Abstract
Objective:
In patients with significant mitral regurgitation (MR) at high risk of mortality and morbidity from mitral valve surgery, transcatheter mitral valve repair with the MitraClip System is associated with a reduction in MR and improved quality-of-life and functional status compared with baseline. The objective was to evaluate the cost-effectiveness of MitraClip therapy compared with standard of care in patients with significant MR at high risk for mitral valve surgery from a Canadian payer perspective.
Methods:
A decision analytic model was developed to estimate the lifetime costs, life years, quality-adjusted life years (QALYs), and incremental cost per life year and QALY gained for patients receiving MitraClip therapy compared with standard of care. Treatment-specific overall survival, risk of clinical events, quality-of-life, and resource utilization were obtained from the Endovascular Valve Edge-to-Edge REpair High Risk Study (EVEREST II HRS). Health utility and unit costs (CAD $2013) were taken from the published literature. Sensitivity analyses were conducted to explore the impact of alternative assumptions and parameter uncertainty on results.
Results:
The base case incremental cost per QALY gained was $23,433. Results were most sensitive to alternative assumptions regarding overall survival, time horizon, and risk of hospitalization for congestive heart failure (CHF). Probabilistic sensitivity analysis showed MitraClip therapy to have a 92% chance of being cost-effective compared with standard of care at a willingness-to-pay threshold of $50,000 per QALY gained.
Study limitations:
Key limitations include the small number of patients included in the EVEREST II HRS which informed the analysis, the limited data available to inform clinical events and disease progression in the concurrent comparator group, and the lack of a comparator group from a randomized control trial.
Conclusion:
MitraClip therapy is likely a cost-effective option for the treatment of patients at high risk for mitral valve surgery with significant MR.
Introduction
Mitral regurgitation (MR) is a condition in which the heart’s mitral valve leaflets fail to close properly and blood flows backward from the heart’s left ventricle into the left atrium. As a consequence, the heart is required to work harder to push blood through the body, which can result in fatigue, shortness of breath, pulmonary edema, congestive heart failure (CHF), and death. MR affects millions of people worldwide. It is the most common type of heart valve insufficiency in the US, with an estimated adjusted prevalence of 1.7%. Prevalence and incidence are similar in Europe, where it is the second most common type of heart valve diseaseCitation1.
MR is classified as primary (degenerative) when caused by structural abnormalities of the mitral valve or as secondary (functional) in the absence of intrinsic mitral valve disease. Causes of primary MR include mitral valve prolapse (MVP), infective endocarditis (IE), connective tissue disorders, rheumatic heart disease, cleft mitral valve, and radiation heart disease, while secondary MR usually results from left ventricular dysfunction due to cardiomyopathy, and ischemic heart diseaseCitation2,Citation3. Secondary MR is typically associated with a worse prognosis which is further complicated by comorbidities in these patients.
For symptomatic patients diagnosed with mild-to-moderate MR, medications are used to manage patient symptoms and to relieve or treat complications. Medicines may include vasodilators, such as angiotensin-converting-enzyme (ACE) inhibitors, to control high blood pressure, diuretics to treat symptoms of heart failure, and anticoagulants to prevent dangerous blood clots. For symptomatic patients diagnosed with moderate–severe (3+) or severe (4+) MR, the current gold standard of treatment is surgical repair or replacement of the mitral valveCitation2,Citation4–6. This typically involves open-heart surgery with the patient on cardiopulmonary bypass and patients may require several months to regain normal physical function and activity. Due to its invasiveness, surgery is judged to be prohibitive for a significant number of patients with significant MR and extensive comorbidities that place them at high risk of surgical morbidity and mortality. It has been estimated that as many as half of all patients with significant MR may not receive surgery for reasons related to prior surgical intervention (e.g. coronary artery bypass graft), significant left ventricular (LV) dysfunction, renal disease, advanced age, and other comorbiditiesCitation1,Citation7,Citation8. While the mortality and quality-of-life benefits of mitral valve surgery are well established in primary MR, they are more uncertain in secondary MRCitation9–12. For patients with significant MR in which the surgical risk is judged to be too high, medical management of symptoms and complications remains the standard of care. Medical management of significant MR is largely palliative, does not alter the progression of disease and is associated with poor prognosisCitation13,Citation14.
Transcatheter mitral valve repair interventions have recently emerged as a possible alternative to conventional mitral valve surgery and/or medical management for patients with significant MRCitation15. The most widely utilized transcatheter device is the MitraClip System (Abbott Vascular, Menlo Park, CA). The MitraClip device is introduced from the femoral vein to the left atrium through a transseptal puncture. The clip arms of the device are opened and advanced into the LV, where the anterior and posterior mitral leaflets are grasped between the clip arms and the grasper arms and the clip is closedCitation16. The Endovascular Valve Edge-to-Edge REpair EVEREST II study was the first randomized controlled study designed to assess the safety and efficacy of the MitraClip device in treating patients with significant (3+ to 4+) MRCitation16,Citation17. The prospective High Risk Study (HRS), a non-randomized arm of the EVEREST II trial, enrolled symptomatic patients with moderate–severe and severe MR for whom the risk of perioperative mortality was estimated to be greater than 12%Citation18. After establishing protocol-based eligibility for the MitraClip procedure with transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), patients were entered into the prospective, multi-centre, single arm study. A concurrent comparator group of patients receiving standard of care with similar degrees of MR, risks and comorbidities who were screened for the EVEREST II HRS but were not enrolled for various reasons were consented retrospectively and followed to determine 30-day and 1-year survival. Overall, the 12-month survival rate was 76% in the MitraClip group and 55% in the concurrent comparator group (p = 0.047). Severity of MR and physical functioning improved in patients who received the MitraClip device. Many observational studies report similar feasibility, efficacy, and safety results using the MitraClip device in patients at high risk for mitral valve surgeryCitation19–38.
A recent meta-analysis comparing the safety, clinical efficacy, and survival outcomes of MitraClip therapy with MV surgery in patients with significant MR highlighted the need for safer alternatives in patients at high risk for mitral valve surgery and supported the indication for MitraClip therapy in these patientsCitation39. The meta-analysis indicated that transcatheter repair with the MitraClip device in patients with significant MR was associated with similar mortality and symptomatic improvement as MV surgery and was a potential alternative for up to half of all patients with significant symptomatic MR who are denied surgeryCitation39.
Transcatheter mitral valve repair is an expensive procedure relative to standard of care for patients with significant MR at high risk for mitral valve surgery. Therefore, despite promising clinical results, the costs and cost-effectiveness of MitraClip transcatheter mitral valve repair must be evaluated to support decisions regarding the efficient allocation of scarce healthcare resources in Canada. The objective of this study was to conduct a cost-effectiveness analysis (CEA) from the perspective of the Canadian payer to compare the costs and benefits of MitraClip therapy vs standard of care alone in patients with significant MR at high risk for mitral valve surgery.
Methods
A Microsoft ExcelCitation40 decision analytic model was constructed to assess the long-term benefits, costs, and cost effectiveness of MitraClip therapy compared to standard of care for the treatment of patients with significant MR at high risk for mitral valve surgery. The analysis was performed for a lifetime horizon from the perspective of the Canadian healthcare payer. Treatment-specific overall survival, risk of clinical events, and quality-of-life data for the CEA was obtained from the intention-to-treat results of the EVEREST II HRSCitation18. The analysis included direct medical costs borne by the Canadian payer, including all costs associated with the MitraClip procedure, hospitalizations for CHF, major adverse events, and physician visits. Health utility estimates and unit costs (expressed in 2013 Canadian dollars) were taken from the published literature and Ontario cost databases. Costs and benefits were discounted at a rate of 5% per yearCitation41.
Final outputs of the analysis included the incremental cost per LY gained and the incremental cost per quality-adjusted life year (QALY) gained for MitraClip therapy vs standard of care. The impact of alternative assumptions and uncertainty around input parameters was tested in one-way sensitivity analyses and probabilistic sensitivity analysis (PSA), respectively.
Overview of the EVEREST II HRS
Patient characteristics and the majority of data for the CEA were taken from the EVEREST II HRS. The design and results of the EVEREST II HRS have been reported previouslyCitation18. Briefly, the EVEREST II HRS enrolled 78 symptomatic patients with moderate–severe and severe (3+ to 4+) MR for whom surgical risk for perioperative mortality was estimated to be ≥12%, using either the Society of Thoracic Surgeons (STS) calculatorCitation42 or surgeon-estimated risk based on pre-specified criteria. Eligibility for the MitraClip procedure was established using TTE and TEE.
The majority of patients were older than 75 years. All patients had a history of CHF attributable to either secondary MR (59%) or primary MR (41%), and the majority (84%) had a history of coronary artery disease. Nearly 90% of patients were NYHA functional class III or IV, indicative of significant functional limitation. The mean predicted perioperative mortality rate was 14.2% based on the calculated STS score, and 18.2% based on surgeon co-investigator pre-specified criteria.
Following the completion of the HRS, the EVEREST II HRS investigators retrospectively identified and included 36 concurrent comparator group patients with MR severity of ≥3+ and a predicted surgical mortality rate of ≥12% screened for enrollment in the HRS who did not enrol or who were not anatomically eligible for the MitraClip deviceCitation18. Twenty-two per cent of these patients (n = 8) met all HRS eligibility criteria, while 19% (n = 7) were determined to be eligible based on TTE assessment of MR severity, but anatomic eligibility based on TTE was never confirmed. The remaining 58% of patients (n = 21) met all the eligibility criteria except for one or more specific anatomic protocol criteria related to MitraClip device placement; MV area <4.0 cm2 (n = 4), jet origin other than A2-P2 (n = 4), flail width of ≥15 mm, or flail gap of ≥10 mm (n = 2), coaptation length of ≥2 mm (n = 3), leaflet calcification (n = 7), or a severely retracted posterior leaflet (n = 1). Patients were requested to participate in the study by consenting for follow-up to 30-day and 1-year determination of survival. All concurrent comparator group patients were treated according to standard of care over the 12-month period, with 86% managed medically and 14% undergoing MV surgery. Baseline characteristics and co-morbidities of the concurrent comparator group were similar to those of the MitraClip group, with a predicted surgical mortality rate of 14.9% based on the calculated STS scores and 17.4% based on surgeon co-investigator pre-specified criteriaCitation18.
MitraClip devices were successfully placed in 96% of patients in the MitraClip group. Overall 30-day mortality rate was 7.7% in MitraClip patients and 8.3% in patients who received standard of care (p = NS). The 12-month survival rate was 76% in the MitraClip group vs 55% in the concurrent comparator group (p = 0.047). Seventy-eight percent of surviving MitraClip patients with matched baseline and 12-month data showed improved echocardiographic MR severity to either mild (1+) or moderate (2+) at 12 months. Functional status as measured by the New York Heart Association (NYHA) classCitation43 significantly improved following MitraClip therapy from class III/IV at baseline in 89% of patients to class I/II at 12 months in 74% of patients (p = 0.0001). The annual rate of hospitalization for CHF in surviving MitraClip patients with matched data decreased significantly from 0.59 at 12 months pre-enrollment to 0.32 at 12-months post-discharge (p = 0.034).
Model description
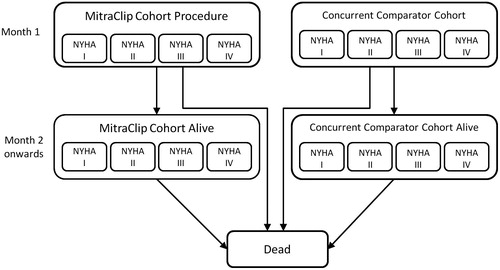
shows the structure of the Markov health state transition model that was used. All patients started the model in the ‘alive’ health state, where patients in the MitraClip arm of the model received the MitraClip procedure and patients in the concurrent comparator arm of the model received standard of care. Each model cycle, patients either remained in the ‘alive’ health state or transitioned to the ‘dead’ health state. Transition probabilities dictating movement from ‘alive’ to ‘dead’ were calculated from treatment-specific overall survival curves from the EVEREST II HRS. The analysis was conducted using a monthly cycle length in order to capture the timing of all relevant outcomes and costs associated with the MitraClip procedure and to capture the granularity provided in the overall survival curves.
Patients in the ‘alive’ states were stratified by NYHA functional class so that health-related quality-of-life (HRQoL) and health utility associated with disease severity could be taken into consideration in the CEACitation44. Health utility decrements from the literature were applied to the MitraClip procedure, hospitalizations for heart failure, and mitral valve surgeries. Patients in the MitraClip and concurrent comparator groups in the ‘alive’ state were considered at risk of clinical events such as CHF-related hospitalization and MV surgery. MitraClip patients were also at risk of 30-day periprocedural complications such as major vascular complication, major bleeding complication, and non-cerebral thromboembolism, and major adverse events (e.g., myocardial infarction [MI], stroke, renal failure, etc.). The risks for CHF hospitalization, MV surgery, 30-day periprocedural complications, and major adverse events were taken from the EVEREST II HRS. For the MitraClip group, resource use and costs associated with the MitraClip procedure, periprocedural complications, adverse events, CHF hospitalizations, MV surgery and physician visits were included in the analysis. For the concurrent comparator group, resource use and costs associated with CHF hospitalizations, MV surgery and physician visits were included in the analysis. Estimates of resource utilization were derived from a variety of sources including the EVEREST II HRS, published literature, and expert clinical opinion. Unit costs were derived from the Ontario Case Costing Initiative (OCCI), Sunnybrook Health Sciences Centre, Ontario Health Insurance (OHIP) Schedule of Benefits and Fees, and the published literature.
Overall survival
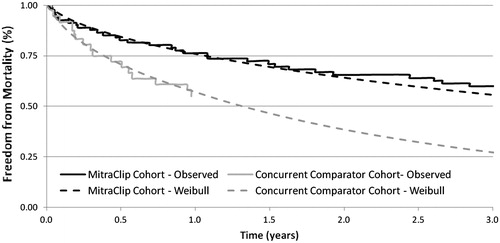
Monthly transitions that informed the proportion of patients that remained in the ‘alive’ health state and the proportion of patients that transitioned to ‘all-cause death’ were informed directly by overall Kaplan-Meier survival data from the EVEREST II HRS. The published results of the EVEREST II HRS provided 12-month survival data for the MitraClip and concurrent comparator groups and accounted for the periprocedural deaths that were observed in the MitraClip groupCitation18. Unpublished data for the MitraClip group was available for an additional 24 months of follow-upCitation45.
In order to estimate benefits and costs beyond the duration of the clinical trial to a lifetime horizon, extrapolation of overall survival from the clinical trial was required. The extrapolation process involved fitting a Weibull regression function of the form S(t) = exp(−λt^γ) to the observed Kaplan-Meier survival curves. The curves were fitted using SigmaPlot 11 and the global curve fitting function, with the shape parameter (γ) assumed to be common for both the MitraClip and concurrent comparator groups. The scale parameter (λ) for the MitraClip curve was estimated by multiplying the scale parameter for the concurrent comparator group by the hazard ratio (0.492) for overall survival for the MitraClip group vs the concurrent comparator groupCitation45. The extrapolated survival curves were further adjusted using age- and sex-adjusted Canadian general population survival to force all patients into the ‘dead’ state by the age of 100 yearsCitation46. presents the Kaplan-Meier curves as they were reported in the EVEREST II HRS and the extrapolated survival curves used in the analysis. The treatment-specific Weibull functions were then used to generate survival estimates for each month in the model.
Clinical events
During each month of the model, MitraClip and concurrent comparator group patients in the ‘alive’ state were at risk of CHF-related hospitalization. The annual rate of hospitalization due to CHF for the MitraClip treatment group was reported in the EVEREST II HRS for the 12 months prior to the MitraClip procedure and for the 12 months following the MitraClip procedureCitation18. The 12-month post-procedure annual rate of 0.36 was used in the model for the MitraClip treatment group. The annual rate of CHF-related hospitalization was not reported for the concurrent comparator group included in the EVEREST II HRS, and no such data was available through the published literature. Therefore, the annual rate of CHF-related hospitalization for MitraClip patients reported for the 12 months prior to the MitraClip procedure (0.65), assumed to be a reasonable estimate of the rate of CHF hospitalization in the concurrent comparator group, was utilized in the base case analysis. The hospitalization rates were converted to monthly probabilities using the formula p = 1 − exp(−rt), where p is the probability, r is the rate, and t is the period of time (). The rate of CHF hospitalization was applied for the duration of the analysis. The 1-year probability of MV surgery (including mitral valve repair or replacement surgery) was also taken from the EVEREST II HRS and was 0% for the MitraClip group and 14% for the concurrent comparator group. The 1-year probability of MV surgery was applied in the first cycle of the model onlyCitation18.
Table 1. Base case model inputs for transition probabilities and probability of events.
During the first month of the model, MitraClip patients were at risk of 30-day periprocedural complications such as major vascular complications, major bleeding complications, and non-cerebral thromboembolism. The probability for each of these complications was applied in the first month of the model according to the event rates reported in the 30-day periprocedural period of the EVEREST II HRS ().
During each month of the model, MitraClip patients were also at risk of major adverse events such as MI, major stroke, renal failure, mechanical ventilation >24 hours, gastrointestinal (GI) complication requiring surgery, septicemia, and blood transfusion requiring ≥2 units of blood. The probabilities for these major adverse events were reported in the EVEREST II HRS for the 30-day periprocedural period and for the 12 months following the MitraClip procedure. The probability of major adverse events for the 30-day periprocedural period was applied in the first month of the model and the probability of major adverse events in the 11 months following the periprocedural period was converted to a monthly probability and was applied for the duration of the analysis (). Data regarding major adverse events was not reported for the concurrent comparator group included in the EVEREST II HRS, and no such data was available through the published literature. In the absence of any reasonable estimates regarding the risk of these events in these patients, major adverse events were excluded from the analysis for the concurrent comparator group.
Table 2. Resource utilization and costs associated with the MitraClip procedure.
Finally, one MitraClip patient in the EVEREST II HRS underwent a second MitraClip procedure ∼6 weeks following the first procedure to place a second MitraClip device. A probability of 0.0128 for a second MitraClip procedure was applied in the first cycle of the model to capture the costs associated with a repeat procedure. Beyond the first cycle of the model, the MitraClip re-intervention rate was assumed to be 0. This assumption was tested in one-way sensitivity analyses.
NYHA functional class
The NYHA functional classification characterizes the extent of heart failure by placing patients in one of four categories based on the degree of limitation during daily physical activities and experience of symptoms of cardiac insufficiency. The limitations/symptoms are in regards to fatigue, shortness of breath, angina pain, and palpitations. NYHA class I is representative of patients with cardiac disease, but no symptoms and no limitation in ordinary physical activity (e.g., shortness of breath when walking, climbing stairs, etc.). NYHA class II is representative of patients with mild symptoms (e.g., mild shortness of breath and/or angina) and slight limitation during ordinary activity. NHYA III characterizes patients with marked limitation in activity due to symptoms, even during less-than-ordinary activity (e.g., walking short distances of 20–100 m), and NYHA IV is associated with severe limitations and symptoms even while at rest. NYHA class was used in the model to assign health utility to patients during each cycle. Baseline NYHA class for patients in the MitraClip group was taken from the 12-month post-procedure NYHA class reported in the EVEREST II HRS ()Citation18. Thirty-three per cent of MitraClip patients were NYHA class I, 41% were NYHA class II, 24% were NYHA class III, and 2% were NYHA IV. Baseline NYHA class for patients in the concurrent comparator group was taken from the baseline assessment of NYHA reported in unpublished data from the EVEREST II HRSCitation45. Three per cent of patients in the concurrent comparator group were NYHA class I, 13% were NYHA class II, 65% were NYHA class III, and 19% were NYHA IV.
The percentage breakdown of patients by NYHA functional class was assumed to remain the same as the baseline for the time horizon of the analysis. For patients receiving the MitraClip device, progression of NYHA was examined at 12 months in the published EVEREST II HRSCitation18 and at 3 years during long-term follow-up of MitraClip patients enrolled in the EVEREST II HRSCitation45. The 12-month and 3-year data demonstrated that NYHA class was sustained in patients receiving the MitraClip device. Progression of NYHA was not reported for concurrent comparator patients included in the EVEREST II HRS, and no such data was available through the published literature. Therefore, patients in the concurrent comparator group were also assumed to remain in the same NYHA class for the duration of the analysis. The assumption of no progression in NYHA was tested in sensitivity analyses.
Quality-of-life and health utility
Patients in the ‘alive’ state were assigned health utility based on NYHA classCitation44. A literature search was conducted to identify published health utilities for NYHA. Briefly, six studies were identified and compared for appropriatenessCitation47–52. For the base case analysis, health state utilities for NYHA functional class were taken from a heart failure population reported by Göhler et al.Citation47 (). The authors reported a health utility of 0.90 for NYHA class I, 0.83 for NYHA class II, 0.74 for NHYA class III, and 0.598 for NYHA class IV. The utility estimates from Göhler et al. were selected as the most appropriate set of values for the base case analysis, as this study was recent (2009), included the largest number of patients (n = 1395), included a population of patients that were most similar to the patients enrolled in the EVEREST II HRS (i.e., heart failure patients), and provided a complete set of health utility estimates (i.e., a utility estimate for each of the NYHA classes). The impact of using alternative estimates of health utilityCitation48 on the results of the CEA was tested via sensitivity analyses.
Health utility decrements were also applied to the MitraClip procedure, hospitalizations for CHF and mitral valve surgeries. A utility decrement of 0.064 was applied for 1 month for patients who experienced a CHF hospitalizationCitation53. The utility decrement for the MitraClip procedure (0.043) was assumed to be the same as that for other percutaneous coronary interventions and was also applied for 1 monthCitation54. The utility decrement for MV surgery (repair or replacement) was applied as a 10% disutility of NYHA class III (0.074) for the first 12 cycles (i.e., 12 months) according to the method reported by Marwick et al.Citation55.
Resource use and unit costs
The CEA incorporated all resource use and costs associated with the MitraClip procedure, including the diagnostic tests to confirm eligibility of the patient for the MitraClip procedure, acquisition cost of the MitraClip device, MitraClip procedure, 30-day periprocedural complications, adverse events, and re-interventions with the MitraClip procedure. No indirect costs such as out-of-pocket costs borne by the patient and/or caregiver or productivity losses were included in the analysis. Resource use was calculated based on the EVEREST II HRS for length of stay following the MitraClip procedure, post-procedural complications and adverse events, and re-interventionCitation18. Resource use was based on clinical experience at a Canadian center for diagnostic testing and the MitraClip procedureCitation56. Costs associated with the MitraClip procedure include the MitraClip device, diagnostic procedures, and procedural costs of MitraClip implantation and in-hospital care, as well as complications and adverse events occurring in the periprocedural period ($42,901) ().
Table 3. Resource utilization and costs associated with clinical events and disease management.
Unit costs for complications and adverse events following the MitraClip procedure were taken from the Ontario Case Costing Initiative (OCCI) database where available, and from the published literatureCitation57–62 (). The OCCI is an undertaking of the Ontario Ministry of Health and Long-Term Care and collects case cost data for acute inpatient, day surgery, and ambulatory care cases, as well as complex continuing care, rehabilitation, mental health, and community care access center casesCitation57. Costs from the OCCI database contain case costs that include nursing, pharmacy, laboratory, and overhead costs. Costs were obtained for post-procedure complications [major bleeding complication ($7117)Citation58, major vascular complication ($5233)Citation59, and non-cerebral thromboembolism ($7142)Citation57], and adverse events [myocardial infarction ($7700)Citation57, stroke ($6890)Citation57, renal failure ($6493)Citation57, mechanical ventilation >48 h ($2888 (assumes two additional days in ICU)), GI complication requiring surgery ($5832)Citation61, septicemia ($13,047)Citation57, and blood transfusion ≥2 U of blood ($535)Citation60].
The aggregate cost for MV surgery ($26,348) was based on the mean cost reported for mitral valve repair by the OCCI (procedure codes 1HU80LA, 1HU80LAFE, and 1HU80LAXXA)Citation57. Similarly, the aggregate cost of an episode of hospitalization for CHF ($7186) was taken from the OCCI (CMG Code 196 - Heart Failure without Cardiac Catheterization)Citation57. The analysis assumed no differences in the unit cost of hospitalization for CHF between the treatment groups. Any differences in the total cost of CHF-related hospitalizations between treatment groups were derived solely from differences between treatment groups in the rate of CHF-related hospitalization derived from the EVEREST II HRSCitation18.
Patients in the MitraClip and concurrent comparator groups received ongoing management costs including routine clinical follow-up and testing. Monthly costs for ongoing management are estimated at $97 for patients in the MitraClip and the concurrent comparator group ()Citation57,Citation63,Citation64.
Sensitivity analyses
One-way deterministic sensitivity analyses were performed to test the impact of alternative assumptions regarding the time horizon, discount rate, overall survival benefit for MitraClip therapy, rate of MitraClip procedure re-intervention, rate of MV surgery, rate of hospitalization for CHF, progression of NYHA beyond the duration of the clinical trial, and utility decrements for NYHA class ().
Table 4. Summary of alternative parameter estimates utilized in one-way sensitivity analyses.
A probabilistic sensitivity analysis was performed to determine the impact of uncertainty using the variability around point estimates used in model parameters (i.e., costs, utilities, clinical event rates, MV surgery rates, and hazard ratio for overall survival) (). Results were presented using a scatter plot of 10,000 runs and a cost-effectiveness acceptability curve (CEAC).
Table 5. Summary of parameter estimates and distributions used in the probability sensitivity analysis.
Results
Base case analysis
The detailed results of the base-case analysis are shown in . Overall, MitraClip treatment resulted in greater life years, QALYs, and costs compared to standard of care. Compared to patients in the concurrent comparator group, MitraClip therapy patients gained on average 1.84 life years and 1.73 QALYs over a lifetime, but incurred additional costs ($40,617) for an incremental cost-effectiveness ratio (ICER) of $22,109 per life year gained, and $23,433 per QALY gained. Over a lifetime time horizon, the MitraClip group accrued incremental costs of $42,901 per patient for the MitraClip procedure, with $528 per patient for MitraClip procedure re-intervention, and an additional $6480 for lifetime clinical management, and savings of $5613 and $3689 related to reductions in heart failure hospitalizations and mitral valve repair/replacement surgeries (). The analysis was primarily driven by improved survival in the MitraClip group.
Table 6. Base case analysis results for MitraClip therapy vs concurrent comparator.
Deterministic sensitivity analyses
The results of the one-way sensitivity analysis scenarios are shown in . Overall, the model was sensitive to overall survival benefit, time horizon, and hospitalization rate for CHF. Alternative assumptions regarding the survival benefit of MitraClip therapy led to a large impact on the ICER. In the base case analysis, the hazard ratio (HR) for survival is 0.49, with 75.9% and 57.1% of modeled patients alive at Year 1 in the MitraClip therapy and concurrent comparator cohorts, respectively. One-way deterministic sensitivity analyses for the efficacy of MitraClip therapy utilizing the upper and lower 95% confidence intervals of the HR demonstrate a significant impact on cost per QALY and cost per LYG. The lower confidence interval (0.25) of the hazard ratio yields an ICER of $15,923 per QALY. In contrast, the upper confidence interval (0.96) gives an ICER of $84,895 per QALY, driven entirely by improvements in quality-of-life.
Table 7. Results for the one-way deterministic sensitivity analyses.
Alternative time horizons demonstrated the sensitivity of the model to duration of the analysis, with shorter time horizons increasing the ICER. Time horizons of 3 and 10 years led to ICERs of $59,248 and $25,752 per QALY, respectively ().
The rates of hospitalization for CHF for both MitraClip and concurrent comparator groups have an impact on model results (). An increased rate of hospitalization in the MitraClip group increases the ICER, while an increase in the rate of hospitalization for the concurrent comparator group reduces the ICER. Conversely, a reduced rate of hospitalization in the MitraClip group decreases the ICER, while a decrease in the rate of hospitalization for the concurrent comparator group increases the ICER.
The rate of second MitraClip procedure intervention was low in the EVEREST II HRS cohort (one patient, 1.28%). In a clinical report from the German TRAMI registry which represents the largest real-world experience in Europe, the MitraClip procedure re-intervention rate was similarly low at 1.6% (8/486)Citation31. That said, rates up to 7.7% were reported in a smaller case seriesCitation22. In the current model, a scenario analysis with a MitraClip procedure re-intervention rate of 7.7% increased the ICER to $24,993 per QALY. Similarly, the rate of MV surgery (mitral valve repair or replacement following MitraClip therapy) was relatively low in the EVEREST II HRS cohort (0%). Compared with clinical experience, there are several European reports of MV surgery rates following MitraClip therapy in the 3–6% rangeCitation22,Citation31,Citation37,Citation65,Citation66. When a 6.3% MV surgery rate (as reported in the ACCESS EU studyCitation37) was assessed in the MitraClip arm of this model, the ICER increased to $25,374 per QALY.
In the base case analysis, NYHA was assumed to remain static from baseline onwards for patients receiving standard of care and MitraClip therapy. For the MitraClip group, this assumption was supported by 4-year results from the EVEREST II study which showed that improvements in NYHA class for patients receiving the MitraClip therapy were sustained for the full 4 years of post-procedure follow-upCitation17. For the concurrent comparator group, this assumption was considered to be conservative against MitraClip therapy since MR is a progressive disease with continual decline in physical functioning. The assumption was tested in an extreme sensitivity analysis in which all patients in the MitraClip and concurrent comparator group declined by one NHYA class per year. For example, patients in NYHA I in year 1 of the model transitioned to NYHA class II in the second year, to class III in the third year, and to class IV in the fourth year, where they remained for the duration of the analysis. Under this assumption, all patients, irrespective of treatment, were in NYHA IV by year 5 of the analysis. The results of the sensitivity analysis (ICER = $31,123 per QALY) demonstrated that the base case results were robust to a significantly less attractive assumption for MitraClip therapy regarding the progression of NYHA.
A sensitivity analysis was performed to examine the impact of MR etiology by evaluating the cost-effectiveness of MitraClip therapy in a group of prohibitive surgical risk primary MR patients. In this analysis, MitraClip patient data was based on 127 prohibitive surgical risk primary MR patientsCitation21 compared with the concurrent comparator cohort (36.1% primary MR) reported in the EVEREST II HRSCitation18. The baseline characteristics of primary MR patients was similar to the MitraClip and concurrent comparator cohorts used in the base case analysisCitation18, although were slightly older (mean age 82.4 years) but with a lower predicted surgical risk (13.2%)Citation21. Using the survival and clinical event data for primary MR patients treated with the MitraClip therapy reported by Lim et al.Citation21 in the model resulted in an ICER similar to that found in the base case analysis (within 5% of the base case analysis ICER).
Probabilistic sensitivity analysis
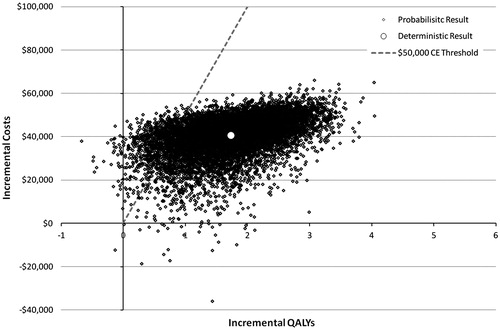
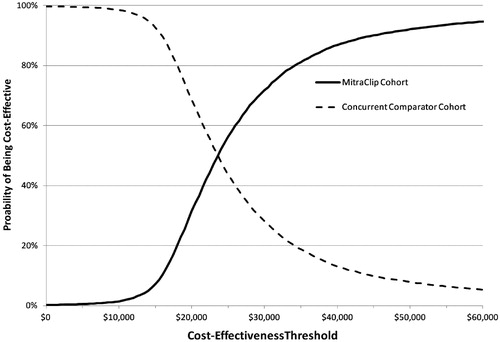
The results of the probabilistic sensitivity analysis are shown in and . Over 99% of results fall in the North-East Quadrant (more costly and more effective); 26 results (0.3%) were dominant (SE quadrant, i.e., less costly and more effective); 38 results (0.4%) were dominated (NW quadrant, i.e., more costly, less effective); and one result (0.01%) was in the SW quadrant (i.e., less costly, less effective). Overall, at a cost-effectiveness threshold of $50,000 per QALY, MitraClip therapy was 92% cost-effective over a lifetime.
Discussion
This study represents the first Canadian CEA of transcatheter mitral valve repair with the MitraClip System vs standard of care in patients with significant MR at high risk for MV surgery. In the base-case analysis the incremental cost per QALY gained and the incremental cost per life year gained were $23,433 and $22,109, respectively. The analysis was driven largely by a statistically significant advantage in overall survival for patients receiving the MitraClip device compared with patients receiving standard of care. Over a lifetime horizon (a maximum of 23 years in this analysis), treatment with the MitraClip System resulted in a gain of 1.8 years of life compared with standard of care; over a 5-year time horizon, treatment with the MitraClip System resulted in a gain of a full life year vs standard of care. One-way sensitivity analyses confirmed the sensitivity of the model to overall survival. In a sensitivity analysis that utilized the upper 95% confidence interval of the HR for MitraClip therapy vs standard of care to construct the extrapolated overall survival curves and to subsequently estimate the transition probabilities, the incremental cost per QALY gained increased significantly to $84,895.
Given that the costs for treatment with the MitraClip System are incurred within the first 30 days, while the health benefits accrue over the remaining lifetime of the patient, a sensitivity analysis that utilized a shorter time horizon of 3 years also resulted in a higher incremental cost per QALY gained for MitraClip therapy compared with standard of care ($59,248). The sensitivity analysis assessing the impact of assuming the maintenance of NYHA class for the duration of the analysis, demonstrated that the base case results were robust to a significantly less attractive assumption for MitraClip therapy regarding the progression of NYHA. The model was robust to all other changes in underlying assumptions and model inputs.
The PSA also demonstrated that the results were robust to the uncertainty in all input parameters, with over 99% of the bootstrap results showing MitraClip therapy to be more costly and more effective than standard of care. Over 92% of bootstrap results fell below a willingness-to-pay cost-effectiveness threshold of $50,000 per QALY gained. Seventy-two per cent of bootstrap results fell below a willingness-to-pay cost-effectiveness threshold of $30,000 per QALY gained.
The only other CEA of MitraClip therapy that has been conducted in a patient population with significant MR high risk for MV surgery was conducted from the perspective of the National Health Service (NHS) in the UK and reported by Mealing et al.Citation67. The UK analysis also utilized the EVEREST II HRSCitation18 to inform overall survival, frequency of adverse events, and NYHA class in their decision analytic modelCitation67. The authors reported a 10-year cost per QALY gained of £14,800 ($26,660 CAD) in their base case analysis that was very similar to the 10-year ICER of $25,752 reported in our sensitivity analysisCitation67–69. Overall, the UK analysis model was reported to be most sensitive to the choice of time horizon, the discount rate applied to benefits, the starting age of the cohort, and the utility decrement associated with NYHA II. The results of the Canadian economic evaluation were within $1000 of those reported by Mealing et al.Citation67–69, providing external validity for the cost-effectiveness analysis of MitraClip therapy vs standard of care.
The base case analysis utilized data from the EVEREST II HRS, which enroled a mixed population of high risk patients with either primary MR or secondary MRCitation18. In Europe, MitraClip therapy has been used since 2008 when it gained CE mark approval, where patients are predominantly high surgical risk secondary MR patients. In the US, the Food and Drug Administration (FDA) approved the use of MitraClip therapy in patients with primary MR who are at prohibitive risk for surgeryCitation70. It is reasonable to anticipate a similar approval for MitraClip therapy from Health Canada. Therefore, it is also important to understand the effectiveness and cost-effectiveness of MitraClip therapy in patients specifically with primary MR. Unfortunately, the design of the EVEREST II HRS was not conducive to conducting sub-group analyses for primary MR patients only. We utilized overall survival data for primary MR patients from the Lim et al.Citation21 study to conduct a sub-group CEA of MitraClip therapy compared with standard of care for patients with primary MR. The sub-group analysis demonstrated that MitraClip therapy was also cost-effective compared to standard of care in high risk primary MR patients at a willingness-to-pay threshold of $50,000. Insufficient data was available at the time of this economic evaluation to conduct a sub-group CEA in patients with secondary MR. Several studies investigating the use of MitraClip therapy in patients with secondary MR suggest that MitraClip therapy is similarly effective in these patientsCitation33,Citation37. Ongoing randomized trials of MitraClip therapy in patients with heart failure and significant secondary MR will provide further insights into the clinical benefits of the therapy and should enable robust CEAs of MitraClip therapy to be conducted in patients with high risk secondary MRCitation71.
Limitations
The primary limitation of the CEA is that it used aggregate data from a relatively small number of MitraClip patients enroled in the EVEREST II HRS (n = 78), and an even smaller number of retrospectively identified non-propensity matched patients in the concurrent comparator group (n = 36)Citation18. The limitations of the clinical study have been described elsewhereCitation18. Importantly, transesophageal echocardiograms were not available for review in all patients included in the concurrent comparator group, and several of the patients did not have appropriate anatomic criteria for MitraClip device placement. The use of a non-randomized, non-propensity matched control cohort raises questions regarding the comparability and generalizability of the patients included in the concurrent comparator group. However, in spite of these limitations, the concurrent comparator cohort from the EVEREST II HRS represented the best source of MR severity, overall survival, and NYHA data available for patients with significant MR at high risk for MV surgery managed with standard of care. The paucity of literature reporting overall survival for patients with high risk significant MR highlights the need for further research to better understand the progression of significant MR, functional decline, and overall survival in medically managed high risk MR patients.
Overall survival data was available from the EVEREST II HRS for 3 years in patients receiving the MitraClip device and for 12 months in patients receiving standard of care. However, in order to estimate the lifetime cost-effectiveness of MitraClip therapy compared with standard of care, Weibull regression analysis was required to extrapolate overall survival beyond the duration of the clinical trial. Extrapolation of survival data is imprecise, and may result in over- or under-estimation of the true efficacy of a given therapy. One-way sensitivity analyses demonstrated that the results were sensitive to alternative assumptions regarding overall survival. When the survival benefit for MitraClip therapy vs standard of care was reduced (upper 95% confidence interval of the hazard ratio), the ICER increased to $84,895 per QALY gained. Follow-up of MitraClip patients in the EVEREST II HRS is ongoing and will provide more certainty regarding the long-term clinical benefits of MitraClip therapy.
Unfortunately, data regarding the progression of NYHA in significant MR patients receiving standard of care was not collected as part of the EVEREST II HRS. In addition, no data was available in the published literature to inform either the progression of NYHA in patients receiving standard of care or the progression of NYHA beyond 3 years in MitraClip patients.
Conclusion
Using the best clinical data available from the EVEREST II HRS, this Canadian CEA demonstrated that transcatheter mitral valve repair with the MitraClip System was cost-effective vs standard care in high surgical risk patients with significant MR. In the base-case analysis the incremental cost per QALY gained and the incremental cost per life year gained were $23,433 and $22,109, respectively. The cost per QALY falls below the typical threshold ranges of $50,000–$100,000 USD per QALY gainedCitation72 and $20,000–$100,000 CAD per QALY gainedCitation73 often cited as representing good value for money in the US and Canada, respectively. While results from clinical studies demonstrate that the MitraClip device can successfully reduce the degree of MR and improve quality-of-life in patients not considered to be suitable candidates for MV surgery, and for whom there are no other treatment options, the results of this analysis demonstrate that MitraClip therapy also offers a cost-effective option for these patients.
Transparency
Declaration of funding
The work reported in this manuscript was funded via a consultancy agreement between Cornerstone Research Group, Inc. and Abbott Vascular.
Declaration of financial/other relationships
HLC and LMB disclose that they are consultants commissioned by Abbott Vascular to perform this study. JH and VG disclose that they are employees of Abbott Vascular. AA has received speakers honoraria from Abbott Vascular. The final manuscript has been read and approved by all authors. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43
- Nishimura R, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2014 (Epub ahead of print)
- de Marchena E, Badiye A, Robalino G, et al. Respective prevalence of the different carpentier classes of mitral regurgitation: a stepping stone for future therapeutic research and development. J Card Surg 2011;26:385-92
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-42
- Jamieson WR, Cartier PC, Allard M, et al. Surgical management of valvular heart disease 2004. Can J Cardiol 2004;20(E Suppl):7E-120E
- Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 2007;28:230-68
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65
- Borger MA, Alam A, Murphy PM, et al. Chronic ischemic mitral regurgitation: repair, replace or rethink? Ann Thorac Surg 2006;81:1153-61
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44
- Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7
- McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24
- Lee LS, Kwon MH, Cevasco M, et al. Postoperative recurrence of mitral regurgitation after annuloplasty for functional mitral regurgitation. Ann Thorac Surg 2012;94:1211-16; discussion 6–7
- Carabello BA. The current therapy for mitral regurgitation. J Am Coll Cardiol 2008;52:319-26
- Agricola E, Ielasi A, Oppizzi M, et al. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail 2009;11:581-7
- Chiam PT, Ruiz CE. Percutaneous transcatheter mitral valve repair: a classification of the technology. JACC Cardiovasc Interv 2011;4:1-13
- Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406
- Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol 2013;62:317-28
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130-9
- Auricchio A, Schillinger W, Meyer S, et al. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011;58:2183-9
- Franzen O, Baldus S, Rudolph V, et al. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J 2010;31:1373-81
- Lim DS, Reynolds MR, Feldman T, et al. Improved functional status and quality of life in prohibitive surgical risk patients with degenerative mitral regurgitation following transcatheter mitral valve repair with the MitraClip(R) System. J Am Coll Cardiol 2013 (Epub ahead of print)
- Rudolph V, Knap M, Franzen O, et al. Echocardiographic and clinical outcomes of MitraClip therapy in patients not amenable to surgery. J Am Coll Cardiol 2011;58:2190-5
- Pleger ST, Mereles D, Schulz-Schonhagen M, et al. Acute safety and 30-day outcome after percutaneous edge-to-edge repair of mitral regurgitation in very high-risk patients. Am J Cardiol 2011;108:1478-82
- Schillinger W, Athanasiou T, Weicken N, et al. Impact of the learning curve on outcomes after percutaneous mitral valve repair with MitraClip and lessons learned after the first 75 consecutive patients. Eur J Heart Fail 2011;13:1331-9
- Tamburino C, Ussia GP, Maisano F, et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J 2010;31:1382-9
- Van den Branden BJ, Swaans MJ, Post MC, et al. Percutaneous edge-to-edge mitral valve repair in high-surgical-risk patients: do we hit the target? JACC Cardiovasc Interv 2012;5:105-11
- Scandura S, Ussia GP, Caggegi A, et al. Percutaneous mitral valve repair in patients with prior cardiac surgery. J Card Surg 2012;27:295-8
- Neuss M, Schau T, Schoepp M, et al. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur J Heart Fail 2013;15:786-95
- Boekstegers P, Hausleiter J, Baldus S, et al. Percutaneous interventional mitral regurgitation treatment using the Mitra-Clip system. Clin Res Cardiol Off J Ger Card Soc 2013;103:85–96
- Schillinger W, Hunlich M, Baldus S, et al. Acute outcomes after MitraClip therapy in highly aged patients: results from the German TRAnscatheter Mitral valve Interventions (TRAMI) Registry. EuroIntervention 2013;9:84-90
- Baldus S, Schillinger W, Franzen O, et al. MitraClip therapy in daily clinical practice: initial results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur J Heart Fail 2012;14:1050-5
- Rogers JH, Low RI. Noncentral mitral regurgitation: a new niche for the MitraClip. J Am Coll Cardiol 2013;62:2378-81
- Taramasso M, Maisano F, Latib A, et al. Clinical outcomes of MitraClip for the treatment of functional mitral regurgitation. EuroIntervention 2014 (Epub ahead of print)
- Yeo KK, Yap J, Yamen E, et al. Percutaneous mitral valve repair with the MitraClip: early results from the MitraClip Asia-Pacific Registry (MARS). EuroIntervention 2014 (Epub ahead of print)
- Reichenspurner H, Schillinger W, Baldus S, et al. Clinical outcomes through 12 months in patients with degenerative mitral regurgitation treated with the MitraClip(R) device in the ACCESS-EUrope Phase I trial. Eur J Cardiothorac Surg 2013;44:e280-8
- Armoiry X, Brochet E, Lefevre T, et al. Initial French experience of percutaneous mitral valve repair with the MitraClip: a multicentre national registry. Arch Cardiovasc Dis 2013;106:287-94
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61
- Chan PH, She HL, Alegria-Barrero E, et al. Real-world experience of MitraClip for treatment of severe mitral regurgitation. Circ J Off J Jpn Circ Soc 2012;76:2488-93
- Wan B, Rahnavardi M, Tian DH, et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann Cardiothorac Surg 2013;2:683-92
- Microsoft Corporation. Redmond, Washington. Microsoft Excel. 2007
- Guidelines for the economic evaluation of health technologies: Canada. 3rd edn. Ottawa: Canadian Agency for Drugs and Technologies in Health. 2006
- Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003;75:1856-64; discussion 64–5
- Hurst JW, Morris DC, Alexander RW. The use of the New York Heart Association's classification of cardiovascular disease as part of the patient's complete Problem List. Clin Cardiol 1999;22:385-90
- Criteria Committee of the American Heart Association (New York City Affiliate). Nomenclature and criteria for diagnosis of disease of the heart and great vessels. 9th edn. Boston, MA: Little Brown & Co, 1994
- Abbott Vascular Structural Heart (Data on File). A Study of the Evalve® Cardiovascular Valve Repair (MitraClip® Endovascular Valve Edge-to-Edge) System (EVEREST II): EVEREST II High Risk Registry 3-Year Clinical Report. 2012
- Statistics Canada. Life Tables, Canada, Provinces and Territories 2000 to 2002, Catalogue no. 84-537-XIE. Ottawa: Health Statistics Division, 2006
- Göhler A, Geisler BP, Manne JM, et al. Utility estimates for decision-analytic modeling in chronic heart failure–health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185-7
- Kirsch J, McGuire A. Establishing health state valuations for disease specific states: an example from heart disease. Health Econ 2000;9:149-58
- McAlister FEJ, Wiebe N, Rowe B, et al. Cardiac resynchronization therapy for congestive heart failure. Evidence Report/Technology Assessment No. 106. (Prepared by the University of Alberta Evidence-based Practice Center) under Contract No. 290-02-0023. Rockville, MD: Agency for Healthcare Research and Quality, 2004
- Lewis EF, Johnson PA, Johnson W, et al. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant 2001;20:1016-24
- Holland R, Rechel B, Stepien K, et al. Patients' self-assessed functional status in heart failure by New York Heart Association class: a prognostic predictor of hospitalizations, quality of life and death. J Card Fail 2010;16:150-6
- Calvert MJ, Freemantle N, Cleland JG. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail 2005;7:243-51
- Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20
- Chaplin S, Scuffham PA, Alon M, et al. Secondary prevention after PCI: the cost-effectiveness of statin therapy in the Netherlands. Neth Heart J 2004;12:331-6
- Marwick TH, Scuffham PA, Hunink MG. Selection for early surgery in asymptomatic mitral regurgitation: a Markov model. Int J Cardiol 2013;165(2):266-72
- Dr. Anita Asgar. Personal communication. 2013
- Ontario Case Costing Initiative. Costing Analysis Tool. 2013. Available from: www.occp.com
- Regier DA, Sunderji R, Lynd LD, et al. Cost-effectiveness of self-managed versus physician-managed oral anticoagulation therapy. CMAJ 2006;174:1847-52
- Resnic FS, Arora N, Matheny M, et al. A cost-minimization analysis of the angio-seal vascular closure device following percutaneous coronary intervention. Am J Cardiol 2007;99:766-70
- Tretiak R, Laupacis A, Riviere M, et al. Cost of allogeneic and autologous blood transfusion in Canada. Canadian Cost of Transfusion Study Group. CMAJ 1996;154:1501-8
- Comay D, Marshall JK. Resource utilization for acute lower gastrointestinal hemorrhage: the Ontario GI bleed study. Can J Gastroenterol 2002;16:677-82
- Sunnybrook Health Sciences Center. Freedom of Information Request 2013-0009-FOI. 2013
- Ontario Ministry of Health and Long-Term Care. Ontario: Schedule of Benefits for Laboratory Services. 1999
- Ontario Ministry of Health and Long-Term Care. Ontario: Schedule of Benefits for Physician Services. 2013
- Treede H, Schirmer J, Rudolph V, et al. A heart team's perspective on interventional mitral valve repair: percutaneous clip implantation as an important adjunct to a surgical mitral valve program for treatment of high-risk patients. J Thorac Cardiovasc Surg 2012;143:78-84
- Conradi L, Treede H, Franzen O, et al. Impact of MitraClip therapy on secondary mitral valve surgery in patients at high surgical risk. Eur J Cardiothorac Surg 2011;40:1521-6
- Mealing S, Feldman T, Eaton J, et al. EVEREST II high risk study based UK cost-effectiveness analysis of MitraClip(R) in patients with severe mitral regurgitation ineligible for conventional repair/replacement surgery. J Med Econ 2013;16:1317-26
- Mealing SE, Eaton J. TCT-795 Abstract: MitraClip for patients with mitral regurgitation who are ineligible for surgical repair or replacement: a UK based cost-utility analysis. J Am Coll Cardiol 2011;58:B212-B
- Mealing SE, Eaton J, Singh M, et al. TCT-795 Poster: MitraClip for patients with mitral regurgitation who are ineligible for surgical repair or replacement: a UK based cost-utility analysis. Presented at the Transcatheter Cardiovascular Therapeutics annual scientific symposium, San Francisco, California, November 7-11, 2011
- Evalve, Abbott Vascular. United States Food and Drug Administration. MitraClip Clip Delivery System – P100009 Device Approval. Silver Sprint, MD: Food and Drug Administration. Available online at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm375149.htm. Accessed January 25, 2014
- Cardiovascular outcomes assessment of the MitraClip Therapy Percutaneous Therapy for High Surgical Risk Patients (COAPT). NCT01626079. Bethesda, MD: National Library of Medicine (US). 2014. Available online at: http://clinicaltrials.gov/ct2/show/NCT01626079. Accessed January 25, 2014
- Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making 2000;20:332-42
- Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81
- British Columbia Ministry of Health Services. British Columbia PharmaCare Formulary. Available online at: http://www.health.gov.bc.ca/pharmacare/benefitslookup/faces/Search.jsp. Accessed January 14, 2014