Abstract
Objectives:
To conduct an economic evaluation of the currently prescribed treatments for stroke prevention in patients with non-valvular atrial fibrillation (NVAF) including warfarin, aspirin, and novel oral anticoagulants (NOACs) from a French payer perspective.
Methods:
A previously published Markov model was adapted in accordance to the new French guidelines of the Commission for Economic Evaluation and Public Health (CEESP), to adopt the recommended efficiency frontier approach. A cohort of patients with NVAF eligible for stroke preventive treatment was simulated over lifetime. Clinical events modeled included strokes, systemic embolism, intracranial hemorrhage, other major bleeds, clinically relevant non-major bleeds, and myocardial infarction. Efficacy and bleeding data for warfarin, apixaban, and aspirin were obtained from ARISTOTLE and AVERROES trials, whilst efficacy data for other NOACs were from published indirect comparisons. Acute medical costs were obtained from a dedicated analysis of the French national hospitalization database (PMSI). Long-term medical costs and utility data were derived from the literature. Univariate and probabilistic sensitivity analyses were performed to assess the robustness of the model projections.
Results:
Warfarin and apixaban were the two optimal treatment choices, as the other five treatment strategies including aspirin, dabigatran 110 mg, dabigatran in sequential dosages, dabigatran 150 mg, and rivaroxaban were strictly dominated on the efficiency frontier. Further, apixaban was a cost-effective alternative vs warfarin with an incremental cost of €2314 and an incremental quality-adjusted life year (QALY) of 0.189, corresponding to an incremental cost-effectiveness ratio (ICER) of €12,227/QALY.
Conclusions:
Apixaban may be the most economically efficient alternative to warfarin in NVAF patients eligible for stroke prevention in France. All other strategies were dominated, yielding apixaban as a less costly yet more effective treatment alternative. As formally requested by the CEESP, these results need to be verified in a French clinical setting using stroke reduction and bleeding safety observed in real-life patient cohorts using these anticoagulants.
Introduction
Atrial fibrillation (AF), a serious and common cardiac disease, affects between 600,000–1,000,000 patients in France, the majority of whom are aged >75 yearsCitation1. AF is one of the main risk factors for strokeCitation1, the third most common cause of mortality in the countryCitation2. Further to the major humanistic consequences, stroke also constitutes an ongoing source of burden for the French healthcare system. For example, a recent study undertaken on behalf of the French Ministry of HealthCitation3 showed that the overall healthcare cost of managing stroke in 2007 was €5.3 billion, mainly from nursing care (€2.4 billion), loss of productivity (€255.9 million), and social benefit payments (€63.3 million)Citation3.
Given the strong association between AF and the occurrence of stroke, the management of AF focuses on the reduction of stroke episodes through the administration of oral anticoagulants (OACs), such as vitamin K antagonists (VKA). According to the International Self-Monitoring Association of Oral Anticoagulated Patients (ISMAAP)Citation4, it is estimated that 600,000 patients receive VKA treatment in France. However, traditional VKA treatment is complicated by potential bleeding episodes in the absence of adequate control, as monitored by the International Normalized Ratio (INR)Citation5, and is often linked with a lower predictability of anticoagulant effects and a higher probability of food and drug interactionsCitation6. As a result, uncontrolled use of VKAs leads to 17,000 hospitalizations and 5000 fatalities per year in FranceCitation7. Such complications have contributed to the under-use of anticoagulant therapy, with a significant proportion of patients being prescribed antiplatelet therapy such as aspirinCitation8,Citation9, which is associated with inferior protection in preventing strokes in comparison to anticoagulant therapyCitation10.
The introduction of novel oral anticoagulants (NOACs) for prevention of stroke in AF patients has been encouraging, due to the lower associated risk of intracerebral hemorrhage and lack of VKA complications such as monitoring food–drug and drug–drug interactionsCitation1. These include the direct thrombin inhibitor dabigatran and direct Factor Xa inhibitors rivaroxaban and apixaban, which may all be an improved means of managing stroke riskCitation5. Data from three large multinational clinical trials, RELYCitation11, ROCKET-AFCitation12, and ARISTOTLECitation13, showed that dabigatran at a dose of 150 mg BID and apixaban at a dose of 5 mg BID were superior to dose-adjusted warfarin in the prevention of stroke events, whilst non-inferiority was shown for dabigatran at a dose of 110 mg BID and rivaroxaban at a dose of 20 mg QD. In terms of safety end-points, dabigatran 110 mg and apixaban resulted in lower risks of intracranial hemorrhages and other major bleeds compared to warfarin, whilst dabigatran 150 mg and rivaroxaban were non-inferior. Of the three NOACs, apixaban is the only NOAC to demonstrate superiority to aspirin in prevention of stroke events in the AVERROES trialCitation14 without significantly increasing the risks of major bleeding.
A formal introduction of physicians to the practical use of the various NOACs was attempted by the European Heart Rhythm Association (EHRA) in 2013, which published a guide outlining the use of the different treatment options available for safer and more effective prescriptionsCitation15. The Association’s 2012 guidelines suggested that NOACs should be considered in place of adjusted dose VKACitation10. Despite this recommendation and improved clinical data observed in NOAC trials, patient surveys have demonstrated a reluctance in some patients to switch therapy from traditional warfarinCitation16–18. This, alongside lack of evidence to support an argument in favor of a particular NOAC in the guidelines, suggests a gap in the evidence and a need for comparative assessment of the overall benefits associated with alternative OAC therapiesCitation16. An assessment of the cost-effectiveness of the various interventions for the prevention of stroke and systemic embolism (SE) in France has not been performed yet, and, given the existing burden of the AF complications for the French healthcare system, a comparison between currently available therapies for AF is needed. To inform decision-making surrounding the optimal treatment, the trade-offs between costs and benefits for each treatment alternative should be considered to aid identification of the treatments that provide most clinical value for a given investment. In France, a decree concerning the medico-economic evaluation of health products was published in 2012, with an implementation in 2013. The medico-economic evaluation becomes an additional determinant for fixing the prices of health products by the Health Products Economic Committee (Comité économique des produits de santé, CEPS) and, thus, modifies the market access conditionsCitation19. The objective of this study was, therefore, to conduct an economic evaluation of the currently prescribed treatments for stroke prevention in patients with AF including warfarin, aspirin, and, per the recent guidelinesCitation15, apixaban, rivaroxaban, and dabigatran from the perspective of the French National Authority for Health (Haute Autorité de Santé, HAS).
Methods
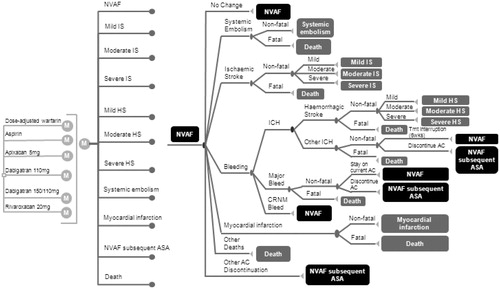
The model used in this study was adapted in accordance to the new French guidelines of the Commission for Economic Evaluation and Public Health (Commission Evaluation Economique et de Santé Publique, CEESP) from a previously published modelCitation20,Citation21. The model was developed in Microsoft Excel using a 6-week cycle Markov approach (). Clinical events considered included: ischemic stroke; hemorrhagic stroke; SE; intracranial hemorrhage (ICH) that is not hemorrhagic stroke (referred to as other ICH); other major bleeds (non-ICH major bleeds); clinically relevant non-major bleeding (CRNMB); myocardial infarction (MI); and death. Treatments included in the analysis were: dose-adjusted warfarin (base case comparator); aspirin; apixaban 5 mg BID; dabigatran 110 mg BID; dabigatran 150 mg BID; and switch to 110 mg BID at the age of 80 years, as indicated by the summary of product characteristics; dabigatran 150 mg BID; and rivaroxaban 20 mg QD.
Figure 1. Schematic of Markov model. ASA, aspirin; CRNM, clinically relevant non-major; ICH, intracranial hemorrhages; NVAF, non-valvular atrial fibrillation; AC, anticoagulant; IS, ischemic stroke; HS, Hemorrhagic stroke.
Model inputs on patient characteristics, and clinical event rates were obtained from a previously published cost-effectiveness analysis. In summary, event rates for warfarin and apixaban were obtained from the ARISTOTLE trialCitation13, while those for aspirin were derived from the AVERROES trialCitation14. Clinical event rates for other comparators were estimated by means of hazard ratios (HRs) relative to apixaban, HRs for rivaroxaban, and dabigatran were from an indirect treatment comparisonCitation20,Citation21, as head-to-head data were not available. These HRs were calculated using the Bucher methodCitation22, in the following studies: ARISTOTLECitation13 (apixaban 5 mg twice daily vs warfarin, dose adjusted to maintain an international normalized ratio [INR] of 2.0–3.0); the ROCKET–AF studyCitation12 (rivaroxaban 20 mg once daily vs warfarin, INR 2.0–3.0); and the RELY studyCitation11 (dabigatran 110 mg twice daily vs dabigatran 150 mg twice daily vs warfarin, INR 2.0–3.0). Details on model design, assumptions, and clinical inputs have been previously describedCitation20,Citation21. A summary of clinical event rates and HRs is presented in .
Table 1. Clinical event rates by treatment.
The model was adapted to the French setting by adjusting background mortality estimates as well as resource use and cost inputs. Mortality was derived from age- and gender-specific life tables of the French population. The analysis was taken from the perspective of the French National Health Insurance, where only direct medical care costs (2012 Euros) were consideredCitation23. The reason for this choice was that patients with AF are eligible to the full coverage or their AF-related medical expenditures by the Social Health Insurance. Clinical event-related costs were obtained from a study estimating the resources associated with AF and anticoagulation complications using the French national hospital database (Programme médicalisé des système d’information, PMSI)Citation24 and the National Hospital TariffCitation25, which also included taxes. Costs of routine care were attained from a cross-sectional study of retrospective data previously collected by general practitioners (GPs) and cardiologists in FranceCitation26. Health and cost outcomes were discounted at 4% per annum as requested by the French guidelinesCitation23. Cost inputs specific to the French healthcare system are presented in .
Table 2. Model inputs on costs (all taxes included).
Utility estimates were obtained from a UK-based EQ-5D catalogueCitation31, as displayed in , similarly to those used in earlier modelsCitation20,Citation21. Utility decrements associated with ischemic and hemorrhagic stroke, SE and MI were permanent, whilst those associated with other events (i.e. CRNMB, other major bleeds, other ICH) were transient. A disutility associated with use of treatment was applied to all patients. A higher utility loss was used for patients treated with warfarin due to monitoring and drug interactions, as obtained from a time-trade-off studyCitation32. The disutility associated with aspirin was obtained from the same study and assumed to be the same for all NOACsCitation32, given the lack of monitoring requirement.
Table 3. Model inputs on utilities.
Analyses
A lifetime horizon was adopted to estimate total number of events among a cohort of 1000 patients with AF, life years, costs, and quality-adjusted life years (QALYs) under each treatment with each anticoagulant. Interventions were ranked in terms of costs (from the cheapest to the most expensive). If an intervention was less effective than the previous less costly intervention, it was considered to be strictly dominated and subsequently excluded from further analysis. Incremental cost-effectiveness ratios (ICERs) were then calculated for each intervention, compared with the next most expensive, non-dominated strategy. If the ICER for an intervention was higher than that of the next most effective intervention, it was ruled out by extended dominance and the process was reiterated to determine the interventions that made up the efficiency frontier. The efficiency frontier was created by plotting each treatment’s costs (horizontal axis) and QALYs (vertical axis), and drawing a line linking treatments that are not dominated by any of the other treatments in considerationCitation23,Citation33.
Univariate sensitivity analysis was conducted to explore the impact of various input parameters including: varying the event risks for apixaban and warfarin, quality of INR control, the HRs for dabigatran, rivaroxaban, and aspirin vs apixaban for all outcomes, as well as cost and utility inputs. Input parameters were varied by their 95% confidence intervals where available. The efficiency frontier was calculated in each sensitivity analysis to determine the parameters that influenced the shape of the frontier. Details of each scenario run can be found in Supplementary Appendix A.
To account for variability in outcomes due to statistical uncertainty in inputs, probabilistic sensitivity analyses were performed. The model inputs were varied across 2000 iterations by sampling from probability distributions. The distributions used for the inputs included a beta distribution for probabilities and utilities, a gamma distribution for rates and costs, and log-normal distributions for hazard ratios. A cost-effectiveness acceptability curve (CEAC) was then produced based on the probability of generating the maximum net benefit across the comparators. In addition, the proportion of simulations in which each treatment appeared on the frontier was calculated.
Results
Deterministic results
As presented in , in a hypothetical cohort of 1000 patients with AF treated with warfarin the model projected the occurrence of 341 strokes (including first and recurrent ischemic and hemorrhagic strokes) or SE events and 254 major bleeds events (including first and recurrent hemorrhagic strokes, other ICH, and other major bleeds). Comparatively, aspirin treatment was associated with an additional 31 stroke and SE events, whilst treatment with dabigatran 110 mg, dabigatran 150 mg, dabigatran (110 mg and 150 mg), rivaroxaban, and apixaban reduced the numbers of stroke and SE events (3, 15, 13, 7, 18, respectively). Treatment with rivaroxaban was predicted to cause three additional major bleeds in comparison to warfarin, whilst all other treatments were predicted to cause fewer major bleeds than warfarin (−114, −61, −43, −41, and −42 in patients treated with aspirin, dabigatran 110 mg, dabigatran 150/110 mg, dabigatran 150 mg, and apixaban, respectively).
Table 4. Base case results—total number of events, costs, life-years, and QALYs.
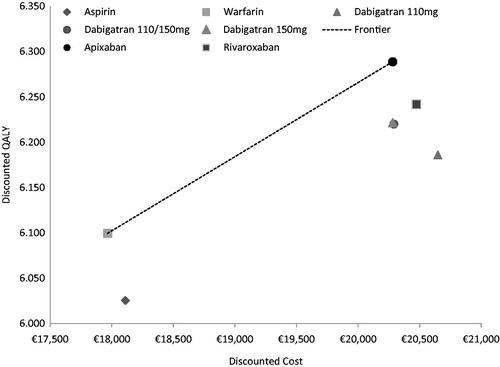
Total discounted costs amongst the different treatment regimens ranged between €17,966–€20,648, with apixaban being the intervention associated with the highest total costs. Discounted QALYs varied between 6.025–6.289, depending on treatment, with apixaban similarly predicted to result in the highest number of QALYs gained ().
The results of the deterministic analysis showed that aspirin was dominated (i.e., higher costs but lower QALYs) by warfarin, dabigatran 110 mg was dominated by dabigatran 110 mg and 150 mg, dabigatran 150 mg, rivaroxaban, and apixaban. Dabigatran 110 mg and 150 mg was also dominated by a treatment strategy with dabigatran 150 mg and apixaban. Similarly, both dabigatran 150 mg and rivaroxaban were dominated by apixaban (). This resulted in only apixaban and warfarin making up the efficiency frontier; the resulting ICER of apixaban vs warfarin was €12,227 per QALY gained.
Sensitivity analyses results
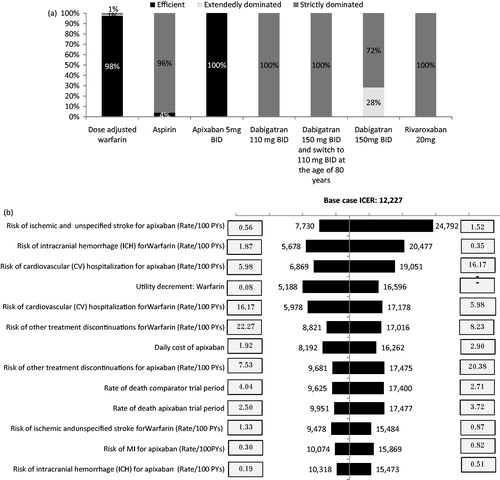
Detailed scenario results can be viewed in Supplementary Appendix A. In most scenarios the shape of the frontier remained unaltered, with apixaban and warfarin emerging as the only efficient alternatives. Apixaban remained the most efficient alternative on the frontier and dominated the other NOACs in all scenarios (). Aspirin appeared on the frontier only in four out of 222 scenarios run, including decreasing the baseline risk of stroke for apixaban, decreasing and increasing the baseline risk of treatment discontinuation for apixaban and warfarin, respectively, and increasing the disutility associated with warfarin. The ICER of apixaban vs the less costly, non-dominated alternative, in most cases vs warfarin, varied between €5978–€27,137 per QALY gained. The results of the univariate sensitivity analysis vs warfarin, i.e., the only non-dominated alternative, are displayed in the form of a tornado diagram (). The ICER of apixaban vs warfarin varied between €5188–€24,792 per QALY gained and was most influenced by the risk of ischemic stroke for apixaban, the risk of ICH for warfarin, and the risk of CV hospitalization for apixaban.
Figure 3. Results of the univariate sensitivity analysis. (a) Percentage of scenarios in which each strategy was efficient, extendedly dominated or strictly dominated. (b) Tornado diagram apixaban vs warfarin. The efficiency frontier was calculated in each sensitivity analysis, where each parameter was varied by their 95% confidence intervals as detailed in Supplementary Appendix A. The percentage of scenarios in which each strategy appeared on the frontier (denoted as efficient) was calculated as the number of scenarios in which the alternative was non-dominated using the number of total scenarios (i.e., 219) as a denominator. The percentage of scenarios in which each strategy was extendedly dominated or strictly dominated was calculated in a similar manner.
The probabilistic analysis results are presented as the proportion of each treatment for all 2000 simulations on the efficiency frontier, and as CEACs vs all alternatives (), as well as vs the non-dominated alternatives (). Aspirin, warfarin, dabigatran 110 mg, dabigatran 150 mg and 110 mg, dabigatran 150 mg, rivaroxaban, and apixaban appeared on the frontier in 41%, 73%, 7%, 8%, 10%, 0%, and 91% of the iterations, respectively.
Figure 4. Results of the probabilistic sensitivity analyses. (a) Multi-way CEAC. (b) CEAC vs warfarin (non-dominated strategy).
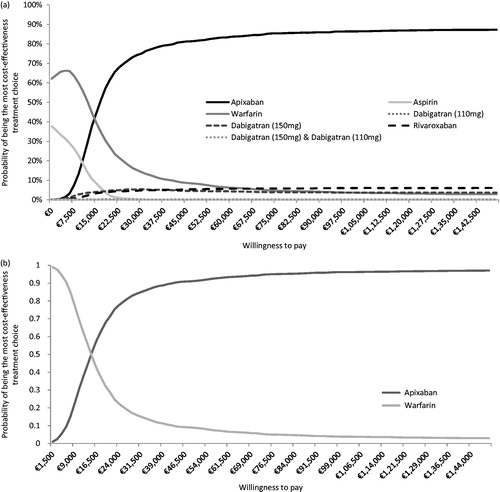
According to the CEACs (), below a willingness-to-pay threshold of €15,000, warfarin is the intervention most likely to be cost-effective, whereas for a threshold above €15,000, apixaban is the most cost-effective treatment option. Assuming a willingness-to-pay threshold of €30,000, aspirin, warfarin, dabigatran 110 mg, dabigatran 150 mg, dabigatran 110 mg and 150 mg, rivaroxaban, and apixaban are likely to be the optimal treatment option at a probability of 0%, 14%, 0%, 6%, 0%, 5%, and 75%, respectively. When comparing apixaban to warfarin only () the CEAC indicated that, at a threshold of €30,000, the probability of apixaban being the most cost-effective strategy was 85%.
Discussion
This economic evaluation is the first to simultaneously compare all currently available anticoagulant treatments as well as aspirin for stroke prevention in patients with NVAF in France. Given the absence of a cost-effectiveness threshold, the HAS methodological guideCitation23 for economic evaluations states that interventions should be presented in terms of dominance, which can be done through the use of the efficiency frontier. Also, only cost-effective interventions are compared with each other (warfarin vs apixaban); thus, taking into consideration whether an intervention provides ‘good value for money’ across all currently available treatments.
In this study, the incremental analysis demonstrated that apixaban dominated all other NOACs and aspirin was dominated by warfarin. This can be attributed to a favorable bleeding profile for apixaban, allowing patients to remain on treatment and benefit from anticoagulant therapy for a longer period of time, thus also reducing the number of stroke events. The overall reduction in clinical events resulted in increased QALY gains in patients treated with apixaban, which was robust when varying the QALY impact of bleeds compared to averted strokes in scenario analysis. In addition, compared to other NOACs in France, apixaban’s net price presents a discount of 5%. Our study, therefore, suggests that, from the available treatments in France, apixaban and warfarin are the most efficient interventions, with apixaban being a cost-effective alternative to warfarin at an ICER of €12,227 per QALY gained. Univariate and probabilistic sensitivity analysis demonstrated that apixaban was consistently the most cost-effective alternative. Apixaban remained cost-effective at a low monitoring cost of €50 annually, but even in comparison to well-controlled warfarin (cTTR ≥ 76.51%; Supplementary Appendix A), consistently with earlier publications of ARISTOTLE highlighting that the benefits of apixaban were similar across ranges of center’s and patients quality of INR controlCitation34. Our analysis confirms the findings of earlier studies using the same modelCitation21 or others conducted in other countriesCitation35–38, suggesting that apixaban is not only a cost-effective alternative to warfarin but also offers higher QALY gains in comparison to other currently available NOACs. The main differences between our study and earlier published models consist of the use of France-specific resource use and cost data. In addition, our study conducted simultaneous assessment of the economic implications associated with all currently available treatments for stroke prevention in AF using the efficiency frontier approach according to the new French CEESP, while earlier evaluations conducted pairwise comparisons between treatment alternativesCitation21; however, results were consistent, highlighting that apixaban was cost-effective against warfarin and other NOACsCitation21. Thus, our study contributes to strengthening the convergent validity of earlier results across different healthcare systems. Furthermore, our study adopted conservative estimates, in particular around the estimation of INR monitoring cost, which was an issue for previous cost-effectiveness analyses of NOACs. In our study, the global cost of INR monitoring used was ∼€130 per year, which did not take into account the new pharmacists’ incentive of €40 per patient, as part of the French 2014 pay for performance program for a better monitoring of VKA regimen.
This cost-effectiveness analysis was evaluated by the dedicated commission of HAS, the CEESP which drafted an experimental appraisal. The methodology and main results of the efficiency frontier were formally considered as validCitation39, thus, providing external validity to our evaluation. However, the CEESP highlighted uncertainty around clinical data used for NOACs. As typically observed in prevention, incremental QALYs between the strategies appeared relatively small on average. The CEESP also emphasized that rivaroxaban was close to apixaban and, considering the uncertainty of indirect comparison, it may have been wrongly excluded from the efficiency frontier in the base case. Indeed, an indirect treatment comparison was used to obtain the treatment effects associated with efficacy and safety outcomes between NOACs, which did not control for the differences in patient baseline characteristics, risk profiles as determined by the CHADS2 risk profile, time in therapeutic range, or trial design. However, the lack of these adjustments can be considered to favor dabigatran and rivaroxaban and, thus, is unlikely to alter treatments that currently are on the efficiency frontier. The open-label design of the RELY trial may over-estimate the effect of dabigatranCitation19,Citation39,Citation40. In addition, although the ROCKET-AF trial included higher-risk patients compared to those who participated in the RELY and ARISTOTLE trials, an indirect treatment comparison that looked at a sub-group of patients with CHADS2 ≥3 revealed that, in relation to apixaban, the degree of risk reduction in the primary efficacy outcome of stroke or SE provided by rivaroxaban was not as great as when all trial patients were included in the analysisCitation41,Citation42. Had these adjustments been made, dabigatran and rivaroxaban would still have been dominated. In the absence of head-to-head evidence, and although it was done on only three clinical trials, we consider the indirect treatment comparisons employed in this study to be the best evidence available to conduct comparisons between treatments. Results from the univariate sensitivity and probabilistic analysis, where treatment effects were varied by their confidence intervals, demonstrated that apixaban consistently remained the most efficient alternative dominating all other NOACs. However, we acknowledge the considerable uncertainty surrounding the clinical inputs and consider the comparative effectiveness of NOACs to be an area of further research through observational real-world data as it was formally requested by the HAS to the manufacturers commercializing these compounds.
Several additional limitations apply to our analysis. First, in absence of France-specific utilities, our analysis employed utilities based on a UK EQ-5D catalogueCitation31, assuming that they would be similar for a French population. The univariate sensitivity analysis, however, demonstrated that efficiency frontier remained unaltered, regardless of assumptions surrounding utility inputs.
Furthermore, in the absence of data to inform the utility decrement associated with NOAC use we assumed the same disutility would apply as that experienced in patients treated with aspirin. This is similar to assumptions used in earlier modelsCitation20,Citation21, given aspirin is also an unmonitored drug. This assumption could be an under-estimate of the utility decrement with NOACs; however, considered reasonable in light of evidence to suggest that patient satisfaction is higher with NOACsCitation17 or unmonitored drugs such as aspirinCitation43. In addition, scenario analysis setting the utility decrement associated with NOACs higher than that associated with warfarin (i.e. setting the decrement for warfarin to 0), demonstrated that the shape of the frontier and conclusions remained unaltered to such assumptions.
Although warfarin is considered as the VKA of reference in our analysis, fluindione is the most prescribed VKA treatment in France, and warfarin only represents 7% of the marketCitation40. This feature is a consequence of a historical management care of AF, which is actually not supported by any clinical evidence. In the absence of evidence to compare directly to fluindione, warfarin was used in our study. Additionally, the treatment effects associated with aspirin were obtained from the AVERROES trial, which consisted of patients who were unsuitable for VKA treatment. Thus, an assumption was made that the same relative clinical effect to apixaban as observed in the VKA unsuitable patients would be applied to the population in this study.
Moreover, the CEESP recommends use of a societal perspective to conduct economic evaluations and, thus, criticized the payer perspective adopted in this study, which did not take into account patients’ out-of-pocket expenses. However, as AF is one of a list of afflictions for which all cases are reimbursed at 100%, the rest of the charge remains marginal for these patients in France. In some cases, patients may pay extra fees for specific consultation (especially for cardiologists), but the extent of such additional costs is difficult to quantify. This feature should not be able to modify the conclusions of this study, but could contribute to a lower ICER for patients treated with apixaban given it was predicted to cause fewer strokes in comparison to other treatments. Similarly to the limitations above, as our analysis adopted a payer perspective we did not consider any potential societal disutility or costs related with the transition from a drug such as warfarin that has been widely used for decades, vs the NOACs that have only recently emerged; thus, lacking the confidence of experience that exists with warfarin. Whilst such consequences could have increased the ICERs, these would likely be offset by consideration of the societal impact of stroke and less constrained treatment in terms of intakes and monitoringCitation16,Citation17 potential shifting preference in those less willing to accept novel anticoagulantsCitation18.
Finally, in addition to the treatment alternatives included in this analysis, a new NOAC, edoxaban, has been evaluated at two different doses vs dose-adjusted warfarinCitation44. The introduction of edoxaban and its subsequent pricing may have implications on the shape of the frontier and treatment alternatives that are considered most cost-effective. However, at the time of this study edoxaban was neither reimbursed or commercialized in France; thus, this remains an implication for future research.
Conclusion
Overall, following the new French methods for economic evaluation, the efficiency frontier approach demonstrated that warfarin and apixaban are efficient therapies in terms of cost, QALYs, and subsequent efficiency for patients with AF in France. Aspirin, dabigatran 110 mg, 150 mg, and 110 mg and 150 mg, as well as rivaroxaban were all under the efficiency frontier, which signifies that these interventions did not provide the most cost-effective option. Based on indirect treatment comparisons, the analyses demonstrated apixaban’s value as an economically justifiable alternative to the other OAC treatments. As requested by the French health authorities, real-world evidence on the comparative effectiveness on NOACs is required to strengthen and validate our findings.
Notice of Correction
The version of this article published online ahead of print on 29 May 2014 contained some errors in the affiliation details and references. Authors F. E. Cotté and A. F. Gaudin should have been affiliated with “Bristol-Myers Squibb, Health Economics & Outcomes Research, Rueil-Malmaison, France” and author I. Kachanar should have been affiliatied with “Pfizer, Health Economics & Outcomes Research, Paris, France”.
Also, references 25, 27, 28 and 30 were replaced and the original reference 39, “Clinical Study Report from Sponsor. A phase 3, randomized, double-blind, placebo-controlled, multi-center study confirming the efficacy and safety of Genz-112638 in patients with Gaucher Disease Type 1 (ENGAGE); 2013:GZGD02507”, was deleted and as a result subsequent references and in-text citations were updated to reflect this change. The errors have been corrected for this version.
Supplementary Material
Download PDF (446.8 KB)Transparency
Declaration of funding
This work was supported by a grant from Pfizer and Bristol-Myers Squibb.
Declaration of financial/other relationships
Pfizer and Bristol-Myers Squibb (BMS) manufacture apixaban. TL and TK are employees of Evidera and were paid consultants to Pfizer and BMS in connection with this study. FEC and AFG are employees of BMS France. IK is an employee of Pfizer France. IDZ is an employee of the Mondor Hospital (Créteil, France) and URC Eco and was a paid consultant to Pfizer and BMS in connection with this study. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Hanon OAP, Belmin J, Collet JP, et al. Expert consensus of the French Society of Geriatrics and Gerontology and the French Society of Cardiology on the management of atrial fibrillation in elderly people. Arch Cardiovasc Dis 2013;106:302-23
- Fery-Lemonnier E. La prévention et la prise en charge des accidents vasculaires cérébraux en France: Synthèse du rapport à Madame la ministre de la santé et des sports. Ministere De La Sante Et Des Sports, 2009. Available at: http://www.sante.gouv.fr/IMG/pdf/AVC_synthese_seule_rapport_final_vf.pdf
- Chevreul K, Durand-Zaleski I, Gouepo A, et al. Cost of stroke in France. Eur J Neurol 2013;20:1094-100
- ISMAAP. International Self Monitoring Association of oral Anticoagulated patients. Available at: http://www.ismaap.org
- Bassand JP. Review of atrial fibrillation outcome trials of oral anticoagulant and antiplatelet agents. Europace 2012;14:312-24
- Fauchier L, Taillandier S, Clementy N. [Antithrombotic management in atrial fibrillation]. Rev Prat 2013;63:199, 201-6
- Le Reste JY CB, Le Floch B, Nabbe P, et al. There are considerable drawbacks to oral anticoagulant for monitoring patients at home which should lead family physicians to discuss alternative or enhanced solutions: a cross-sectional study. BMC Cardiovasc Disord 2013;13:71
- Liard F, Le Heuzey JY, Aliot E, et al. [Atrial fibrillation and anticoagulation: general practitioner, cardiologist and patient's points of view]. Presse Med 2013;42:e259-70
- Mabo P, Leenhardt A, Jaillon P, et al. [Management of atrial fibrillation in France: the observational FACTUEL study]. Ann Cardiol Angeiol (Paris) 2009;58:151-8
- Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17
- Heidbuchel HPV, Alings M, Antz M, et al. EHRA Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013;34:2094-106
- Elewa HF, Deremer CE, Keller K, et al. Patients satisfaction with warfarin and willingness to switch to dabigatran: a patient survey. J Thromb Thrombolysis 2013 (epub ahead of print)
- Choi JC, Dibonaventura MD, Kopenhafer L, et al. Survey of the use of warfarin and the newer anticoagulant dabigatran in patients with atrial fibrillation. Patient Prefer Adherence 2014;8:167-77
- Attaya S, Bornstein T, Ronquillo N, et al. Study of warfarin patients investigating attitudes toward therapy change (SWITCH Survey). Am J Ther 2012;19:432-5
- Dervaux B, Baseilhac E, Fagon JY, et al. Medico-economic evaluation of health products in the context of the Social Security Financing Act for 2012. Therapie 2013;68:253-63
- Dorian P, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. Eur Heart J 2014 (epub ahead of print)
- Lip GY, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
- Haute autorité de santé (HAS). A methodological guide. Choices in methods for economic evaluation. Department of Economics and Public Health Assessment, 2012. Available at: http://www.has-sante.fr
- Cotte FE, Chaize G, Kachaner I, et al. Incidence and cost of stroke and hemorrhage in patients diagnosed with atrial fibrillation in France. J Stroke Cerebrovasc Dis 2014;23:e73-83
- Ministry of Health. National Hospital Tariff. Official Journal of the French Republic. 2011. Available at: http://www.journal-officiel.gouv.fr.
- Cohen A, Dallongeville J, Durand-Zaleski I, et al. Characteristics and management of outpatients with history of or current atrial fibrillation: the observational French EPHA study. Arch Cardiovasc Dis 2010;103:376-87
- Pricing Comittee (CEPS). Drug price database for France. Available at: http://www.medicprix.sante.gouv.fr. Last accessed 31st December 2013
- National Health Insurance Fund for Salaried Worker (CNAMTS). National tariffs for biological and nursing acts. 2011. Available at: http://www.ameli.fr
- Haute autorité de santé (HAS). Fibrillation auriculaire. Available at: www.has-sante.fr. Accessed Juillet 200.
- Ministry of Health. Prevention and management of stroke in France. 2009. Available at: http://www.sante.gouv.fr/IMG/pdf/AVC_rapport_final_vf.pdf
- Sullivan P, Slejko J, Sculpher M, et al. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31:800-4
- Gage BF CA, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. JAMA Intern Med 1996;156:1829-36
- Caro JJ, Nord E, Siebert U, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ 2010;19:1117-27
- Wallentin L, Lopes RD, Hanna M, et al. Efficacy and safety of apixaban compared with warfarin at different levels of predicted international normalized ratio control for stroke prevention in atrial fibrillation. Circulation 2013;127:2166-76
- Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes 2012;5:7119
- Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health 2013;16:498-506
- Harrington AR, Armstrong EP, Nolan PE, Jr., et al. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676-81
- Pink J, Pirmohamed M, Hughes DA. Comparative effectiveness of dabigatran, rivaroxaban, apixaban, and warfarin in the management of patients with nonvalvular atrial fibrillation. Clin Pharmacol Ther 2013;94:269-76
- Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA 2010;304:793-4
- Carrasco J, Cotté FE, Duprat Lomon I, et al. Anticoagulation treatment with VKA in France, Italy, Germany, Spain and United Kingdom. Results from the React AF study. Value in Health 2013;16:A516
- Lip GY, Larsen TB, Skjoth F, et al. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2012;60:738-46
- Schneeweiss S, Gagne JJ, Patrick AR, et al. Comparative efficacy and safety of new oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2012;5:480-6
- Davis NJ, Billett HH, Cohen HW, et al. Impact of adherence, knowledge, and quality of life on anticoagulation control. Ann Pharmacother 2005;39:632-6
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104