Abstract
Objective:
Achieving therapeutic goals in multiple sclerosis (MS) requires strict adherence to treatment schedules. This retrospective study analyzed persistence with, and adherence to, fingolimod compared with injectable/infusible disease-modifying therapies (DMTs) in patients with MS.
Methods:
Patients in the PharMetrics Plus™ US administrative claims database with at least one prescription for, or administration of, fingolimod, glatiramer acetate (GA), interferon (IFN), or natalizumab (index DMT) between October 1, 2010 and September 30, 2011 were included. Patients were naïve to index DMT (no claim in the previous 360 days) and had an MS diagnosis code within 360 days of the first index DMT prescription. Outcomes were persistence, risk of discontinuing index DMT (evaluated by a Cox proportional hazards model), adherence (measured using the medication possession ratio [MPR] and proportion of days covered [PDC] in patients with at least two index DMT prescriptions), and the risk of being non-adherent (MPR <80% and PDC <80%, assessed using a logistic regression model).
Results:
The study included 3750 patients (fingolimod, n = 889; GA, n = 1233; any IFN, n = 1341; natalizumab, n = 287). Discontinuation rates (fingolimod, 27.9%; GA, 39.5%; IFN, 43.7%; natalizumab, 39.5%; all p < 0.001) and risk of discontinuation were significantly higher (hazard ratios vs fingolimod [95% confidence interval]: GA, 1.75 [1.49–2.07]; IFN, 2.01 [1.71–2.37]; natalizumab, 1.53 [1.22–1.91]) for patients receiving other DMTs compared with fingolimod. The risk of being non-adherent was also lower for patients in the fingolimod cohort than the other treatment cohorts, irrespective of whether non-adherence was defined as MPR <80% (p < 0.05 for all) or PDC <80% (p < 0.05 for GA and IFN).
Limitations:
As with all studies assessing real-world treatment patterns it is unclear if medications were used as prescribed.
Conclusions:
In a real-world setting, persistence with, and adherence to, oral fingolimod was higher than for injectable and infusible DMTs.
Introduction
Multiple sclerosis (MS) is an incurable, chronic, inflammatory, degenerative disease of the central nervous system (CNS)Citation1,Citation2. It is the leading cause of disability in young and middle-aged people in the developed worldCitation3, affecting ∼2.1 million people worldwide and 400 000 people in the USCitation4. Most patients (80–85%) present with the relapsing–remitting form of MS, which is characterized by self-limiting episodes of neurological dysfunction (relapses) followed by periods of remission and recoveryCitation5. The course of MS is highly unpredictable but, as endogenous repair mechanisms fail to restore damaged CNS tissueCitation6, the disease progressively worsens to result in irreversible disability and premature deathCitation7,Citation8.
Current treatments for MS aim to prevent relapses, slow disease progression, and postpone long-term disability. Glatiramer acetate (GA) and interferons (IFNs) are recommended as first-line disease-modifying therapies (DMTs) for MSCitation9–11, but these injectable therapies are increasingly perceived to have only partial effectiveness and a sub-optimal tolerability profile, mainly driven by influenza-like symptoms or injection site reactionsCitation12. Several new therapies with different mechanisms of action and modes of administration have been introduced in recent yearsCitation12,Citation13. These include natalizumab, a recombinant anti-alpha 4 monoclonal antibody that is administered by monthly infusionsCitation14,Citation15, and fingolimod, a sphingosine 1-phosphate receptor modulator, which is the first oral therapy approved for the treatment of relapsing forms of MSCitation16.
Achieving therapeutic goals requires prompt treatment initiation and stringent adherence to both the medication dose and administration scheduleCitation17–20. Adherence is a measure of the extent to which patients take medication in accordance with the prescribed regimenCitation21. Real-world insight into medication-taking behaviors can be gained from analyses of medical claims data. Claims databases also provide valuable information on persistence, which provides a complementary assessment of medication use, measuring the duration of continuous use rather than the proportion of days that a patient took the prescribed medicationCitation21.
Several studies have shown that adherence to, and persistence with, DMTs are associated with a reduced risk of relapseCitation22,Citation23, reduced healthcare resource utilizationCitation22, reduced incidence of MS-related hospitalizationCitation19, and improved health-related quality-of-lifeCitation24,Citation25 in patients with MS. First-line injectable DMTs are associated with sub-optimal rates of adherence and persistenceCitation20,Citation22,Citation26–29, and treatment-related issues, such as unresponsiveness and intolerance to GA or IFNs, prompt patients to discontinue or switch therapyCitation30,Citation31. A review of DMT discontinuation rates across several countries found that 16–27% of patients discontinued GA or IFN therapy prematurely within 24 months of follow-up, increasing to 43% for GA and up to 34% for IFNs when patients were followed for longer than 24 monthsCitation32. The most common reasons given for IFN discontinuation were side-effects and lack of effectivenessCitation32. A US claims database analysis reported discontinuation rates of 43.8% for GA and 44.8–53.9% for IFNs for treatment-naïve patients within 12 months of treatment initiationCitation33. A recent retrospective claims database analysis of nearly 7000 patients with MS in the US reported that only 55% and 52–62% of patients were adherent (measured as a medication possession ratio [MPR] ≥80%) to GA and IFNs, respectively, over 12–36 monthsCitation27. Another study that included only IFNs reported that 27–41% of patients were adherent (MPR ≥85%) in each year of the study, with only 4% of patients being continuously adherent throughout the 3-year studyCitation22. Natalizumab is administered as an intravenous infusion once monthly and has improved adherence compared with the injectable DMTs, with one study reporting a mean adherence rate (measured using MPR) of 85% compared with 73–78% for GA and IFNsCitation26. The authors of that study suggested that this improved adherence was probably due to the requirement for active physician involvement and monitoring during natalizumab administrationCitation26.
Effective management of MS is an ongoing process in which factors that support patient persistence and adherence are important. Phase 3, randomized, controlled trials have shown that fingolimod is more effective than IFN or placebo at reducing relapses, slowing disability progression and improving magnetic resonance imaging (MRI) and brain atrophy outcomesCitation34,Citation35. In addition, the favorable tolerability profileCitation36,Citation37 and once-daily oral administration of fingolimod may result in improved medication-taking behaviors compared with injectable/infusible DMTs. A recent claims database analysis demonstrated that patients with MS initiating fingolimod therapy had higher rates of persistence (discussed above) and adherence than patients using GA and IFNs (MPR ≥80% in treatment-naïve patients; fingolimod, 87.4%; GA, 81.5%; IFNs, 72.8–80.2%; all p < 0.05)Citation33. However, that study did not include patients treated with natalizumab.
The objective of this retrospective cohort analysis of a US health insurance claims database was to assess persistence and adherence rates among patients with MS treated with once-daily, oral fingolimod in comparison with injectable (GA and IFNs) and infusible (natalizumab) DMTs in a real-world setting.
Patients and methods
Data source
Adjudicated medical and pharmacy claims data from the PharMetrics Plus™ claims database were used in this retrospective cohort analysis. More than 87 million health-plan members across the US have been enrolled in the database from 2006 onwards, providing longitudinal data on ∼22 million patients with at least 4 years of continuous enrollment in their health plan. The database has broad geographical coverage, with patients in each three-digit zip code in the US, and is representative of the US commercially insured population. It includes data from 80% of all US doctors and 90% of US hospitals, and representation from 85% of the Fortune 100 companies. By including integrated claims data (i.e. data from multiple places of service [inpatient, outpatient and pharmacy]) for over 100 000 patients with MS in 2011, the database is also considered to be generally representative of the population of patients with MS in the US.
Inpatient and outpatient diagnoses were recorded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; other data recorded include outpatient and inpatient procedures, detailed information on pharmacy and medical benefits (co-payment/co-insurance amount, deductible, in-network vs out-of-network), inpatient stay (admitting vs other diagnoses, admission type and source, discharge status) and provider details (specialty, zip code, attending, referring, rendering, prescribing, primary care provider), and retail and mail-order prescription records. ICD-9-CM diagnosis and procedural codes were available for each hospital stay, although names and descriptions of specific medications during inpatient stays were not available. Demographic variables (age, gender, and geographical region), insurance product type (e.g. health maintenance and preferred provider organizations), payer type (e.g. commercial, self-pay) and start and stop dates of health-plan enrollment were additional data elements considered. Amounts charged by providers, and amounts allowed and paid by health plans, were available for all services rendered, and included the dates of all services. The names and descriptions of specific medications used during hospital stays were not available, however, making it impossible to identify DMTs used during an inpatient stay. DMTs administered in an outpatient clinic or infusion center could be identified when an overnight hospital stay was not required. Dates of service for all claims, and fees charged by providers (and amounts allowed and paid by health plans) were available for all services. Other recorded fields included demographic data (patient age, gender, and geographical region), insurance product type (e.g. health maintenance and preferred provider organizations), payer type (e.g. commercial, self-pay), and start and stop dates of health-plan membership.
The database is fully compliant with the Health Insurance Portability and Accountability Act (HIPAA) privacy regulations. Patient informed consent and Institutional Review Board approval were not required for this study because all patient-level data are certified to be anonymous and no direct contact with the patient was required.
The study was designed, implemented, and reported in accordance with the ethical principles laid down in the Declaration of Helsinki, the Guidelines for Good Pharmacoepidemiology Practices (GPP) of the International Society for Pharmacoepidemiology, and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelinesCitation38.
Patient selection
Patients who had at least one prescription or administrative claim for fingolimod (Gilenya® ), GA (Copaxone®Footnote† ), IFN therapy (IFN beta-1a [intramuscular Avonex®Footnote‡ or subcutaneous Rebif®Footnote§ ] or IFN beta-1b [Extavia®Footnote¶ and Betaseron®Footnote** , both administered subcutaneously]) or natalizumab (Tysabri®Footnote†† ) between October 1, 2010 and September 30, 2011 (index period) were identified in the database. National Drug Codes used are listed in Supplementary Table 1 and procedural codes for administering DMTs in the clinical setting are listed in Supplementary Table 2. The first prescribed DMT in this period was defined as the index DMT, and the date on which the index DMT was first prescribed was defined as the index date. Patients with evidence of both fingolimod and another DMT were preferentially assigned to the fingolimod cohort to preserve the sample size, while the other treatment cohorts were assigned based on the first observed DMT. Medical and pharmacy records for eligible patients were then studied for 360 days following the index date.
For inclusion, patients needed to have continuous health-plan enrollment for a minimum of 360 days before and after the index date (the pre- and post-index periods, respectively); to have at least one claim with an MS diagnosis (ICD-9-CM code 340) within 360 days of the index date (pre- or post-index); and to be aged 18 years or older on the index date. Patients were excluded from the analysis if they had received more than one DMT on the index date, had a claim for the index DMT in the previous 360 days (i.e. patients must have been naïve to index DMT), had data quality issues (e.g. invalid enrollment date, missing or invalid age or missing gender; or coverage information) or had a gap in the claims data indicative of missing days of supply information for the index therapy (). Days’ supply is not reported on claims for physician-administered injectable/infusible treatments so, in order to measure persistence, days’ supply was derived from the prescribing information for each treatment. Administration of once-daily GA equalled 1 days’ supply; administration of 4-weekly natalizumab equalled 28 days’ supply; administration of once-weekly Avonex® equalled 7 days’ supply; administration of Betaseron® and Extavia® (prescribed for use every other day) equalled 2 days’ supply; and Rebif® administered 3-times weekly equalled 2.3 days’ supply.
Study measures
The primary measures of interest in this study were persistence and adherence rates in the fingolimod, GA, IFN, and natalizumab cohorts. Additional analyses were performed for the patient cohorts receiving IFN beta-1a and IFN beta-1b. Persistence was measured, based on treatment practice patterns, as the time in consecutive days from index DMT monotherapy initiation until discontinuation, receipt of another DMT of interest (fingolimod, GA, IFN, or natalizumab), or the end of available data (360 days post-index), whichever occurred first. Discontinuation was defined as a gap in exposure to the index DMT of at least 60 days following the date when the index DMT should next have been dispensed or administered.
The proportion of patients persistent with therapy, total number of therapy claims, and total number of persistence days were assessed for all patients and for those patients with at least two claims for the index DMT. Adherence to index DMTs was assessed over the post-index persistence period in the sub-set of patients with at least two claims for their index DMT. It was assessed using two measures.
MPR was defined as the total number of days supply of the index DMT during the persistence period divided by the number of days between the first prescription fill and the last refill plus the days supply of the last refill.
Proportion of days covered (PDC) was defined as the number of days on the index DMT during the persistence period divided by the number of days that a patient was persistent with the index DMT. Compared with the fixed-interval MPR, the PDC provides a more conservative estimate, as it counts only the number of days with index therapy. If a patient refills a prescription early, any days with double counting or overlapping from the early refill would be counted only once.
For each index DMT, MPR and PDC were presented as a point estimate and were provided for those patients with at least two claims for their index DMT. Patients with an MPR or PDC of at least 80% were considered adherent and those with an estimated MPR or PDC of less than 80% were considered non-adherent.
Other data recorded included patient demographics and baseline characteristics, which were evaluated during the pre-index period. These included age at index date, gender, previous use of non-index DMT, use of dalfampridine, Charlson comorbidity index scoreCitation39,Citation40, comorbidities of interest (e.g. dyslipidemia, depression), evidence of any MS relapse, and number of MS relapses.
Statistical analyses
For categorical measures, data are presented as counts and proportions. Continuous variables were summarized by providing the mean, 95% confidence interval (CI), standard deviation (SD) and median. Differences in the distribution of these variables were tested for statistical significance using χ2 tests for categorical variables and the Wilcoxon rank-sum test for continuous variables. The risk of discontinuing treatment with the index DMT was estimated using a Cox proportional hazards model, adjusting for differences in pre-index demographic and clinical characteristics. Variables included in the final model were age group, gender, payer type, physician/prescriber specialty, pre-index total healthcare cost (as a logarithmic continuous measure), pre-index number of relapses (two or more vs one), pre-index use of dalfampridine, and comorbidities and symptoms present in at least 5% of patients and those thought to be associated with the dependent variable. Other demographic and clinical characteristics were originally included, but later removed from the model due to multi-collinearity. The risk of being non-adherent to the index DMT during the persistence period was estimated in patients with at least two claims during the persistence period with a logistic regression model using a similar approach. The dependent variable was non-adherence to DMT, with MPR/PDC <80% = 1 and MPR/PDC ≥80% = 0.
Results
Study attrition
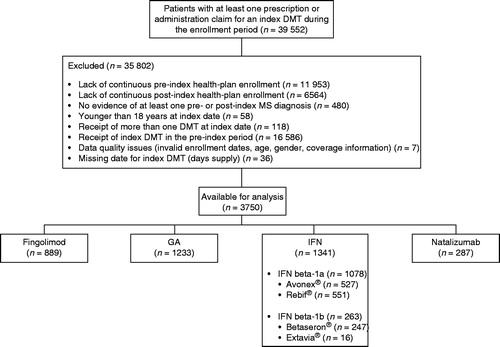
In total, 39 552 patients were initially identified, of whom 35 802 (90.5%) were excluded for the reasons listed in . Of the 3750 eligible patients, 889 (23.7%) received fingolimod as their index DMT, 1233 (32.9%) received GA, 1341 (35.8%) received an IFN (Avonex®, n = 527; Rebif®, n = 551; Betaseron®, n = 247; Extavia®, n = 16) and 287 (7.7%) received natalizumab.
Baseline demographics and clinical characteristics
The baseline demographics and clinical characteristics of the fingolimod, GA, IFN and natalizumab cohorts are shown in . Over three-quarters (77.0%) of the patients included in the study were women. Patients in the fingolimod cohort were significantly older (all p < 0.01) and were more likely to have previously used DMTs (all p < 0.001) than patients in the other DMT cohorts. In addition, patients treated with fingolimod had lower Charlson comorbidity index scores than patients treated with the other DMTs.
Table 1. Baseline demographics and clinical characteristics (in the 360-day pre-index period) of patients with MS included in the index cohorts.
A history of dyslipidaemia was significantly more common in the fingolimod cohort than in the GA and IFN cohorts, but a significantly lower proportion of patients in the fingolimod cohort had a history of CVD compared with patients in the GA and IFN cohorts. A history of depression was more common in the fingolimod and natalizumab cohorts than in the GA and IFN cohorts. During the pre-index period, significantly more patients in the fingolimod and natalizumab cohorts experienced at least one relapse compared with patients in the GA and IFN cohorts (fingolimod, 41.4%; GA, 32.0%; IFN, 31.8%; natalizumab, 45.3%). The mean number of relapses in the pre-index period was highest in the natalizumab cohort (0.74), followed by 0.67 in the fingolimod cohort and 0.39 in both the GA and IFN cohorts.
Discontinuation of index DMT
Of the 3750 patients included in the study, 38.2% discontinued their index therapy during the post-index period. The proportion of patients who discontinued their index therapy was significantly lower in the fingolimod cohort (27.9%) than in the other DMT cohorts (GA, 39.5%; IFN, 43.7%; natalizumab, 39.0%; all p < 0.001; ). Similar observations were made for the individual IFN sub-cohorts, with 44.2% of patients in the IFN beta-1a sub-cohort and 41.8% of patients in the IFN beta-1b sub-cohort discontinuing therapy (all p < 0.0001 vs fingolimod).
Figure 2. Proportion of patients with MS who discontinue index DMT within 360 days of initiating therapy. The risk of discontinuing treatment with the index DMT was estimated using a Cox proportional hazards model, adjusting for pre-index demographic and clinical characteristics of interest. DMT, disease-modifying therapy; GA, glatiramer acetate; IFN, interferon; MS, multiple sclerosis.
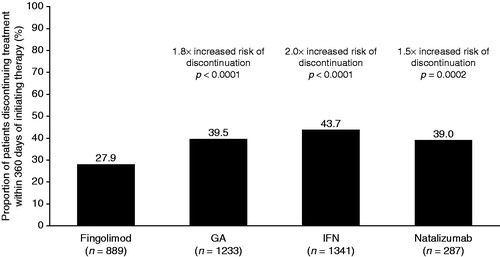
After controlling for baseline characteristics, the risk of discontinuation from index DMT was 1.5–2-fold higher in patients taking GA, IFN, or natalizumab than in those taking fingolimod (hazard ratios vs fingolimod [95% CI]: GA, 1.75 [1.49–2.07]; IFN, 2.01 [1.71–2.37]; natalizumab, 1.53 [1.22–1.91]; ). The adjusted estimates for the risk of discontinuing are very similar to the unadjusted estimates (). Additional multivariate analyses assessing the impact of independent variables on the risk of discontinuation showed that the hazard ratio is influenced by gender, number of pre-index relapses, pre-index total healthcare costs, and several symptoms (numbness, visual symptoms, bowel dysfunction and pain; ).
Table 2. Cox proportional hazards model of variables affecting risk of discontinuing index disease-modifying therapy in patients with MS.
Non-adherence to index DMT
Adherence to index DMT was assessed over the post-index persistence period in the sub-set of patients with at least two fills/administrations of their index DMT; the mean persistence period for these patients was longer than that for the full study population (fingolimod, 318 [SD = 88 days]; GA, 301 [SD = 99 days]; IFN, 290 [SD = 105 days]; natalizumab, 288 [SD = 110 days]). Using an MPR of less than 80% as a measure of non-adherence, a lower proportion of patients receiving fingolimod were non-adherent over the post-index persistence period than patients receiving other DMTs (). When the individual IFNs were considered as separate sub-cohorts, 11.2% in the IFN beta-1a sub-cohort and 14.5% of patients in the IFN beta-1b sub-cohort had an MPR of less than 80%. In multivariate adjusted analyses, the risk of being non-adherent (MPR <80%) to GA, IFN, or natalizumab was 1.9–2.3-fold higher than fingolimod (odds ratio vs fingolimod [95% CI]: GA, 2.30 [1.60–3.30], p < 0.0001; IFN, 2.12 [1.48–3.04], p < 0.0001; natalizumab, 1.88 [1.16–3.05], p = 0.0103). Similar results were observed when non-adherence was defined as a PDC of less than 80%. The proportion of non-adherent patients was lower in the fingolimod cohort than in the other treatment cohorts (). When the individual IFNs were considered as separate sub-cohorts, 14.2% in the IFN beta-1a sub-cohort and 17.6% of patients in the IFN beta-1b sub-cohort had a PDC of less than 80%. Furthermore, after adjusting for baseline differences patients receiving GA or IFN had a 1.4–1.8-fold increased risk of being non-adherent (PDC <80%) over the 360-day follow-up than patients receiving fingolimod (odds ratio vs fingolimod [95% CI]: GA, 1.76 [1.30–2.38], p = 0.0002; IFN, 1.52 [1.13–2.06], p = 0.0060; natalizumab, 1.36 [0.89–2.08], p = 0.1534). The adjusted estimates for the risk of non-adherence are very similar to the unadjusted estimates ().
Figure 3. Proportion of non-adherent patients with MS, measured as MPR <80% (a) or PDC <80% (b) during the post-index persistence period and the risk of being adherent to therapy for each cohort over the 360-day follow-up. The risk of being non-adherent to index DMT was estimated in patients with at least two claims during the persistence period using a logistic regression model, adjusting for pre-index demographic and clinical characteristics of interest. GA, glatiramer acetate; IFN, interferon; MPR, medication possession ratio; MS, multiple sclerosis; PDC, proportion of days covered.
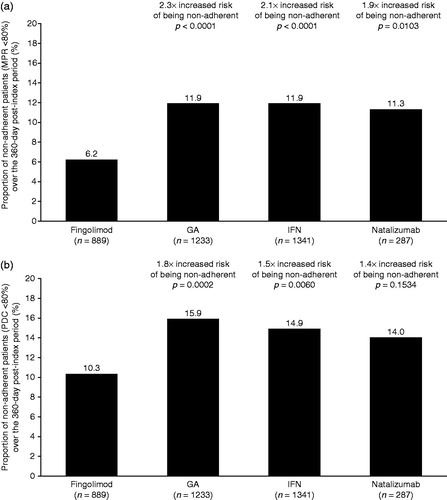
Table 3. Regression analysis of variables affecting odds of being non-adherent (MPR <80%, PDC <80%) to index DMT in patients with MS with two or more claims for their index DMT.
Multivariate adjusted analyses assessing the impact of independent variables on non-adherence to therapy showed that predictors of non-adherence were different to predictors for the risk of discontinuation. Odds ratios for the risk of being non-adherent (common to both MPR and PDC) were age, gender, and numbness (). Significant predictors also differed between the two measures of adherence, with age, gender, headache, and numbness influencing adherence measured by MPR, and age, gender, pre-index healthcare costs, pre-index use of dalfampridine, and numbness influencing adherence measured by PDC.
Discussion
This retrospective cohort database analysis showed that rates of persistence and adherence in a real-world setting were significantly higher for patients with MS treated with fingolimod than for those treated with injectable/infusible DMTs. In this US managed care population, the risk of discontinuing treatment was lower with fingolimod than with GA, IFNs, and natalizumab. Adherence outcomes, as assessed by both MPR and PDC, were also significantly improved for the fingolimod cohort compared with the cohorts for the other DMTs.
By reporting on both persistence and adherence, this study provides a comprehensive assessment of DMT-taking behavior in a real-world setting. The higher rates of persistence and adherence observed in patients receiving fingolimod than in those receiving injectable/infusible DMTs in our study may reflect the favorable tolerability profile of fingolimodCitation36,Citation37 and the convenience of its once-daily oral administration. In addition, the superior efficacy of fingolimod over IFN beta-1a i.m. with regard to reducing relapses, and improving MRI and brain atrophy outcomes, as demonstrated in the phase 3, randomized, controlled TRANSFORMS studyCitation34, may contribute to improved rates of medication use.
The main reasons for discontinuing IFN and GA treatment are a perceived lack of efficacyCitation30 and the occurrence of adverse eventsCitation30,Citation32. Discontinuations due to these reasons can lead to a switch in DMT. Switches can result in increased non-pharmacy costsCitation41 and may be associated with a break in disease control, as some DMTs take time to become fully effective; for example, GA may take to up 9 months to reach its maximum effectivenessCitation42. However, switches can also be beneficial to patients, particularly if they have switched due to experiencing breakthrough disease on injectable therapies to a more efficacious therapy. A switch to a more efficacious therapy may promote adherence, which has been shown to be associated with improved clinical outcomesCitation19, reduced healthcare resource utilizationCitation22, reduced incidence of MS-related hospitalizationCitation19, and improved health-related quality-of-lifeCitation24,Citation25. Tan et al.Citation19 found that patients who were adherent (measured as an MPR of ≥0.80) had significantly lower odds of relapse than non-adherent patients (odds ratio = 0.71; 95% CI = 0.59–0.85). These findings suggest that treatment with highly effective and tolerable DMTs that promote patient adherence, such as fingolimod, may result in better clinical outcomes.
Only one other US claims database study, by Agashivala et al.Citation33, has compared discontinuation and adherence rates for fingolimod with those for the first-line injectable DMTs. However, that study did not include patients treated with natalizumab. Agashivala et al. reported that, in patients who had previously used DMTs, those treated with fingolimod had lower rates of discontinuation than those treated with injectable DMTs (fingolimod, 25.7%; GA, 37.4%; IFNs, 45.9–57.1%; p < 0.05). These rates are comparable to those in our study, which also showed that significantly fewer patients in the fingolimod cohort discontinued therapy than in the other DMT cohorts (fingolimod, 27.9%; GA, 39.5%; IFN, 43.7%; natalizumab, 39.0%; all p < 0.001). Agashivala et al. also assessed rates of adherence using both MPR and PDC, and reported that, among patients who had previously used DMTs, those treated with fingolimod had higher rates of adherence than patients treated with injectable DMTs. Rates of adherence using MPR in the Agashivala et al. study are generally in agreement with those observed in our study, although rates using a PDC of at least 80% to identify adherent patients are lower than those in our study (fingolimod, 73.7% vs 89.7%, respectively). This could be explained by the differences in calculating PDC between the two studies; in our study, PDC was calculated using the number of days that a patient was persistent to the index DMT, while Agashivala et al. calculated PDC over a specified time interval, regardless of whether the patient was persistent.
The rates of adherence reported for GA and IFN in our study are higher than those reported in previous studies that used an MPR of at least 80% as the definition of adherence (88% for both GA and IFN). Halpern et al.Citation26 reported lower rates of adherence for GA and IFN (52.2–62.3% of patients) and Tan et al.Citation19 reported a combined rate of 59.6%; however, both of these studies required patients to have only one claim for the index DMT (not at least two claims as in our study), and both had substantially longer follow-up periods than in our study (1–3 years and 4 years, respectively). Another 4-year study reported adherence rates of 52.2–62.3% for GA and IFNsCitation27. Similar rates were also reported in a review that included 24 studies of adherence to DMTs, with weighted mean adherence rates for GA and IFNs ranging between 56.8% and 69.4%Citation43. This review also noted that the highest rates of adherence were seen with the DMTs that required least frequent administrationCitation43, indicating that patients may be averse to frequent injections and that DMTs with a convenient mode of administration and dosing schedule may improve patient adherence.
To our knowledge, no other retrospective claims database analyses have compared rates of discontinuation and non-adherence in patients treated with fingolimod and natalizumab. Fingolimod was associated with lower rates of discontinuation and non-adherence than natalizumab during the post-index period in our study. While the reasons for changes in medication use are not recorded in the database, the difference in these rates for fingolimod and natalizumab may reflect the convenience of oral administration or potentially the limitations of long-term natalizumab treatment due to increasing risk of progressive multi-focal leukoencephalopathy (PML) over timeCitation44. In addition, there may be a stronger incentive for patients to stay on fingolimod compared with natalizumab owing to the requirement for cardiovascular monitoring for at least 6 h after the first dose of fingolimod and the need for this to be repeated with any lapse in therapy of over 2 weeksCitation45. Our study has demonstrated using multivariate adjusted analyses that the risk of discontinuation or non-adherence is influenced by age, gender, some clinical characteristics, and several symptoms. It is possible that other factors not accounted for in this study, such as formulary management and out-of-pocket expenses, could also influence persistence with and adherence to DMTs.
Our results for adherence with natalizumab are slightly higher than those reported by Halpern et al.Citation26 in their retrospective claims analysis comparing persistence with, and adherence to, second-line DMTs. Our study found that rates of persistence and adherence with natalizumab were similar to those observed with IFN and GA, whereas Halpern et al. showed that, when used as second-line treatments, rates are significantly improved for natalizumab compared with the injectable DMTs.Citation26 These differences may be explained by subtle differences in the calculation of persistence between the two studies.
This retrospective database analysis has both strengths and limitations relating to the study design. A major strength was that data were derived from a large US administrative health-plan database, which comprises adjudicated claims for more than 60 million active patients across the US, including over 100 000 patients with MS, and is considered to be representative of the US national, commercially insured population. These data provide an excellent resource for assessing treatment patterns and outcomes in a real-world setting.
Most of the limitations of this database analysis are inherent in all retrospective claims database analyses. In particular, results are based on pharmacy and physician outpatient claims and do not provide information on whether medications were used as prescribed. There is the potential for the miscoding of diagnosis codes by clinicians and administrators, and chart review and verification of data were not possible. However, the likelihood of including non-MS patients in this study is minimized owing to the requirement for both a diagnosis of MS and a prescription for/administration of a DMT.
To preserve the sample size of the fingolimod cohort, patients with claims for both fingolimod and another DMT during the index period were preferentially assigned to the fingolimod cohort. However, as most of the preferentially assigned patients would have been excluded from the GA and IFN cohorts owing to not being newly treated with their index DMT or not having pre-index continuous enrollment, this assignment does not significantly affect the size of cohorts for the other DMTs. In addition, there was an absence of clinical measures, such as MS severity and the risk of disease progression, which were not readily available in the claims database. Such end-points would have provided additional detail and insight into treatment patterns reported in this study.
Finally, the analytical focus was on patients who met continuous enrollment criteria (12 months pre- and post-index therapy), which may have excluded patients with different treatment patterns incompatible with enrollment criteria. However, this type of continuous enrollment restriction was necessary to ensure that full details of patients’ treatment were captured in the pre- and post-index periods, and to allow comparison between cohorts.
Conclusions
This real-world analysis demonstrated that patients prescribed fingolimod benefited from significantly improved rates of persistence and adherence over all injectable/infusible DMTs. These improved outcomes are likely to facilitate the core MS treatment goals of preventing relapses, slowing disease progression and postponing disability.
Transparency
Declaration of funding
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Declaration of financial/other relationships
NB, RL, and GC are paid employees of Novartis Pharma AG, Basel, Switzerland. NA and AP are paid employees of Novartis Pharmaceuticals Corporation, East Hanover, NJ. JRK, AAP, SUK, CBM, and CM are paid employees of IMS Health, Plymouth Meeting, PA. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Supplementary Material
Download PDF (25 KB)Acknowledgments
The authors take full responsibility for the content of the paper. The authors thank Dr Gemma Carter and Sebastian Reynolds (Oxford PharmaGenesis, Ltd) for medical writing support, editorial assistance, and collation and incorporation of comments from all authors.
Notes
*Gilenya is a registered trademark of Novartis Pharma AG, Basel, Switzerland.
†Copaxone is a registered trademark of Teva Pharmaceuticals, Petach Tikva, Israel.
‡Avonex is a registered trademark of Biogen Idec, Cambridge, MA, USA.
§Rebif is a registered trademark of Merck Serono SA, Genf, Switzerland.
¶Extavia is a registered trademark of Novartis Pharma AG, Basel, Switzerland.
**Betaseron is a registered trademark of Bayer Healthcare, Leverkusen, Germany.
††Tysabri is a registered trademark of Biogen Idec, Cambridge, MA, USA.
References
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221-31
- Rolak LA. Multiple sclerosis: it's not the disease you thought it was. Clin Med Res 2003;1:57-60
- Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520-32
- National Multiple Sclerosis Society. MS prevalence. http://www.nationalmssociety.org/about-the-society/ms-prevalence/index.aspx. Accessed June 22, 2013
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502-17
- Piaton G, Williams A, Seilhean D, et al. Remyelination in multiple sclerosis. Prog Brain Res 2009;175:453-64
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med 1997;29:101-6
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess 2002;6:1-73
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655-61
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group. Mult Scler 2000;6:255-66
- Derwenskus J. Current disease-modifying treatment of multiple sclerosis. Mt Sinai J Med 2011;78:161-75
- Thone J, Ellrichmann G. Oral available agents in the treatment of relapsing remitting multiple sclerosis: an overview of merits and culprits. Drug Healthc Patient Saf 2013;5:37-47
- FDA. Natalizumab (marketed as Tysabri) Prescribing and Labeling information: latest label approved 20/01/2012. http:///www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf. Accessed March 8, 2012
- EMA. Tysabri summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000603/WC500044686.pdf. Accessed March 8, 2012
- Chun J, Hartung HP. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin Neuropharmacol [Review] 2010;33:91-101
- Flachenecker P, Rieckmann P. Early intervention in multiple sclerosis: better outcomes for patients and society? Drugs 2003;63:1525-33
- Comi G. Shifting the paradigm toward earlier treatment of multiple sclerosis with interferon beta. Clin Ther 2009;31:1142-57
- Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011;28:51-61
- Ivanova JI, Bergman RE, Birnbaum HG, et al. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ 2012;15:601-9
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44-7
- Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 2010;30:89-100
- Oleen-Burkey MA, Dor A, Castelli-Haley J, et al. The relationship between alternative medication possession ratio thresholds and outcomes: evidence from the use of glatiramer acetate. J Med Econ 2011;14:739-47
- Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2011;18:69-77
- Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol 2009;256:568-76
- Halpern R, Agarwal S, Borton L, et al. Adherence and persistence among multiple sclerosis patients after one immunomodulatory therapy failure: retrospective claims analysis. Adv Ther 2011;28:761-75
- Halpern R, Agarwal S, Dembek C, et al. Comparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence 2011;5:73-84
- Portaccio E, Amato MP. Improving compliance with interferon-beta therapy in patients with multiple sclerosis. CNS Drugs 2009;23:453-62
- Wong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci 2011;38:429-33
- Clerico M, Barbero P, Contessa G, et al. Adherence to interferon-beta treatment and results of therapy switching. J Neurol Sci 2007;259:104-8
- Rio J, Porcel J, Tellez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler 2005;11:306-9
- Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler 2012;18:932-46
- Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 2013;13:138
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15
- Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- Collins W, Cohen J, O’Connor P, et al. Long-term safety of oral fingolimod (FTY720) in relapsing multiple sclerosis: integrated analyses of phase 2 and 3 studies. Mult Scler [Abstract] 2010;16:S295
- Ontaneda D, Hara-Cleaver C, Rudick RA, et al. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci 2012;323:167-72
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9
- Roos LL, Stranc L, James RC, et al. Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res 1997;32:229-38; discussion 39–42
- D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993;32:382-7
- Reynolds MW, Stephen R, Seaman C, et al. Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ 2010;13:90-8
- Rich SR, Coleman IC, Cook R, et al. Stepped-care approach to treating MS: a managed care treatment algorithm. J Manag Care Pharm 2004;10:S26-32
- Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013;19:S24-40
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870-80
- FDA. FDA Drug Safety Communication: revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolimod). 2011. http://www.fda.gov/drugs/drugsafety/ucm303192.htm. Accessed June 9, 2014