Abstract
Objective:
To estimate the real-world economic impact of switching hypertensive patients from metoprolol, a commonly prescribed, generic, non-vasodilatory β1-blocker, to nebivolol, a branded-protected vasodilatory β1-blocker.
Methods:
Retrospective analysis with a pre–post study design was conducted using the MarketScan database (2007–2011). Hypertensive patients continuously treated with metoprolol for ≥6 months (pre-period) and then switched to nebivolol for ≥6 months (post-period) were identified. The index date for switching was defined as the first nebivolol dispensing date. Data were collected for the two 6-month periods pre- and post-switching. Monthly healthcare resource utilization and healthcare costs pre- and post-switching were calculated and compared using Wilcoxon test and paired t-test. Medical costs at different years were inflated to the 2011 dollar.
Results:
In total, 2259 patients (mean age: 60 years; male: 52%; cardiovascular [CV] disease: 37%) met the selection criteria. Switching to nebivolol was associated with statistically significant reductions in the number of all-cause hospitalization (−33%; p < 0.01), CV-related hospitalizations (−60%; p < 0.01), and outpatient visits (−7%; p < 0.01). Monthly inpatient costs were reduced by $111 (p < 0.01), while monthly drug costs increased by $52 (p < 0.01). No statistically significant differences were found in overall costs and costs of outpatient or ER visits. Sensitivity analyses, conducted using various lengths of medication exposure, controlling for spill-over effect or excluding patients with compelling indications for metoprolol, all found some level of reduction in resource utilization and no significant difference in overall healthcare costs.
Conclusions:
This real-world study suggests that switching from metoprolol to nebivolol is associated with an increase in medication costs and significant reductions in hospitalizations and outpatient visits upon switching, resulting in an overall neutral effect on healthcare costs. These results may be interpreted with caution due to lack of a comparator group and confounding control caused by design and limitations inherent in insurance claims data.
Introduction
Drugs from the same class can have a variety of pharmacologic properties; therefore, their efficacy and tolerability in individual patients can differ significantlyCitation1–4. Beta-adrenergic receptor antagonists, for example, are a heterogeneous class of compounds, because its members differ in their adrenergic receptor selectivity, intrinsic sympathomimetic activity, and vasodilatory propertiesCitation3. Those differences are then reflected in the mechanism of action and tolerability of those agentsCitation3,Citation5.
Healthcare systems throughout the world are under increasing pressure to control and minimize costs. Substitution of brand-protected drugs with generic compounds within the same class is often believed to be a means of cost control and the opposite, a within-class therapy switch from a generic compound to a brand-protected drug, is often discouraged due to cost considerations. However, evidence for the assumed cost impact of those within-class substitutions is mixed, possibly due to heterogeneity among individual drugs. In cases of hypertension, there are studies that suggest both savingsCitation6,Citation7 and long-term cost increaseCitation8,Citation9 when substituting a branded angiotensin II receptor blocker [ARB] with a generic ARB; similar data for β-blockers are largely unavailable.
Metoprolol is a β1-selective blocker of β-adrenergic receptors, with a 74-fold preference for the β1 over β2 receptorCitation3,Citation10; it is the most frequently used β-blocker for the treatment of hypertension and the second most frequently used anti-hypertensive drug overallCitation11. In the US, metoprolol has been available as a generic medication since 2006. Nebivolol is the most recently approved β-blocker for hypertension and still under patent protection. It is highly β1-selective (β1/β2 affinity ratio: 321) and has a nitric oxide (NO)-mediated vasodilatory effectCitation5,Citation10,Citation12. A meta-analysis of head-to-head randomized trials showed that the tolerability of nebivolol is superior to that of other β1-selective blockers, including metoprololCitation1, and a retrospective claims analysis suggested that initiation of nebivolol treatment was associated with a lower risk of discontinuation or switching to another anti-hypertensive agent, compared with other β-blockers, including metoprololCitation13.
In this retrospective analysis of a medical database, we sought to examine the economic impact of switching from metoprolol to nebivolol in patients with hypertension.
Methods
Data source
Data were collected from the Truven Health MarketScan® Research Database (2007–2011)Citation14, which captures person-specific clinical utilization, expenditures and enrollment across inpatient, outpatient, prescription drug, and carve-out services from a selection of large employers, health plans, and government and public organizations in the US. The MarketScan Databases link paid claims and encounter data to detailed patient information across sites and types of providers and over time. This database includes both commercial claims and encounters data and Medicare supplemental and co-ordination of benefits data. All census regions are represented, predominantly the South and North Central (Midwest) regions.
Study design
This was a real-world, retrospective data analysis of the same set of patients before and after an index date (pre/post design). Patients with hypertension who were continuously treated with metoprolol ≥6 months (‘metoprolol period’), then switched to nebivolol and continuously treated with nebivolol for ≥6 months after switching (‘nebivolol period’) were identified. Index date was defined as the first day of dispensing nebivolol. Data were collected for the periods of 6 months before and after index date and compared in terms of health resources utilization (HRU) and costs (see Health economics outcomes below).
Eligibility criteria
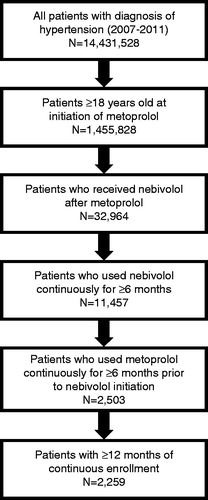
The Truven Health Analytics MarketScan® DatabaseCitation14 was used to collect blinded data on individuals ≥18 years of age who initiated metoprolol treatment in the period 2007–2011 and had one or more inpatient or outpatient claims with a primary or non-primary diagnosis of hypertension (ICD-9-CM 401.xx-405.xx). In addition, patients had to be treated with metoprolol (tartrate or succinate) continuously for ≥6 months prior to switching to nebivolol. The switch was defined as discontinuation of metoprolol and filling of a nebivolol prescription; the patients were required to initiate nebivolol treatment no earlier than 15 days before the last metoprolol supply date and no later than 30 days thereafter. Patients had to have ≥12 months of continuous enrollment, of which ≥6 months was prior to and ≥6 months was following nebivolol initiation.
Health economics outcomes
The HRU parameters comprised the numbers of all-cause hospitalizations, cardiovascular (CV)-related hospitalizations (DRG code: 216-316), all-cause emergency room (ER) visits, CV-related ER visits, outpatient visits, and medications. Costs data ($2011, adjusted for inflation) were collected for hospitalizations, ER visits, outpatient visits, and medications.
Statistical analysis
Demographic and clinical characteristics were summarized using descriptive statistics. Comparison of HRU data between metoprolol and nebivolol periods was performed using a Wilcoxon signed rank test; paired t-test was used for cost variables. Statistical significance was at the 1% level (two-sided). In addition to the primary analysis (≥6 months of exposure to metoprolol vs ≥6 months of exposure to nebivolol), sensitivity analyses were conducted to examine the potential influence of (a) duration of drug exposure (>0 months, ≥3 months, and ≥9 months), (b) duration of follow-up (both periods were extended to 12 months), (c) spill-over effect from metoprolol treatment (data from the first post-index date week were excluded; monthly HRU data in the nebivolol period were calculated based on 3–6 months post index date), and (d) compelling indications for metoprolol use (patients diagnosed with angina pectoris, acute myocardial infarction, or congestive heart failure during the 6-month period preceding the index date were excluded).
Results
Patient characteristics
Out of 14,431,528 patients with hypertension captured in the database in the period 2007–2011, a total of 2259 satisfied the eligibility criteria (). The mean age on index date was 60.3 years (range = 21–96 years); 51.8% of eligible patients were men, 56.8% had preferred provider organization (PPO) insurance, and the most common comorbidities were CV disease (37.0%) and diabetes (19.3%). Demographic and clinical characteristics on index date are summarized in .
Table 1. Demographic and clinical characteristics of eligible patients on index date (n = 2259).
Diuretics, calcium channel blockers (CCBs), and angiotensin-converting enzyme inhibitors (ACEIs) were the most commonly used background anti-hypertensive therapies (); changes in background regimen after index date were negligible (data not shown).
Table 2. Background anti-hypertensive treatment before (metoprolol period) and after (nebivolol period) index date (n = 2259).
Primary analysis
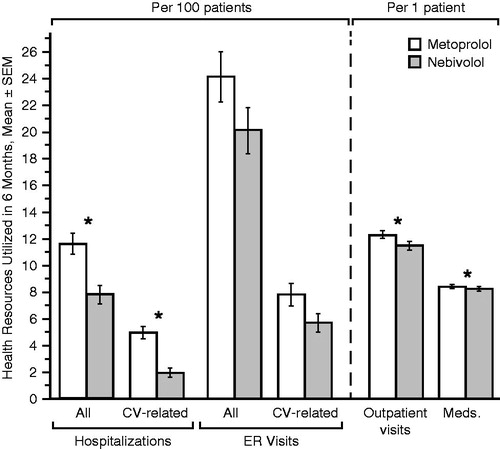
Switching from metoprolol to nebivolol was associated with statistically significant reductions in the number of all-cause hospitalizations (by 33%: from 11.7 per 100 patients to 7.9 per 100 patients, p < 0.001), CV-related hospitalizations (by 60%: from 5.0 per 100 patients to 2.0 per 100 patients, p < 0.001), and outpatient visits (by 7%: from 12.4 per patient to 11.5 per patient, p < 0.001) ().
Figure 2. Six-month health resources utilization before (metoprolol period) and after (nebivolol period) index date. *p < 0.01 (Wilcoxon signed rank test). CV, cardiovascular; ER, emergency room; Meds, medications, SEM, standard error of the mean.
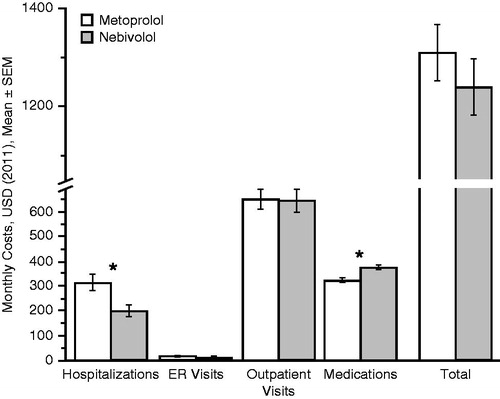
In addition, switching from metoprolol to nebivolol was associated with a statistically significant reduction in the monthly hospitalization costs ($111 per patient: from $314 to $205, p = 0.003) and a statistically significant increase in monthly medication costs ($52 per patient: from $326 to $379, p < 0.001). The reduction in total monthly costs of $69 per patient in the nebivolol period was not statistically significant (from $1310 to $1241, p = 0.230) ().
Sensitivity analyses
Switching from metoprolol to nebivolol in patients with any duration of exposure to either drug (>0 months; n = 10538) resulted in significantly reduced HRUs for all parameters assessed () and significant reductions in costs of hospitalizations, ER visits, outpatient visits, and total costs, as well as a significant increase in medication costs (). Other sensitivity analyses were generally in agreement with the primary findings (data not shown).
Figure 4. Monthly health resources utilization before (metoprolol period) and after (nebivolol period) index date (≥0 months of treatment with either drug, n = 10 538). *p < 0.01 (Wilcoxon signed rank test). CV, cardiovascular; ER, emergency room; SEM, standard error of the mean.
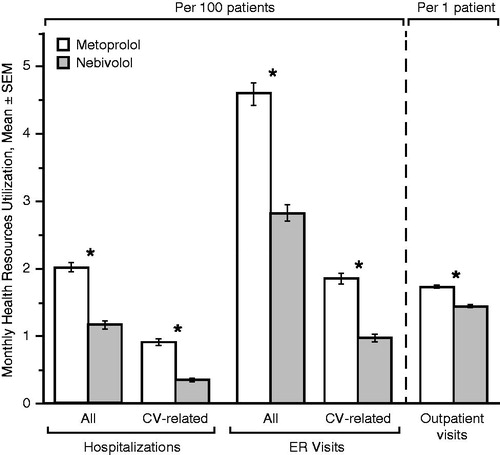
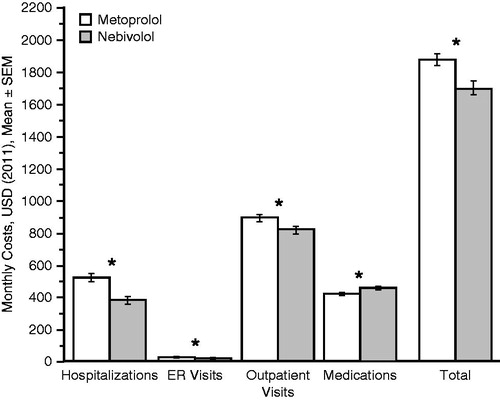
Figure 5. Monthly costs before (metoprolol period) and after (nebivolol period) index date (≥0 months of treatment with either drug, n = 10 538). *p < 0.01 (paired t-test). ER, emergency room; SEM, standard error of the mean; USD, US dollar.
Finally, exclusion of the 222 patients who had a compelling indication for metoprolol use (a diagnosis of angina pectoris, acute myocardial infarction, or congestive heart failure) in the 6-month period preceding the index date created a population of 2037 patients (90.2% of the original data set), in whom switching from metoprolol to nebivolol did not result in significant changes in overall costs or HRU, except for the number of 6-month outpatient visits per patient (mean ± SEM, from 11.5 ± 0.3 per patient to 10.9 ± 0.3 per patient; p < 0.01).
Discussion
To our knowledge, this was the first study to investigate the pharmacoeconomic consequences of switching from a generic to branded β-blocker in patients with hypertension. The results suggest that, in patients with hypertension, switching from metoprolol, a generic, non-vasodilatory, β1-selective blocker, to nebivolol, a brand-protected, vasodilatory, β1-blocker, is not associated with an overall increase in costs of care, despite a significant increase in the cost of anti-hypertensive medication. This observation was confirmed in sensitivity analyses, even upon removal of data from patients with angina, myocardial infarction, and heart failure.
The data also suggest that the switch from metoprolol to nebivolol was associated with a lower rate of health resources utilization (i.e. hospitalizations and outpatient visits). Benefits on hospitalization appear to be concentrated in patients with a compelling indication for metoprolol use (angina pectoris, acute myocardial infarction, congestive heart failure), which is consistent with a relatively high cost and high demand on health resources associated with those conditionsCitation15. Studies that examined the effects of switching from a brand-protected to a generic ARB also suggested an association between costs and the rate of CV eventsCitation8,Citation9. Benefits on outpatient visits were observed among patients with or without other indications for metoprolol.
While the causal pathway of reduction in healthcare resources utilization was not explored in this study, existing literature suggests some possible reasons, including favorable efficacy, tolerability, and treatment persistence associated with nebivolol use. Head-to-head randomized trials showed that nebivolol was better tolerated than other β1-selective blockers, including metoprololCitation1,Citation16. An open-label prospective study (n = 1468) found that nebivolol, prescribed as an initial, add-on, or switch therapy to patients with hypertension, was associated with significant improvements in quality-of-lifeCitation17. A large retrospective claims analysis (n = 173,200) demonstrated a greater drug persistence (a proxy measure of tolerability) with nebivolol compared with other β-blockers, including metoprololCitation13.
There were several limitations to our approach, most of which related to the use of claims databases. First, in the absence of blood pressure and tolerability data, it is impossible to construct a control group that would have matching clinical characteristics but stay on metoprolol instead of switching to nebivolol. During the study period, patients may have experienced changes in conditions not related to hypertension and, without a control group, we were not able to fully assess cost impacts of those changes. In addition, assessing CV-related events might shed some light on the hypertension-related benefits of switching, but it remains unclear to what extent are those due to switching to nebivolol and to what extent a consequence of mere metoprolol discontinuation. Second, data were derived from a geographically diverse population, covered by large employer-sponsored private health insurance programs, and, therefore, may not be representative of populations with different characteristics—in particular, Medicare and Medicaid recipients. Third, medication consumption was assessed using dispensing records, which means that information on whether patients really took their medication was not available. These limitations should be addressed in future studies.
Conclusions
This study suggests that switching from metoprolol, a generic β1-selective blocker, to nebivolol, a brand-protected β1-selective blocker, may be associated with lower healthcare resources utilization, which could offset the increase in medication costs. Despite higher drug acquisition cost, switching to nebivolol was not associated with increased overall healthcare costs. Formulary policies that discourage switching from generic metoprolol to branded nebivolol in order to reduce costs may, therefore, lead to an increase in healthcare resource utilization, not to cost-saving. Physicians should continue to focus on individual patient needs when considering this therapy substitution. Further studies, aimed at identifying specific reasons for the observed benefits of switching from metoprolol to nebivolol, will help inform future prescription and formulary decisions.
Transparency
Declaration of funding and financial relationships
This study was sponsored by Forest Research Institute (FRI), a subsidiary of Forest Laboratories, the US marketer of nebivolol.
Declaration of financial relationships
SC and ST are employees of Forest Research Institute. TL was a Forest Research Institute employee at the time the study was conceived and conducted. In addition, all authors own (or used to own - TL) Forest stock. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors wish to acknowledge medical writing assistance provided by Leah Richmond, Bill Sterling, and Vojislav Pejović of Prescott Medical Communications Group (Chicago, IL), a contractor of the Forest Research Institute. The Prescott Group assisted with editing, literature searches, optimization of graphs and tables, and formatting of manuscript for submission.
References
- Ambrosioni E, Borghi C. Tolerability of nebivolol in head-to-head clinical trials versus other cardioselective B-blockers in the treatment of hypertension. High Blood Press Cardiovasc Prev 2005;12:27-35
- Belz GG. Pharmacological differences among angiotensin II receptor antagonists. Blood Press Suppl 2001;2:13-18
- Frishman WH, Saunders E. beta-Adrenergic blockers. J Clin Hypertens (Greenwich) 2011;13:649-53
- Mancia G. Clinical differences among angiotensin II receptor antagonists. Blood Press Suppl 2001;2:19-24
- Vanhoutte PM, Gao Y. Beta blockers, nitric oxide, and cardiovascular disease. Curr Opin Pharmacol 2013;13:265-73
- Usher-Smith J, Ramsbottom T, Pearmain H, et al. Evaluation of the clinical outcomes of switching patients from atorvastatin to simvastatin and losartan to candesartan in a primary care setting: 2 years on. Int J Clin Pract 2008;62:480-4
- Usher-Smith JA, Ramsbottom T, Pearmain H, et al. Evaluation of the cost savings and clinical outcomes of switching patients from atorvastatin to simvastatin and losartan to candesartan in a Primary Care setting. Int J Clin Pract 2007;61:15-23
- Baker TM, Goh J, Johnston A, et al. Cost-effectiveness analysis of valsartan versus losartan and the effect of switching. J Med Econ 2012;15:253-60
- Signorovitch J, Zhang J, Wu EQ, et al. Economic impact of switching from valsartan to other angiotensin receptor blockers in patients with hypertension. Curr Med Res Opin 2010;26:849-60
- Münzel T, Gori T. Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol 2009;54:1491-9
- Gu Q, Burt VL, Dillon CF, et al. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation 2012;126:2105-14
- Gao Y, Vanhoutte PM. Nebivolol: an endothelium-friendly selective beta1-adrenoceptor blocker. J Cardiovasc Pharmacol 2012;59:16-21
- Signorovitch JE, Samuelson TM, Ramakrishnan K, et al. Persistence with nebivolol in the treatment of hypertension: a retrospective claims analysis. Curr Med Res Opin 2012;28:591-9
- Truven Health Analytics. Ann Arbor, MI.
- Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care 2009;15(2 Suppl):S36-41
- Van Bortel LM, Fici F, Mascagni F. Efficacy and tolerability of nebivolol compared with other antihypertensive drugs: a meta-analysis. Am J Cardiovasc Drugs 2008;8:35-44
- Hermans MP, De Coster O, Seidel L, et al. Quality of life and efficacy of nebivolol in an open-label study in hypertensive patients. the QoLaN study. Blood Press Suppl 2009;1:5-14