Abstract
Objective:
To develop a decision-analytic model to estimate the cost-effectiveness of initiating maintenance treatment with aripiprazole once-monthly (AOM) vs paliperidone long-acting injectable (PLAI) once-monthly among patients with schizophrenia in the US.
Methods:
A decision-analytic model was developed to evaluate a hypothetical cohort of patients initiating maintenance treatment with AOM or PLAI. Rates of relapse, adverse events (AEs), and direct medical costs were estimated for 1 year. Patients either remained on initial treatment or discontinued treatment due to lack of efficacy, AEs, or other reasons, including non-adherence. Data from placebo-controlled pivotal trials and product prescribing information (PI) were used to estimate treatment efficacy and AEs. Analyses were performed assuming dosing of clinical trials, real-world practice, PIs, and highest therapeutic dose available, because of variation in practice settings. The main outcome of interest was incremental cost per schizophrenia hospitalization averted with AOM vs PLAI.
Results:
Based on placebo-controlled pivotal trials’ dosing, AOM improved clinical outcomes by reducing schizophrenia relapses vs PLAI (0.181 vs 0.277 per person per year [pppy]) at an additional cost of US$1276 pppy, resulting in an incremental cost-effectiveness ratio (ICER) of US$13,280/relapse averted. When PI dosing was assumed, this ICER increased to US$19,968/relapse averted. When real-world dosing and highest available dosing were assumed, AOM was associated with fewer relapses and lower overall treatment costs vs PLAI.
Conclusions:
AOM consistently provided favorable clinical benefits. Under various dosing scenarios, AOM results indicated fewer relapses at lower overall costs or a reasonable cost-effectiveness threshold (i.e., less than the cost of a hospitalization relapse) vs PLAI. Given the heterogeneous nature of schizophrenia and variability in treatment response, health plans may consider open access for treatments like AOM. Since model inputs were based on data from separate placebo-controlled trials, generalization of results to the real-world setting is limited.
Introduction
Schizophrenia presents a significant economic burden to society and healthcare plans with an estimated US healthcare cost of US$22.7 billion in 2002 based on direct healthcare costs (drug acquisition, inpatient, outpatient, and long-term care), which is equivalent to US$46.7 billion in 2012 (when adjusted for inflation using the US Consumer Price Index Medical Care Category, 2013)Citation1. Relapses in patients with schizophrenia often require hospitalization, contributing substantially to the total healthcare costs associated with this diseaseCitation2, highlighting the importance of relapse prevention. Such relapses may also involve intensive management of patients in the form of emergency room visits, visits to general physicians and psychiatric care, and other outpatient careCitation1. With the prevalence of schizophrenia in the US estimated at 2.4 million adults (or 1.1% of the adult patient population)Citation3 and relapse rates at 40–50% at 1 year and 80% at 5 yearsCitation4–6, an important aim of treatment is to reduce the risk of relapse-related hospitalizations, possibly by improving adherence to antipsychotic therapyCitation7,Citation8.
US and international guidelines recommend that clinicians consider patient preference and potential benefits of long-acting injectable (LAI) antipsychotic medications when making decisions about choice of medication in schizophreniaCitation9–14. LAI antipsychotic medications have been reported to reduce schizophrenia relapse relative to oral agents in retrospective studiesCitation15,Citation16, and several second-generation (atypical) LAI antipsychotic medications are now available, adding to the treatment choices for individual patientsCitation17. There are multiple potential clinical benefits of LAI treatment. In addition to the fact that LAIs provide sustained drug availability due to the drug formulation benefits of long-acting dosage forms, patients treated with LAIs must visit the clinic every 1–6 weeksCitation18. This additional interaction with healthcare professionals may serve to facilitate treatment persistence and adherence, and help to reduce the risk of future relapses and hospitalizationsCitation19. However, despite potential advantages with LAIs, use of LAI treatment can still be optimized in clinical practice in the USCitation14.
Currently, only two once-monthly, second-generation LAIs that do not require special post-injection monitoring are available: aripiprazole once-monthly (AOM)Citation20 and paliperidone palmitate LAI (PLAI)Citation21; risperidone is twice monthly and, hence, not included in this list. AOM is the only LAI antipsychotic with partial agonist activity at dopamine D2 and serotonin 5-HT1A receptors. Aripiprazole’s pharmacologic profile thus differs from currently marketed LAI atypical antipsychotics, which are all full antagonists at the dopamine D2 receptor.
The choice of initial LAI for the treatment of patients with schizophrenia is of paramount importance since relapse prevention is a major goal of antipsychotic therapy. Patients who discontinue treatment with an LAI (e.g., due to relapse or adverse events) and are switched to a different medication may be less likely to continue taking the second treatment for a variety of reasons, including the perception that the newer treatment will be ineffective. Consequently, understanding which LAI is most likely to be taken consistently without requiring a medication switch to aid patient treatment persistence is of value to health plans in the US, since they evaluate drugs for various levels of formulary placement. Further, selection of an appropriate treatment for patients with schizophrenia should be based on effectiveness and total cost of care, i.e., both the drug price (acquisition cost) and total medical costs of managing a patient while on treatment, including cost of outcomes such as hospitalization due to relapses and drug-related adverse events.
Given the high costs of schizophrenia care, a key consideration for healthcare decision-makers is the assessment of cost-effectiveness of treatments among patients receiving LAIs. Decision analytic modeling provides a methodological framework for policy and healthcare decision-makers to gain insights into the clinical benefits and associated costs of various treatment options. Such models have been used in the evaluation of LAIs for schizophrenia treatmentCitation22. A measure that is useful for decision-makers is the incremental cost-effectiveness ratio (ICER): incremental cost/incremental effectiveness, where the former is the costs associated with use of drug A minus that of drug B, and the latter is the difference between number of relapses associated with drugs A and BCitation22,Citation23. Previous cost-effectiveness evaluations regarding treatments for schizophrenia have focused on risperidone LAI (administered every 2 weeks)Citation24 and PLAICitation22.
To further our understanding of the potential clinical and economic trade-offs associated with AOM vs PLAI treatment, a decision-analytic model was developed to compare direct medical costs, relapse-related hospitalizations, and adverse events between treatments.
Methods
Model overview
To increase the utility and clinical relevance of this cost-effectiveness model to US payers, the following factors were considered in model development. First, US payers are primarily interested in economic models that quantify the benefit of treatments over a short time horizon. This is especially relevant in modeling schizophrenia outcomes, since clinical benefits due to relapse prevention are typically seen within the first year of treatment. The current model, therefore, compared cost-effectiveness of AOM vs PLAI, both administered monthly, over a 1-year time horizon. Additionally, US payers prefer economic models that are transparent, clinically meaningful, intuitive, and valid. The current model thus used efficacy and adverse event data directly from AOMCitation25 and PLAICitation26 placebo-controlled trials and prescribing informationCitation20,Citation21. Because of a lack of comparative placebo-controlled data, risperidone LAI was not included in the model as an initial therapeutic choice, but it was included under the category of other LAI treatments. Finally, because there is significant dosing variability in clinical practice and clinical trial settings, model results are presented for multiple dosing assumptions.
Model structure
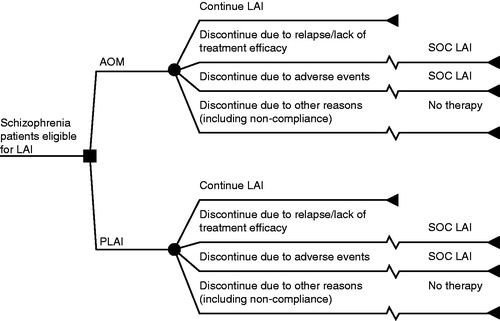
A 1-year decision-analytic model was developed to estimate the cost-effectiveness of initiating treatment with AOM vs PLAI, with results reported in costs per schizophrenia relapse-related hospitalizations averted in the US. The model evaluated a cohort of patients with schizophrenia who were eligible to receive an LAI and compared patients initiating AOM or PLAI once-monthly as index therapies. TreeAge Pro 2012 software (TreeAge Software Inc., Williamstown, MA) was used to develop the model and shows a schematic representation of the model used.
Figure 1. One-year schematic decision tree model for antipsychotic drugs in patients with schizophrenia. AOM, aripiprazole once-monthly; LAI, long-acting injectable; P, paliperidone once-monthly; SOC, standard-of-care. SOC LAI includes fluphenazine, haloperidol, and risperidone, and the model assumes the average of relapse rates and adverse events for SOC.
Model assumptions
Patient progression through treatment pathways was based on evidence from pivotal clinical trials used in the drug approval processCitation25,Citation26. Patients either remained on initial treatment throughout the course of 1 year or discontinued treatment at 6 months because of relapse/lack of treatment efficacy, adverse events, or other reasons, including non-adherence. In the model, treatment discontinuation is assigned at 6 months because the model estimates an average duration of treatment that ranges from a short period to up to 1 year; this assumption was consistent between the two treatment arms. Those who discontinued treatment because of relapse/lack of treatment efficacy or adverse events switched from their initial treatment to a different standard-of-care (SOC) LAI, whereas those who discontinued treatment because of other reasons did not receive additional therapy. The SOC LAI treatment option consisted of commonly used LAIs in the US, including fluphenazine, haloperidol, and risperidone.
Patients could experience adverse events while on treatment and, at all points in the model, patients could experience schizophrenia relapse resulting in hospitalization. Clinical estimates including relapse rates for patients on SOC LAICitation27 and no treatmentCitation28, and adverse event ratesCitation29,Citation30, were derived from published literature as described in more detail later. All actively treated patients were assumed to require one office visit per month for receiving their injection (i.e., were considered to be clinically stable and not requiring more frequent visits). Patients who switched treatments accrued the cost of three additional office visits to reflect routine clinical practice where physicians may monitor patients at the time of switching treatments in search of the optimal treatment and dosage.
Clinical and cost model inputs
Relapse rates
Patients were at risk of disease relapse throughout the course of a year, regardless of treatment type. shows the different relapse rates used in the model for each index therapy, SOC LAI, and no treatment based on the literature. For patients on index therapies, relapse rates were based on registrational, placebo-controlled clinical trial dataCitation25,Citation26.
Table 1. Efficacy and safety model inputs.
Because patients who changed treatment did so after 6 months of index therapy, relapse rates estimated from the literature were converted to 6-month probabilities as appropriate. For patients on each of the three therapies included in the SOC LAI, the risk of relapse was reported as 6-month ratesCitation27, and averaged before converting to a single 6-month probability for use in the model. For patients who did not receive any treatment, the relapse rate was based on data from a study conducted by Viguera et al.Citation28, where higher relapse rates were reported in patients without treatment.
Adverse events
The following clinically relevant adverse events reported in the treatment prescribing information were included in the model: akathisia, weight gain, hyperlipidemia, and extrapyramidal symptoms. Hyperprolactinemia was also added in the model, since prolactin-related side effects can be of significant concern in patients treated with LAIsCitation33. Short-term trials of the oral version of index therapies were used to base model rates of adverse events, since these trials may provide a better perspective of safety and tolerability. Rates from the trials were applied annually, regardless of the duration of the trial; hence, the events reported were assumed to occur throughout the course of a year without further extrapolation.
Indirect comparisons were conducted using data from each product’s prescribing information to determine the rates of adverse events for all therapies. AOM was selected as the reference drug, and the adverse event rates for PLAI, fluphenazine LAI, haloperidol LAI, and risperidone LAI were calculated. Since no cases of hyperprolactinemia were reported in the trial informing AOM rates, indirect comparisons were not possible, and the rates reported for all other therapies were not adjusted. The results of the indirect comparisons for the three SOC LAI products were averaged for use in the model. describes the rates of adverse events for patients on AOM, PLAI, and SOC LAIs.
Costs
Costs were estimated using publicly available databasesCitation34–36. The cost of drugs and treatment administration were assumed to be fixed and not vary by geographic location or contracted rates. Costs were captured in the model by multiplying resource utilization by unit costs, where costs were reported in 2013 US dollars and inflated using the medical care component of the Consumer Price IndexCitation37 (). Additionally, because the model was a 1-year time horizon model, no discount rate was applied to the costs or benefits. Therapy acquisition costs were based on published wholesale acquisition cost pricing. Therapy administration costs were estimated assuming 1-h of nurse time per injection and using wage estimates from the US Bureau of Labor StatisticsCitation35,Citation39 (). Costs of relapse included those of hospitalization and other services including: emergency room visits, physician visits, mental health clinic visits, home care, and social/group interventionsCitation39,Citation40 (). Adverse event costs were based on treatments previously reportedCitation30,Citation38.
Table 2. Model cost estimates.
Table 3. Cost of relapse.
Cost-effectiveness
Cost-effectiveness of AOM compared with PLAI was evaluated by calculating the additional costs per relapse averted as follows: (1) costs and number of relapses for patients starting on each index therapy, and (2) incremental costs and the decrease in number of relapses when using AOM compared with PLAI. Dividing these differences resulted in the additional cost per relapse averted.
Given the variation in dosing based on practice setting, four different dosing strategies were considered (). These dosing strategies were: dosing observed in the Kane et al.Citation25 and Hough et al.Citation26 trials; real-world dosing based on observed utilization for both index therapies, as described in ; prescribing information dosing; and highest available dosing with equivalent treatment efficacy. Finally, one-way sensitivity analyses were conducted on key model parameters such as relapse rates and cost of adverse events to assess the impact of these parameters on ICER results.
Table 4. Drug cost model inputs by dosing strategy.
Results
A summary of the cost-effectiveness analyses using the four dosing strategies is depicted in . Using dosing from the Kane et al.Citation25 and Hough et al.Citation26 trials, the use of AOM was found to reduce schizophrenia relapses compared with PLAI by 0.096 per person per year (0.181 vs 0.277; ). This additional clinical benefit of fewer relapses with AOM came at an increased cost of US$1276 per person per year (US$21,653 for AOM vs US$20,377 for PLAI), generating an ICER of US$13,280 per relapse averted. In using dosing from the Kane et al.Citation25 and Hough et al.Citation26 trials, the assumptions were conservative with respect to AOM, since doses did not include a commonly prescribed high dose (234 mg) of PLAI. For PLAI, dosing observed in the Hough et al.Citation26 trial was above that in the prescribing informationCitation21, but below that found in the real-world settingCitation41. Thus, when real-world dosing was assumed, PLAI was more costly than when dosing from the Hough et al.Citation26 trial was used, resulting in AOM being the dominant strategy (more efficacious, less costly) compared with PLAI (). When dosing from prescribing information was used, AOM continued to have few relapses per dosing, as observed in the Kane et al.Citation25 and Hough et al.Citation26 trials, but with higher overall costs for an ICER of US$19,968 per relapse averted (). When the highest available dosing and equivalent treatment efficacy was assumed for both index drugs, AOM was the dominant strategy compared with PLAI ().
Figure 2. Overall summary of the cost-effectiveness of AOM compared with PLAI for the four dosing strategies. AOM, aripiprazole once-monthly; LAI, long-acting injectable; P, paliperidone; PPPY, per patient per year. * Total cost of AOM minus total cost of PLAI treatment strategy PPPY; †‘Dominant’ defined as fewer relapses and lower total costs; ‡Relapses in AOM minus relapses in PLAI treatment strategy PPPY.
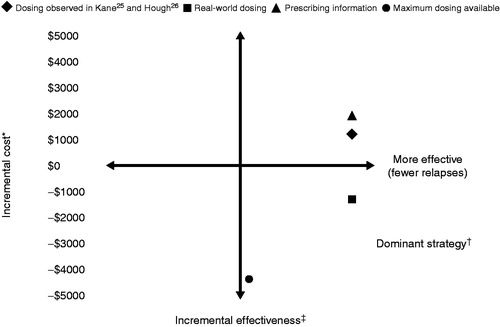
Table 5. Dosing strategy analyses: USD/relapse averted.
In one-way sensitivity analyses, results were most sensitive to index treatment dose. Parameters such as cost of relapse-related hospitalizations and cost of adverse events were also investigated. In all such sensitivity analyses, AOM was found to be a cost-effective treatment alternative compared with PLAI.
Discussion
Cost-effectiveness analysis is a method for objectively assessing the value of treatments by evaluating incremental clinical benefit with respect to incremental costs. Ultimately, cost-effectiveness models enable healthcare decision-makers to make informed choices regarding treatment access. Since the use of LAIs in patients with schizophrenia are associated with a reduction in relapses based on improved disease management and better treatment adherenceCitation22, they may be considered earlier in the course of illness. However, due to high acquisition and administration costs of LAIs, their use may be limitedCitation22; robust pharmacoeconomic evaluations of these medications are, thus, warranted. Our aim was to develop a cost-effectiveness model to estimate the value of using AOM compared with PLAI administered monthly after considering the clinical effectiveness and costs associated with each treatment.
In the analysis using dosing from the Kane et al.Citation25 and Hough et al.Citation26 trials, model results indicate that the total cost of the AOM treatment strategy was US$21,653 compared with US$20,377 for PLAI. It is important to note that, although the model input drug costs were higher by US$3839 for AOM (US$17,323 for AOM minus US$13,484 for PLAI), the model output indicates that the total cost differential, including medical and drugs costs between the two treatments, is only US$1276 per patient per year (US$21,653 for AOM minus US$20,377 for PLAI). This result highlights the cost-offset opportunity presented by AOM due to fewer relapse and costs associated with adverse events compared with PLAI. Hence, the use of AOM in patients with schizophrenia improves clinical outcomes by reducing relapses at an increased cost of US$13,280 per relapse averted. The higher drug costs for AOM were partially attributed to longer time on treatment (i.e., additional monthly injections) because 75% of patients initiating AOM remained on treatment throughout the year compared with 67% of patients initiating PLAI. Similar results were predicted using dosing from the prescribing information, further substantiating AOM as a cost-effective treatment. In the absence of a widely accepted cost-effectiveness threshold in the US for incremental cost per schizophrenia relapse averted, it can be argued that, since the ICER for AOM is US$13,280, which is approximately half the cost of an inpatient relapse (US$25,395), AOM offers improved clinical benefit compared with PLAI at a reasonable cost to payers. Overall, results based on four different dosing assumptions indicate that, depending on the dosing strategy, treatment with AOM was either the dominant strategy (i.e., fewer relapses and lower total costs) or a strategy that was associated with fewer relapses at a reasonable incremental cost compared with PLAI.
Given the heterogeneous characteristics of schizophrenia and patient treatment response, including tolerability, treatment access barriers such as step-edits and prior-authorization policies may restrict physicians from selecting appropriate treatments for their patientsCitation42. Several studies evaluating the impact of restrictive access policies on outcomes suggest that restrictive policies may negatively impact patient outcomes and result in higher medical costsCitation43–45 and also lead to higher rates of treatment discontinuationCitation44. Health plans expect access barriers to increase cost savings; however, previous studies demonstrated no overall impact of the restrictive policy on resource utilization and costsCitation42,Citation46.
Since short gaps in medication adherence increase the risk for relapseCitation47, health plans should avoid imposing treatment access barriers for patients with severe mental illnesses. Considering that step-edits and prior-authorization policies may not positively affect outcomes such as resource use, costs, and treatment adherence among patients with schizophrenia, providing open access to cost-effective treatments such as AOM may be a suitable formulary management policy for health plans.
Another important consideration for health plans is the use of real-world data for decision-making. Real-world monthly maintenance dosing of PLAI in patients with schizophrenia has been reported to range from 157 mg to 172 mg by Kamut et alCitation41 compared with a recommended maintenance dose of 117 mg in the prescribing informationCitation21. These results provide evidence for the treatment-related cost advantage of AOM and, as such, an alternative treatment choice for patients with schizophrenia.
While there were a number of model variables that had some amount of uncertainty, the main model input that resulted in variation in results was in the dose of each index therapy used. Since the severity of a relapse imposes a great burden on the patient and informal caregivers, it can be challenging to fully demonstrate the affect of treatments on clinical and quality-of-life benefits. Considering that AOM improved clinical outcomes by reducing relapses, the results emphasize the value of AOM treatment for patients with schizophrenia.
The outcome of cost per schizophrenia relapse averted was selected since the results may be more accessible to patients, clinicians, and payers than dollars per quality adjusted life year (QALY). In the US, the use of QALY as an outcome is not widely accepted in routine formulary decision-making, due to the substantial assumptions made when considering this measure, and data on quality adjustments are limited and/or inconsistent between sources. Further, QALYs may not be the most appropriate effectiveness measure in the context of a short-term (1-year) evaluation model in the US.
Overall, the results showed that AOM is a cost-effective treatment compared with PLAI. In technical cost-effectiveness terms, AOM was the dominant strategy compared with PLAI when real-world dosing and highest available dosing with equivalent treatment efficacy were assumed. AOM remained a cost-effective treatment option compared with PLAI when more conservative dosing strategies were considered in the analysis.
AOM and PLAI, despite both being categorized as second-generation antipsychotics, are quite different in terms of their overall pharmacodynamic profiles. These profiles may translate to differences in efficacy and tolerability when treating individual patientsCitation48. A patient’s past history of sensitivity toward specific adverse events may influence their values and preferences regarding antipsychotic choice. These include adverse events such as alterations in weight and metabolic variables, sedation, extrapyramidal symptoms, and akathisia, and the effects of elevations in plasma prolactin.
Limitations
Results of this analysis must be considered in light of its limitations. The analysis was based on data from separate registrational placebo-controlled clinical trials, where adherence is optimized. It is possible that results would be different if compared in real-world settings. The analysis should also be considered in light of the assumptions made regarding drug dosing. Real-world PLAI dosing is higher than that used in the clinical trial data, which was used to inform model efficacyCitation26, and dosing of AOM has been shown to be lower than dosing reported in the trial used for estimating AOM model efficacy ()Citation25. However, the precise differential efficacy of PLAI at higher doses is not known, and, if higher doses result in increased treatment efficacy, this could have biased the results of the scenario analysis in favor of AOM.
There was additional uncertainty about the composition and efficacy of the SOC LAI. In defining the treatments after discontinuation of the index therapy, a simplifying assumption was that patients receiving the SOC LAI were equally distributed between using fluphenazine LAI, haloperidol LAI, and risperidone LAI. In clinical practice, there are additional treatments that patients may receive after discontinuing index therapy, that were not considered in the analysis. In sensitivity analyses, the cost of the SOC LAI was varied, and results were reasonably stable even when making extreme assumptions about the composition of the SOC LAI. In addition, it was assumed that patients remained on these treatments for the remainder of the year without the possibility of discontinuation, which may not reflect true clinical practice.
This study may not be generalized to other populations in other treatment settings and/or countries where costs are substantially different. Additionally, this study did not include societal costs (i.e., those not borne by the payer). While these costs are an important consideration, they are difficult to quantify and may bring in unwarranted subjectivity into the interpretation of the results.
Conclusions
In terms of cost-effectiveness, results ranged from AOM either having fewer relapses and lower costs or providing clinical benefits at a reasonable cost compared with PLAI. Given the negative impact that restrictive access policies may have on patient outcomes, health plans may consider providing open access to cost-effective treatments such as AOM based on its favorable clinical and economic benefits in the treatment of schizophrenia.
Transparency
Declaration of funding
This study was sponsored by Otsuka America Pharmaceutical, Inc., Princeton, NJ, USA; Otsuka Pharmaceutical Development and Commercialization, Inc., Princeton, NJ, USA; and H. Lundbeck A/S, Copenhagen, Denmark.
Declaration of financial/other relationships
Leslie Citrome has engaged in collaborative research with, or received consulting or speaking fees from: Alexza, Alkermes, AstraZeneca, Avanir, Bristol-Myers Squibb, Eli Lilly, Envivo, Forest, Genentech, Janssen, Lundbeck, Merck, Mylan, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, and Valeant in the past 36 months, and owns a small number of shares of common stock in Bristol-Myers Squibb, Eli Lilly, J & J, Merck, and Pfizer. Ross A. Baker is an employee of Otsuka Pharmaceutical Development and Commercialization, Inc. Christophe Sapin and Karina Hansen are employees of H. Lundbeck A/S. Anna Eramo is an employee of Lundbeck. Benjamin Gutierrez and Siddhesh A. Kamat are employees of Otsuka America Pharmaceutical, Inc. Jesse Ortendahl and Tanya G. K. Bentley are employees of Partnership for Health Analytic Research, LLC, which has received financial funds from Otsuka America Pharmaceutical, Inc., in connection with conduction of this study.
Acknowledgments
Medical writing and editorial support for the preparation of this manuscript was provided by Scientific Connexions, Inc., Lyndhurst, NJ, funded by Otsuka America Pharmaceutical, Inc., Princeton, NJ, and H. Lundbeck A/S, Copenhagen, Denmark.
References
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122–9
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry 2010;10:2
- National Institute of Mental Health. Numbers count: mental disorders in America. 2013. www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-in-america/index.shtml#Schizophrenia. Accessed December 19, 2013
- Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241–7
- Schooler NR. Relapse and rehospitalization: comparing oral and depot antipsychotics. J Clin Psychiatry 2003;64(16 Suppl):14–17
- Schennach R, Obermeier M, Meyer S, et al. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv 2012;63:87–90
- Hasan A, Falkai P, Wobrock T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 2013;14:2–44
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. American Psychiatric Association, http://psychiatryonline.org/content.aspx?bookID=28§ionID=1665359. Accessed December 20, 2013
- Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56
- Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193–217
- McEvoy JP, Scheifler PL, Frances A. Expert consensus guideline series: treatment of schizophrenia 1999. J Clin Psychiatry 1999;60(11 Suppl):1–80
- International Psychopharmacology Algorithm Project. www.ipap.org. Accessed December 19, 2013
- Moore TA. Schizophrenia treatment guidelines in the United States. Clin Schizophr Relat Psychoses 2011;5:40–9
- Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry Suppl 2009;52:S63–7
- Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 2013;74:957–65
- Rossi G, Frediani S, Rossi R, et al. Long-acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations. BMC Psychiatry 2012;12:122
- Citrome L. New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Rev Neurother 2013;13:767–83
- Peng X, Ascher-Svanum H, Faries D, et al. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res 2011;3:9–14
- Olfson M, Marcus SC, Ascher-Svanum H. Treatment of schizophrenia with long-acting fluphenazine, haloperidol, or risperidone. Schizophr Bull 2007;33:1379–87
- Otsuka Pharmaceutical Company Inc. Aripiprazole prescribing information. 2013. Available at: http://www.otsuka-us.com/Documents/Abilify.PI.pdf. [Last accessed April 2014]
- Janssen Pharmaceuticals Inc. Paliperidone palmitate injectable prescribing information. 2012. Available at: http://www.invegasustenna.com/pdf/invegasustenna-prescribing-info.pdf [Last accessed April 2014]
- Achilla E, McCrone P. The cost effectiveness of long-acting/extended-release antipsychotics for the treatment of schizophrenia a systematic review of economic evaluations. Appl Health Econ Health Policy 2013;11:95–106
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn. New York: Oxford University Press, 2005
- Janssen Pharmaceuticals Inc. Risperidone long-acting injection prescribing information. 2012. Available at: http://www.janssencns.com/risperdal/risperdal-prescribing-information. [Last accessed April 2014]
- Kane J, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012;75:617–24
- Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 2010;116:107–17
- Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull 2014;40:192–213
- Viguera AC, Baldessarini RJ, Hegarty JD, et al. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry 1997;54:49–55
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizoph Res 2004;71:155–65
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin 2011;27:713–30
- APP Pharmaceuticals LLC. Fluphenazine decanoate injection prescribing information. 2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/071413s019lbl.pdf [Last accessed April 2014]
- Ortho-McNeil-Janssen Pharmaceuticals. Haloperidol decanoate injection prescribing information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018701s054lbl.pdf [Last accessed April 2014]
- Kelly DL, Wehring HJ, Earl AK, et al. Treating symptomatic hyperprolactinemia in women with schizophrenia: presentation of the ongoing DAAMSEL clinical trial (Dopamine partial Agonist, Aripiprazole, for the Management of Symptomatic Elevated prolactin). BMC Psychiatry 2013;13:214
- Medi-Span Price Rx.® https://pricerx.medispan.com/ProductName.aspx. Accessed December 19, 2013
- US Department of Labor. Bureau of Labor Statistics. Archived consumer price index detailed report information. www.bls.gov/cpi/cpi_dr.htm. Accessed December 19, 2013
- US Department of Health & Human Services. Agency for Healthcare Research and Quality. HCUPnet. http://hcupnet.ahrq.gov. Accessed December 19, 2013
- US Department of Labor. Consumer Price Inflator. www.bls.gov/cpi#data. Accessed March 28, 2014
- Edwards NC, Locklear JC, Rupnow MF, et al. Cost effectiveness of long-acting risperidone injection versus alternative antipsychotic agents in patients with schizophrenia in the USA. Pharmacoeconomics 2005;23(1 Suppl):75–89
- US Department of Labor. Occupational employment statistics. www.bls.gov/oes/current/oes_nat.htm#29-0000. Accessed March 2, 2014
- US Department of Health and Human Services. Agency of Healthcare Research home page. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=D19601906527E991&Form=SelCROSSTAB&JS=Y&Action=%3E%3ENext%3E%3E&_Oneway=Yes. Accessed March 28, 2014
- Kamat S, Gutierrez B, Eramo A, et al. Initial assessment of real-world usage of extended-release injectable paliperidone palmitate among medicaid insured schizophrenia patients. Value Health 2013;16:A61
- Abouzaid S, Jutkowitz E, Foley KA, et al. Economic impact of prior authorization policies for atypical antipsychotics in the treatment of schizophrenia. Popul Health Manag 2010;13:247–54
- Gitlin M, Neuchterlein K, Subotnik KL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001;158:1835–42
- Soumerai SB, Zhang F, Ross-Degnan D, et al. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Aff (Millwood) 2008;27:w185–95
- Law MR, Ross-Degnan D, Soumerai SB. Effect of prior authorization of second-generation antipsychotic agents on pharmacy utilization and reimbursements. Psychiatr Serv 2008;59:540–6
- Tunis SL, Faries DE, Nyhuis AW, et al. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trial. Value Health 2006;9:77–89
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886–91
- Volavka J, Citrome L. Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother 2009;10:1917–28
