional care group continued to receive conventional treatment and did not achieve any additional QALY improvements over the course of the study (corresponding with a FLEXX trial inclusion criteria stipulating that patients did not respond, or responded poorly to usual care treatment), while the BioHA group achieved average QALY changes as observed in the FLEXX Trial. This allowed for a strict comparison using the same patient population that either maintained their conventional, baseline treatment, or added BioHA treatment to the treatment regimen.
The Model 2 study also used a decision analytic model to compare the costs and utilities in patients with OA of the knee treated with BioHA vs conventional therapy (). In this model, the conventional therapy group was based on the appropriate care group from a study by Raynauld et al.Citation28, which allowed for a comparison between treatment with BioHA and typical conventional treatment, up to and including total knee replacement (TKR). For Model 2, treatment outcome was patient response based on the Raynauld study criterion, defined as achieving a ≥20% improvement from baseline on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scoreCitation28.
Data source for outcome probabilities
BioHA treatment response rates for both Model 1 and Model 2 were based on the FLEXX Trial and Extension StudyCitation16,Citation17. The FLEXX Trial was a randomized, double-blind, US multi-center, placebo (saline)-controlled study, which investigated the efficacy and safety of BioHA for the treatment of mild-to-moderate OA knee pain in patients who did not respond adequately to conventional therapiesCitation16. Inclusion criteria included OA of the knee, a moderate-to-severe pain score on the 100 mm VAS immediately following a 50-foot walk test, bilateral standing anterior–posterior radiograph demonstrating Kellgren-Lawrence grade 2 or 3 OA of the target knee, ability and willingness to use only acetaminophen as the study rescue medication, and unassisted walking 50 feet on level ground and going up and down stairs. Patients received a course of 3-weekly injections of either BioHA or saline. The primary efficacy outcome measure was the least-squares mean difference between BioHA and saline in patients’ changes in knee pain from baseline to week 26 on a 100 mm VAS following a 50-foot walk test. In addition, changes in WOMAC subscales of pain, stiffness, and physical function were also assessed at baseline and follow-up. In this trial, intra-articular BioHA therapy resulted in significant OA knee pain relief with a mean reduction from baseline of 53% for intra-articular BioHA vs 38% for intra-articular saline (p = 0.002)Citation16. Patients treated with BioHA also experienced significant improvements in joint function, treatment satisfaction, and general HRQoLCitation16.
In the 26-week, open-label FLEXX Trial Extension Study, patients who completed the FLEXX Trial and elected to participate continued to be masked to their treatment assignment, and received either an initial course of BioHA injections (if they received saline in the FLEXX Trial; n = 214) or a second course of BioHA injections (n = 219)Citation17. Extension Study results show that a repeated BioHA injection series was safe and well tolerated. No patients reported joint effusion over the course of the 52-week FLEXX Trial and Extension Study. The outcomes of BioHA treatment were assessed for the 214 patients in the intent-to-treat population who received BioHA in both the FLEXX Trial and the FLEXX Trial Extension StudyCitation17.
The response rate for conventional treatment in Model 2 was adopted from the appropriate care arm of a prospective, randomized, open-label, 1-year multicenter trial conducted in Canada, comparing appropriate care with CL-HA to appropriate care without CL-HACitation28. Patient inclusion criteria included age ≥40 years, radiologically verified OA, VAS pain score >175 mm of 500 mm on the WOMAC scale despite treatment with acetaminophen or NSAIDs, and ambulatory statusCitation28. Appropriate care included analgesics; NSAIDs; corticosteroid injections; supportive measures such as education and counseling, weight loss, joint rest, application of heat or ice, and use of devices, physical therapy, and arthroscopy; and total joint replacementCitation28.
Derivation of utility scores
Utility represents the value, or weight, that patients place on their health status outcomes. In this study, QALYs were assessed by combining the weights calculated for health states achieved with an intervention, alongside the time spent in those health states. In essence, any QALY gains observed were the product of 2 variables: the increase in HRQoL afforded by the intervention and any additional lifespan gained by an individual due to treatment. Combining the QALYs gained with the respective incremental costs of healthcare resources, consumption resulted in cost-per-QALY estimates.
Since health utility was not directly measured in the FLEXX Trial or Extension Study, a method developed by Grootendorst et al.Citation29 was employed to derive health-state utilities. This approach used a widely used measure of health-state utilities, the Health Utilities Index Mark 3 (HUI-3), which was predicted using WOMAC pain, stiffness, and function subscales, demographic variables, and duration of OA as inputs in a multiple regression model. In the FLEXX Trial and Extension Study, WOMAC was measured at baseline and at weeks 1, 2, 3, 6, 12, 18, 26, 27, 28, 41, and 52. For patients with missing WOMAC data, the last observation carried forward method was used to impute missing values.
Model 1 assumed that all patients who continued on the conventional, baseline treatment would be non-responders with no additional gain in QALY. Therefore, Model 2 is more conservative because it was assumed that there will still be some responders in the conventional treatment arm; the response rate for Model 2 was adapted from published literatureCitation28.
OA treatment costs
The estimated cost of a course of 3-weekly BioHA injections was $342 in 2012. BioHA-treated patients received a total of 2 treatment courses over the 52-week study period. Since patients had to visit the physician’s office for BioHA injections (as per the clinical study protocol and preparation labeling), fees for a physician visit and 2 courses of 3 injectable drug administrations were added to the cost of BioHA treatment. To calculate these costs, the median 2012 fee for all carriers and localities of the Centers for Medicare & Medicaid Services Physician Fee Schedule rates for office visits ($42.55) and injectable drug administration ($69.78) was appliedCitation30.
Several studies have reported on the direct health economic impact of OA in community-based settingsCitation23,Citation31,Citation32. In this study, Model 1 adopted conventional cost figures reported by Waddell et al.Citation23 because this research grouped OA costs specifically by treatment types that closely resembled modalities used in the current study (i.e., conventional treatment with NSAIDs or analgesics). Waddell et al.Citation23 compiled treatment costs from a large claims database with 2 million members, and included costs directly related to OA of the knee. Also included was the cost of OA treatment-related side effects such as gastrointestinal bleeding from NSAID use. The authors reported total annual costs for the group that received conventional treatment at $2622 in 1998 US dollarsCitation23. This was equivalent to $4562 in 2012 dollars after adjustment using the Medical Care Consumer Price Index (CPI).
In Model 2, the costs of conventional OA treatment were taken from a 2013 study by Losina et al.Citation33 that examined the cost-effectiveness of disease-modifying OA drugs. This study included 4 levels of conventional care: NSAIDs, acetaminophen, physical therapy, and assistive devices. For this analysis, the cost estimate for the first level of conventional care was used, amounting to an annual cost of $483 per patient in 2010 dollars. After adjustment to 2012 dollars using CPI data, the average annual treatment cost for an OA patient receiving conventional care was $516.
In both Models 1 and 2, 50% of the costs associated with conventional OA treatment were added to the costs of BioHA. The justification for this came from 2 previous studies. In the first study (1996), Lussier et al.Citation34 reported that approximately one-half of patients who used an HA-based preparation were able to reduce their NSAID use. In the second study (2011), Berger et al.Citation35 examined healthcare utilization costs prior to TKR and found that mean healthcare costs increased by 50% between the eighth calendar quarter and the quarter immediately preceding surgery. These costs were largely attributable to prescription NSAIDs, opioids, and injectable corticosteroids, as well as physician office visits and emergency department visits. Therefore, the BioHA arm was assumed to incur half the costs of NSAID/analgesic treatment, while the conventional care group was assumed to incur the full costs of conventional treatment. However, because Models 1 and 2 used different sources for conventional costs, the amounts added to the BioHA costs varied between models. Costs and other parameters used in the base case decision analysis model are summarized in .
Table 1. Models 1 and 2: Input parameters for the base case and ranges of the parameters for sensitivity analysis.
Incremental cost-effectiveness ratio
Treatment with BioHA was compared with conventional care by calculating the ICER (the ratio of incremental costs over incremental changes in utility during the 52-week study period for the 2 treatments under comparison). The Model 1 analysis computed the incremental cost per QALY of the FLEXX BioHA-treated group compared with the conventional care group, assumed to be maintained on their existing pre-study therapy. The Model 2 analysis computed the incremental cost per QALY of the FLEXX BioHA-treated group compared with the conventional care group (using response rates and costs from Raynauld et al.Citation28 and Losina et al.Citation33, respectively).
Sensitivity analysis
For both models, one-way sensitivity analyses were performed to test the robustness of study results by varying baseline costs and utilities by ±20%. Input variables in the sensitivity analyses were BioHA costs and annual healthcare costs for conventional treatment with NSAIDs and analgesics. For Model 1, the sensitivity analysis also tested variance in QALYs gained for the BioHA arm (95% confidence interval [CI]). For Model 2, response rates for both BioHA and conventional care were varied by ±20% from the base case scenario. Also in Model 2, for probabilistic sensitivity analysis, a Monte Carlo Simulation was performed using 10,000 joint distribution samples of the three parameters (costs, utility, and response rates). The ICER distributions resulting from these simulations were reported, and acceptability curves were charted for the 2 treatments under various willingness-to-pay assumptions. All decision model and sensitivity analyses were carried out using TreeAge Pro Interactive, decision analysis Software (TreeAge Software, Inc., Williamstown, MA). In addition to base case values, low and high parameter values for the sensitivity analyses of both Models 1 and 2 are shown in .
Results
Patient baseline characteristics
At baseline, mean patient age in the FLEXX Trial and Extension Study was 61.7 years, and mean body mass index (BMI) was 32.8 kg/m2 Citation17. Other baseline demographic information, including Kellgren-Lawrence grade and prior OA treatment, are shown in Citation16,Citation17.
Table 2. FLEXX Trial baseline demographicsCitation16.
The Raynauld et al.Citation28 study was used as the conventional treatment arm in Model 2. In that study, mean patient age was 63 years, mean BMI was 32.5 kg/m2, and mean WOMAC pain was 11.7 on a scale of 0–20. The mean duration of OA symptoms in the study knee was 9.45 years, with the majority of patients (60%) showing radiological Grade 3 or 4 in the year prior to enrollment in the study. At baseline, 69% of patients rated their global assessment of OA in the study knee as either “poor” or “very poor”.
Model 1
QALYs gained over 52 weeks
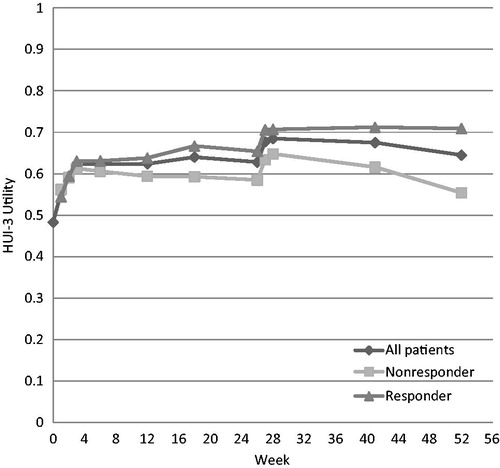
At the end of the FLEXX Extension Study (week 52), the estimated average QALYs gained were 0.163 (95% CI = −0.162 to 0.488) for the 214 patients who received 2 courses of 3-weekly BioHA injections. The HUI-3 scores for the BioHA-treated patients during the 52-week follow-up are plotted in . For the conventional care group, patients were maintained on their original OA care and did not gain any QALYs.
Base case cost-utility analysis scenario
Total treatment costs over 1 year were $3469 for the BioHA group and $4562 for conventional care with NSAIDs. Because BioHA treatment was less costly and more effective than conventional care, BioHA was the dominant strategy, and no ICER was calculated ().
Table 3. Model 1: Cost effectiveness of intra-articular BioHA vs conventional care.
Sensitivity analysis
Results from one-way sensitivity analyses showed that BioHA remained the dominant strategy (both less expensive and more effective) when BioHA and conventional care treatment costs were set at ±20% (). BioHA also remained dominant when QALYs gained were set at the high end of the 95% CI. The only scenario in which BioHA was not dominant was when the QALYs gained with intra-articular BioHA were assumed to be at the lowest end (−0.162).
Table 4. Model 1: Results of one-way sensitivity analysis.
Model 2
Response rates
Patients who received two courses of intra-articular BioHA in the FLEXX Trial and Extension Study achieved a response rate of 56% by the end of the 52-week period. The response rate for conventional treatment was 40%, as reported by Raynauld et al.Citation28.
QALYs gained over 52 weeks
Among patients achieving a response after two courses of intra-articular BioHA, an average of 0.23 QALYs were gained over the 1-year period. Among non-responders, there was an average of 0.08 QALYs gained over the same time period. These utility values were applied to a cost-effectiveness model for responders and non-responders in both the BioHA and conventional care arms ().
Base case CEA scenario
Total treatment costs over 1 year were $1446 for the BioHA group and $516 for patients receiving conventional treatment. Quality-adjusted life year gained was 0.16 for BioHA and 0.14 for conventional treatment. The average cost-effectiveness ratio was $8816 per QALY for BioHA treatment and $3686 per QALY for conventional treatment. The ICER of BioHA, with conventional treatment as the baseline strategy, was $38,741 per QALY gained ().
Table 5. Model 2: Base case scenario results.
Sensitivity analyses
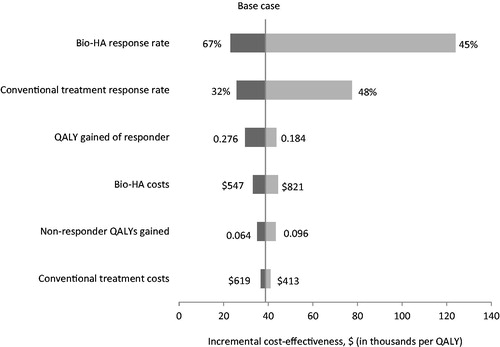
Results from one-way sensitivity analyses showed that the ICER calculated for BioHA was most sensitive to response rates in both the BioHA and the conventional treatment groups. When the BioHA response rate was at the lowest end (45%), the BioHA ICER was nearly $124,000 per QALY. Similarly, if the response rate for conventional care was set high (48%), the BioHA ICER was $77,500 per QALY. The average QALYs gained per responder also affected the BioHA ICER. When the QALY gained per responder was low (0.184), the BioHA ICER was $55,876 per QALY. However, when the QALY gained was high (0.276), the ICER was $29,649 per QALY ().
Figure 3. Model 2: Results of one-way sensitivity analysis. BioHA, bioengineered hyaluronic acid; QALY, quality-adjusted life year.
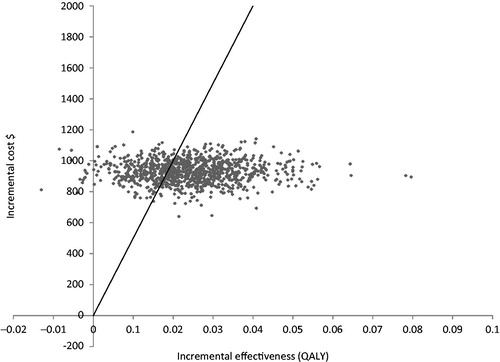
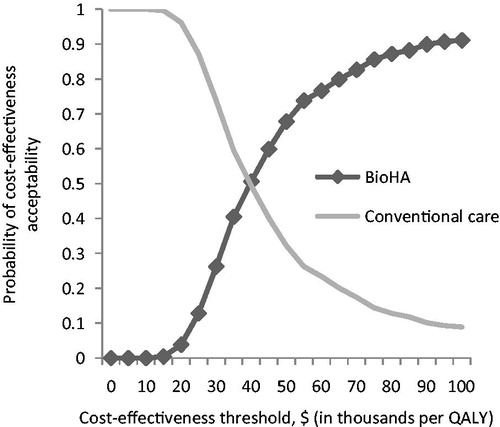
In a probabilistic sensitivity analysis with Monte Carlo Simulation, when the willingness-to-pay level was set at $50,000 per QALY, BioHA was shown to be a cost-effective strategy for OA treatment in ∼70% of simulations (). This simulation was run under various willingness-to-pay assumptions, and corresponding acceptability curves were derived for the 2 treatments (). If the willingness-to-pay threshold was increased to $100,000 per QALY, then the acceptability of BioHA reached 91%, while that of the conventional treatment declined to 9% ().
Discussion
In 2004, the US Food and Drug Administration approved BioHA for the treatment of OA knee pain in patients who do not receive adequate relief from simple pain medication (e.g., acetaminophen) or from exercise and physical therapy. In this cost-effectiveness analysis (Model 1), when outcomes for a BioHA-treated group were compared with patients continuing on conventional care with NSAIDs or other conventional OA treatments (Model 1), BioHA was the dominant strategy. The approach used in Model 1 allowed for a direct comparison of the treatment of non-responding conventional care patients to the addition of BioHA vs ongoing conventional treatment. Sensitivity analyses varying the QALYs gained from BioHA treatment and costs of BioHA and conventional care demonstrated the robustness of these study results. BioHA dominance was eliminated only when the QALYs gained from BioHA were of negative value, an unlikely scenario.
Similarly, when a secondary analysis was run using response rates and cost estimates from alternate sources (Model 2), results still showed BioHA to be more cost-effective than conventional care. Model 2 provides a useful adjunct to the primary study in that it used different inputs for the conventional care group. In Model 2, conventional care was less costly and included all ACR-recommended treatments (except viscosupplementation), including non-pharmacologic, pharmacologic, and surgical approachesCitation28. For example, by study end, 70% of patients randomized to the appropriate care group in Model 2 (using data from Raynauld et al.Citation28) had received corticosteroid injections to the study knee. In contrast, the FLEXX Trial required all patients to stop corticosteroid injections 3 months before study start, and corticosteroids were not permitted for the study duration. Therefore, the conventional treatment response rate used in Model 2 was likely inflated compared with Model 1. Nonetheless, when compared against a more cost-conservative and aggressively treated conventional care group in Model 2, BioHA still met the $50,000 per QALY cost-effectiveness threshold. These secondary results were maintained under the majority of sensitivity analysis scenarios.
It is noteworthy that the conservative cost estimates used in both of the models in this study may have biased the outcomes against BioHA. Although there are several economic studies reporting the treatment costs of conventional care for OA, most are at least 10 years old, making the cost figures obsoleteCitation21,Citation23,Citation31–33,Citation36. In addition, there is a wide range of reported costs in the published literature, with those reported by Waddell et al.Citation23 (applied to Model 1) among the highest estimates in 2012 US dollars ($4562), and the lowest (reported by Losina et al.Citation33, applied to Model 2) at $516.
The use of conventional OA treatment costs from the study by Waddell et al.Citation23 as the input for Model 1 was based in part on the specificity of the costing data reported by these researchers, and in part because these selected costs were conservative estimates. For instance, Mapel et al.Citation37 reported average annual outpatient costs of $4684 in 2001 for OA patients, while Gabriel et al.Citation38 estimated average direct medical charges for OA patients at $2654 in 1997 (adjusted to $4694 for 2012)Citation11. If higher costs for conventional treatment with NSAIDs and analgesics were used in Model 1, it is likely that treatment with BioHA would show even more substantial dominance.
Model 2 used data from Losina et al.Citation33 as the basis for conventional OA treatment costs; this represented ∼11% of the costs reported by Waddell et al.Citation23 With this approach, the BioHA arm was further handicapped by adding 50% of the costs of conventional care to total BioHA costs. Thus, this study’s ICER of $38,741 for BioHA is the most conservative cost estimate of all published research.
It is also worth noting that cost computations for the BioHA group did not include all cost consequences associated with NSAID and analgesic treatments, e.g., the potential for improved patient HRQoL due to the elimination of NSAID treatment side effects. Also, not considered in Model 1 were any downstream consequences from conventional OA therapies. For example, previous studies show that improved outcomes due to viscosupplementation may delay the need for TKR in OA patients (typically reserved as a “last resort” for patients experiencing severe symptoms, functional limitations, and quality-of-life reductions)Citation3,Citation39. An analysis by Waddell and BrickerCitation39 suggests that an average cost of $1420 per knee treated with viscosupplementation could delay TKR by a median of 2.1 years. In addition to quality-of-life improvements, delaying initial TKR may reduce the need for eventual revision TKR by reducing the amount of time the patient has the new joint. Again, if these factors were considered in Model 1, the total costs associated with BioHA would likely be even lower than those observed. Furthermore, Model 2 of this study used conventional care response rates based on Raynauld et al.Citation28; in this case, TKR was included in conventional care inputs and is considered in the cost-effectiveness findings.
The costs of potential AEs due to BioHA treatment were not included in these analytic models because these AEs are generally localized (e.g., pain and swelling of the injected joint) and infrequent, and normally resolve spontaneously or with conventional treatment of symptomsCitation40. Moreover, recent guidelines and reviews by Osteoarthritis Research Society International point out that HA treatments do not have appreciable safety issues, except for transient pain at the injection siteCitation2. Arnold et al.Citation41 reasoned that, in light of systemic side effects of therapies for OA of the knee (e.g., hepatoxicity with acetaminophen, gastrointestinal bleeding with NSAIDs, etc.), patients may prefer local therapy for their joint disease. As such, viscosupplementation therapy with HA in the managed care setting may improve patient outcomes and the efficient use of healthcare resources.
Study limitations
There are several limitations to this study. First, it is important to note that, for both models, the reported cost-effectiveness ratios were dependent on the method used to convert WOMAC scores to HUI-3 values to determine QALYsCitation29. Second, the FLEXX Trial and Extension Study, the clinical trial used as the source for the efficacy assumptions of both models, did not collect downstream healthcare resource utilization data, requiring the use of published literature to identify the costs of conventional treatments. Because the FLEXX trial did not include a conventional treatment comparison arm, Model 1 used data from pre-study conventional treatment. This provided a strict comparison of the same patient group before or after the introduction of BioHA treatment. The analysis favors BioHA under the assumption that the condition of the conventional care group did not either improve or deteriorate over time. However, Model 2 addresses this limitation. A 40% conventional treatment response rate was obtained from a 1-year, multicenter trial conducted by Raynauld et al.Citation28 comparing appropriate care plus CL-HA against appropriate care without CL-HA. In this trial, conventional care included the full range of OA treatments, including NSAIDs and analgesics, corticosteroid injections, and surgical options such as TKR. Thus, Model 2 provides a more “real-world” treatment comparison.
Conclusions
Results from this cost-effectiveness analysis demonstrate that BioHA injection in patients with OA of the knee with inadequate response to conventional therapies is a viable option in terms of both efficacy and cost. When compared with conventional care with NSAIDs and analgesics, BioHA was a dominant treatment strategy. When compared with conventional care with NSAIDs, analgesics, corticosteroids, and surgical options, BioHA was still the cost-effective strategy.
Transparency
Declaration of funding
Financial support for this study and for assistance with medical editing was provided by Ferring Pharmaceuticals Inc.
Declaration of financial/other relationships
Hind T. Hatoum, PhD, is a paid consultant for Ferring Pharmaceuticals Inc., the marketer of one of the study drugs. Funding for the study was provided by Ferring Pharmaceuticals Inc. through a contract with Hind T. Hatoum & Company. Anke L. Fierlinger, MD, is a paid employee of Ferring Pharmaceuticals Inc. Swu-Jane Lin, PhD, is a paid consultant for Hind T. Hatoum & Company. Roy D. Altman, MD, is a paid consultant for Ferring Pharmaceuticals Inc., Abbott Theralogix, LLC, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Rotta Pharmaceuticals Inc., Toltec Pharmaceuticals, LLC, Iroko Pharmaceuticals, LLC, and Novartis Pharmaceuticals Corporation, and also receives clinical trial funding from Ferring Pharmaceuticals Inc. and Novartis Pharmaceuticals Corporation. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors of this study acknowledge the medical writing and editorial assistance of Alanna Kennedy, PhD, at Educational Alliances in Clinical Medicine (Manhasset, NY) for preparation of this manuscript and Ferring Pharmaceuticals Inc. for providing funding for editorial assistance.
Notes
*Euflexxa is a registered trademark of Ferring B.V., Parsippany, NJ, USA.
*Synvisc is a registered trademark of Genzyme Corporation, Ridgefield, NJ, USA.
References
- Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26-35
- Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137-62
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115-26
- Pereira D, Peleteiro B, Araujo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270-85
- Bitton R. The economic burden of osteoarthritis. Am J Manag Care 2009;15(8 Suppl):S230-5
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: a population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs 2012;31:85-91
- Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008;59:1207-13
- Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum 2006;54:226-9
- Centers for Disease Control and Prevention (CDC). Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morb Mortal Wkly Rep 2009;58:421-6
- Centers for Disease Control and Prevention. National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions–United States, 2003. MMWR Morb Mortal Wkly Rep 2007;56:4-7
- US Department of Labor. Bureau of Labor Statistics. CPI Inflation Calculator—2012. Washington, DC. Available at: http://www.bls.gov/data/inflation_calculator.htm. Accessed April 16, 2013
- Torio CM, Andrews RM. National inpatient hospital costs: the most expensive conditions by payer, 2011. HCUP Statistical Brief #160. Rockville, MD: Agency for Healthcare Research and Quality, August 2013
- Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2:CD005321
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465-74
- Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2006;14:154-62
- Altman RD, Rosen JE, Bloch DA, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum 2009;39:1-9
- Altman RD, Rosen JE, Bloch DA, et al. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage 2011;19:1169-75
- Hatoum HT, Rosen JE, Fierlinger AL, et al. Assessment of the health-related quality of life impact of EUFLEXXA® (1% Sodium Hyaluronate) using short form 36 (SF-36) data collected in a randomized clinical trial evaluating treatment of osteoarthritis knee pain. 2013 World Congress on Osteoarthritis; April 18–21, 2013; Philadelphia, PA
- Ramsey S, Willke R, Briggs A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521-33
- Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health 2003;6:9-17
- Torrance GW, Raynauld JP, Walker V, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 2 of 2): economic results. Osteoarthritis Cartilage 2002;10:518-27
- Kahan A, Lleu PL, Salin L. Prospective randomized study comparing the medicoeconomic benefits of Hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine 2003;70:276-81
- Waddell D, Rein A, Panarites C, et al. Cost implications of introducing an alternative treatment for patients with osteoarthritis of the knee in a managed care setting. Am J Manag Care 2001;7:981-91
- Mazieres B, Bard H, Ligier M, et al. Medicoeconomic evaluation of hyaluronic acid for knee osteoarthritis in everyday practice: the MESSAGE study. Joint Bone Spine 2007;74:453-60
- Turajane T, Labpiboonpong V, Maungsiri S. Cost analysis of intra-articular sodium hyaluronate treatment in knee osteoarthritis patients who failed conservative treatment. J Med Assoc Thai 2007;90:1839-44
- Yen ZS, Lai MS, Wang CT, et al. Cost-effectiveness of treatment strategies for osteoarthritis of the knee in Taiwan. J Rheumatol 2004;31:1797-803
- Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res 2008;8:165-78
- Raynauld JP, Torrance GW, Band PA, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): clinical results. Osteoarthritis Cartilage 2002;10:506-17
- Grootendorst P, Marshall D, Pericak D, et al. A model to estimate health utilities index mark 3 utility scores from WOMAC index scores in patients with osteoarthritis of the knee. J Rheumatol 2007;34:534-42
- Centers for Medicare & Medicaid Services. Physician fee schedule look-up. Baltimore, MD. Available at: http://www.cms.hhs.gov/PFSlookup/. Accessed April 16, 2013
- Lee DW, Meyer JW, Clouse J. Implications of controlling for comorbid conditions in cost-of-illness estimates: a case study of osteoarthritis from a managed care system perspective. Value Health 2001;4:329-34
- MacLean CH, Knight K, Paulus H, et al. Costs attributable to osteoarthritis. J Rheumatol 1998;25:2213-8
- Losina E, Daigle ME, Suter LG, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage 2013;21:655-67
- Lussier A, Cividino AA, McFarlane CA, et al. Viscosupplementation with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol 1996;23:1579-85
- Berger A, Bozic K, Stacey B, et al. Patterns of pharmacotherapy and health care utilization and costs prior to total hip or total knee replacement in patients with osteoarthritis. Arthritis Rheum 2011;63:2268-75
- Kamath CC, Kremers HM, Vanness DJ, et al. The cost-effectiveness of acetaminophen, NSAIDs, and selective COX-2 inhibitors in the treatment of symptomatic knee osteoarthritis. Value Health 2003;6:144-57
- Mapel DW, Shainline M, Paez K, et al. Hospital, pharmacy, and outpatient costs for osteoarthritis and chronic back pain. J Rheumatol 2004;31:573-83
- Gabriel SE, Crowson CS, Campion ME, et al. Direct medical costs unique to people with arthritis. J Rheumatol 1997;24:719-25
- Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm 2007;13:113-21
- Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging 2007;24:629-42
- Arnold W, Fullerton DS, Holder S, et al. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm 2007;13(4 Suppl):S3-19; quiz S20–22.