Abstract
Background:
Hospitalized patients with complicated skin and soft tissue infections (cSSTI) present a substantial economic burden, and resource use can vary according to the presence of comorbidities, choice of antibiotic agent, and the requirement for initial treatment modification. REACH (NCT01293435) was a retrospective, observational study aimed at collecting empirical data on current (year 2010–2011) management strategies of cSSTI in 10 European countries.
Methods:
Patients (n = 1995) were aged ≥18 years, hospitalized with a cSSTI and receiving intravenous antibiotics. Data, collected via electronic Case Report Forms, detailed patient characteristics, medical history, disease characteristics, microbiological diagnosis, disease course and outcomes, treatments before and during hospitalization, and health resource consumption.
Results:
For the analysis population, mean length of hospital stay (including duration of hospitalizations for patients with recurrences) was 18.5 days (median 12.0). Increased length of hospital stay was found for patients with comorbidities vs those without (mean = 19.9; [median = 14.0] days vs 13.3 [median = 8.0] days), for patients with methicillin-resistant Staphylococcus aureus compared with patients with methicillin-sensitive S. aureus (mean = 27.7 [median = 19.5] days vs 18.4 [median = 13.0] days) and for patients requiring surgery (mean = 24.4 [median = 16.0] days vs 15.0 [median = 11.0] days). Patients requiring modification of their initial antibiotic treatment had an associated increase in mean length of hospital stay of 10.9 days (median = 6.5) and additional associated hospital resource use. A multivariate analysis confirmed the association of nosocomial infections, comorbidities, directed treatment, recurrent infections, diabetes, recent surgery, and older age (≥65 years), with longer hospital stay.
Conclusions:
This study provides real-life data on factors that are expected to impact length of hospital stay, to guide clinical decision-making to improve outcomes, and reduce resource use in patients with cSSTI.
Introduction
Complicated skin and soft tissue infections (cSSTI) represent a heterogeneous range of diseases, including abscesses, cellulitis, fasciitis, diabetic foot infections, and post-trauma and surgical site infections, as well as superficial infections or abscesses in an anatomical site where the risk of anaerobic or Gram-negative pathogen involvement is high, such as the rectal areaCitation1–3. When infections involve deep layers of skin and supporting structures, they are associated with considerable morbidity and mortality and represent a major economic problem as they are resource intensive and incur high healthcare costsCitation4. cSSTI are often difficult to manage for a variety of reasons—the presence of comorbidities such as diabetesCitation1, the need for hospitalization and intravenous (IV) antibiotic therapyCitation4, and frequent need for surgical interventionCitation5. Outcomes of treatment are often worse in elderly or immunocompromised patientsCitation1 and may be further impeded by increasing resistance to standard antibiotic therapiesCitation6. The lack of a validated severity assessment tool specific for cSSTI further confounds the selection of appropriate and effective initial therapyCitation3.
Costs of treating cSSTI, particularly those caused by Staphylococcus aureus, can be substantial in hospitalized patients and vary according to the presence of comorbidities, choice of antibiotic agent, and the requirement for modification of initial antibiotic therapyCitation7. In some infections, including ventilator-associated pneumonia, intra-abdominal infections, and bacteraemia, there is clear evidence to show that inappropriate initial antibiotic therapy is associated with a higher risk of unsuccessful outcomes, including a requirement for additional antibiotic therapy, increased length of hospital stay, and deathCitation8. However, in cSSTI the available evidence is not consistent. In a US study, inappropriate initial antibiotic treatment in adults hospitalized with healthcare-associated cSSTI and treated with IV antibiotic therapy was associated with longer length of stayCitation9, whereas a study by Zervos et al.Citation10 did not find an association and concluded that more studies are needed to examine the impact of healthcare-associated cSSTI and inappropriate initial therapy on outcomes.
Data describing resource use in cSSTI in Europe are scarce; however, a recently published retrospective observational medical chart review study of patients with documented methicillin-resistant S. aureus (MRSA) cSSTI who were hospitalized in Europe noted that treatment duration (9.3 ± 6.5 vs 14.6 ± 9.9 days; p < 0.001) and length of hospital stay (19.1 ± 12.9 vs 21.0 ± 18.2 days; p = 0.162) tended to be shorter for patients switched from IV to oral treatment than for patients who received IV treatment onlyCitation11. A recent retrospective analysis in the US examining treatment patterns and costs in hospitalized patients with a diagnosis of cSSTI caused by S. aureus found that the mean length of hospital stay was 6.1 (standard deviation [SD] = 6.0; median = 5.0) days and mean cost per patient was US$6830 (SD = US$7100; 2005 costs)Citation12. In-hospital mortality, switches in antibiotic regimen, and presence of complications or comorbidities were associated with increased length of hospital stay and costsCitation12. Further US studies have found increased costs for the treatment of cSSTI in patients with mixed pathogens compared with those with cSSTI due to single pathogensCitation13, and for patients with treatment failureCitation6.
The selection of initial antibiotic therapy in cSSTI clearly impacts the effectiveness of treatment, but also has economic consequences. The REtrospective Study to Assess the Clinical Management of Patients With Moderate-to-severe cSSTI or CAP Infections in the Hospital Setting (REACH) was a multi-national, multi-center, observational retrospective study conducted in a range of hospitals across Europe (NCT01293435). The study aimed to provide healthcare administrators, researchers, and physicians with current, real-life data on clinical management and resource use in the treatment of community-acquired pneumonia (CAP) and cSSTI. The assessment of clinical management strategies for cSSTI and real-life outcomes has been published in the primary publication for this studyCitation14. We report here findings from an analysis of resource use in cSSTI. The insights derived from these data will become increasingly important to inform management strategies in the current economic climate, where there is considerable pressure to reduce hospitalization costs by selecting the most appropriate treatment regimen for initial treatment. The CAP data are reported separately.
Methods
Patients aged ≥18 years (n = 1995), hospitalized with a cSSTI and receiving IV antibiotic therapy were enrolled from 129 sites in 10 participating countries across Europe between March 2010 and February 2011 (Belgium, France, Germany, Greece, Italy, the Netherlands, Portugal, Spain, Turkey, and the UK). Patients were required to have an infection affecting deeper soft tissue and/or requiring significant surgical intervention, infection developing on a lower limb in subjects with diabetes mellitus or well-documented peripheral vascular disease, a major abscess, infected ulcer, or deep and extensive cellulitis. In addition, the presence of at least two local signs of cSSTI and at least one systemic sign was required. Patients were excluded if they had uncomplicated SSTIs or cSSTI with a high cure rate after surgical incision alone or after aggressive local skin care. Data detailing patient characteristics, medical history, disease characteristics, microbiological diagnosis, disease course and outcomes, treatments before and during hospitalization and health resource consumption were collected via an electronic Case Report Form. Study design, and further patient inclusion and exclusion criteria are described in the primary publication for this studyCitation14.
Initial antibiotic treatment modification was defined as the need for a change in initial antibiotic treatment due to insufficient response, adverse reaction, interaction with other drugs, non-suitability of the initial antibiotic based on the results of microbiological tests, or changes to antibiotic therapy or additions of further agents alone or in combination. Cases of streamlining, defined as change to narrower-spectrum antibiotics upon patient improvement or confirmed microbiological diagnosis, were recorded separately. Cases of patient death while on initial antibiotic treatment were also recorded.
We focus on hospital length of stay for resource use comparisons between sub-groups, as this has been shown to be one of the most important drivers of cost in cSSTI treatment, accounting for as much as 81% in the treatment of MRSA infectionsCitation15.
This was a retrospective non-interventional, observational study, using a descriptive analysis approach to assess clinical management and clinical outcomes. The limited sample size precludes stratified analyses, and no formal statistical comparisons were performed as the study was not designed to test hypotheses. Consequently, comparisons between groups refer to numerical differences rather than to statistically significant differences.
An exploratory multivariate analysis was performed using a backwards-selection procedure. To avoid over-saturation of the starting model, variables of interest with at least a p-value <0.20 from the univariate analysis were considered as candidates in the multivariate model. At each iteration, variables were then required to have a p-value <0.10 to remain in the model, removing one at a time until the final model was selected. For each variable remaining in the final model, an interaction analysis was performed to investigate possible interactions between the significant baseline variable (antibiotics in the 3 months prior to hospitalization and prior to index visit, sex, peripheral vascular disease, congestive heart disease, and cancer/malignancy) and selected post-baseline variables (nosocomial cSSTI, comorbidities, modality of treatment, recurrence of cSSTI, diabetes, surgery in the 3 months prior to index visit and age). Hazard ratios (HRs) were defined as part of the final model (after non-relevant variables were excluded).
Estimation of costs associated with resource use
Hospitalization costs for each of the countries involved were determined using estimated unit cost values for primary and secondary healthcare services derived from the World Health Organization (WHO) CHOosing Interventions that are Cost-Effective (CHOICE) project (http://www.who.int/choice/country/country_specific/en/index.html)Citation16. The values are ‘averages’ of unit costs for the country, based on specific assumptions regarding the organization of health services and operational capacity and are included in order to provide an estimate of the cost implication of increased resource use in the participating countries and to allow for comparison. The rationale for using this information was to provide data that could be compared across the different countries in both Euros and US$. The WHO CHOICE data provided a convenient resource for illustration purposes. These estimates represent only the ‘hotel’ component of hospital costs, i.e. excluding the cost of drugs and diagnostic tests but including costs such as personnel, capital, and food costsCitation16.
Results
Patient population
The analysis population included 1995 patients. A detailed breakdown of information on the study population is provided in the primary publication for this studyCitation14. In brief, patients were recruited from sites in 10 countries: Belgium, France, Germany, Greece, Italy, the Netherlands, Portugal, Spain, Turkey, and the UK. The mean age of patients was 60.6 years; 45.2% patients were ≥65 years old and 57.7% were male. There was a high degree of comorbidity, with 78.0% of patients reporting ≥1 condition, the most frequent being diabetes mellitus and peripheral vascular disease ().
Table 1. Patient demographics, medical history, and disease characteristics.
Hospital stay and resource use
Clinical outcomes data are given in full in the primary publication for this studyCitation14. The mean length of hospital stay (including duration of hospitalizations for patients with recurrences [hospitalizations due to a subsequent cSSTI episode in a patient who had a previous cSSTI diagnosis] and in cases of nosocomial infection, calculated starting on the date of cSSTI diagnosis) was 18.5 days (SD = 19.9; median = 12.0). Furthermore, if admitted to the intensive care unit (ICU), the mean length of hospital stay was much higher (12.8 days [SD = 16.3; median = 5.0] vs 5.8 days [SD = 7.6; median = 3.0]). The overall rate of initial antibiotic treatment modification for the full population of patients hospitalized with cSSTI was 39.6%. Patients with initial antibiotic treatment modification remained in hospital for longer (mean = 25.0 days [SD = 24.0; median = 16.5] vs 14.1 days [SD = 15.2; median = 10.0]) and were more likely to be admitted to the ICU (9.2% vs 4.7%) than patients without initial antibiotic treatment modification ().
Table 2. Hospital stay and resource use analyzed by clinical outcomes, and disease and patient characteristics (all countries).
Resource use for the full analysis set, and by selected disease and patient characteristics at baseline, are shown in . Of the 1995 patients, 6.5% required admission to the ICU for a mean duration of 9.7 days (SD = 13.5; median = 4.5). Surgical intervention was required by 37% of patients. Patients requiring surgical intervention had longer mean hospital stays compared with those without (24.4 days [SD = 26.0; median = 16.0] vs 15.0 days [SD = 14.2; median = 11.0]), and were more likely to be admitted to the ICU.
Patients with comorbidities had longer mean length of hospital stay compared with patients without (19.9 days [SD = 20.4; median = 14.0] vs 13.3 days [SD = 16.9; median = 8.0]) and were more likely to require admission to the ICU, experience septic shock, and require home-based care (). Older patients (≥65 years) also experienced similar mean hospital stay compared with younger patients (<65 years) (19.6 days [SD = 20.6; median = 14.0] vs 17.5 days [SD = 19.2; median = 11.0]) and similar rates of antibiotic treatment modification (40.7% vs 38.8%) ().
Patients experiencing a recurrence of the original infection had longer total mean hospital stays than patients without (35.7 days [SD = 27.5; median = 29.0] vs 16.4 days [SD = 17.5; median = 11.0]). The data also suggest that patients were also more likely to be admitted to the ICU and to require surgical intervention and home-based care after discharge than patients without a recurrent infection (). The need for surgery may be the main driver for increased length of hospital stays in the recurrent patient group.
Patients with confirmed diagnoses of MRSA infection had longer mean hospital stays than patients with methicillin-sensitive S. aureus (MSSA) infection (27.7 days [SD = 23.9; median = 19.5] vs 18.4 days [SD = 19.9; median = 13.0]) and were more likely to be admitted to the ICU. Patients with MRSA also had a high requirement for initial treatment modification compared with patients with MSSA (56.9% vs 41.4%) and a higher mortality rate ().
Patients developing septic shock during treatment had longer mean hospital stays (36.1 days [SD = 44.7; median = 20.5] vs 18.0 days [SD = 18.6; median = 12.0]) were more likely to be admitted to the ICU, had a longer mean length of stay in the ICU, and had a higher requirement for surgery after diagnosis compared with patients without septic shock.
An exploratory multivariate analysis showed that certain baseline characteristics, including nosocomial infections, comorbidities, directed treatment, recurrent infections, diabetes, surgery in the 3 months prior to index visit, and older age (≥65 years), were associated with longer hospital stay (). Strong associations were observed with nosocomial infections (HR: 1.40; p < 0.0001; ), comorbidities (HR: 1.36; p < 0.0001; ), directed treatment (HR: 0.68; p < 0.0001; ), and recurrent infections (HR: 1.16; p = 0.0009; ). All others variables (diabetes, surgery in the 3 months prior to index visit, age group) met the criteria to remain in the final model (p < 0.10), but did not demonstrate a strong association (p > 0.05). Additional variables, such as antibiotic treatment in the 3 months prior to hospitalization, sex, hospitalization in the 3 months prior to index visit, peripheral vascular disease, congestive heart disease, and cancer/malignancy, were also assessed and found not to be associated with length of hospital stay ().
Figure 1. Kaplan–Meier plots showing the association between length of hospital stay and (a) nosocomial infections; (b) comorbidities; (c) modality of antibiotic treatment; and (d) recurrent infections.
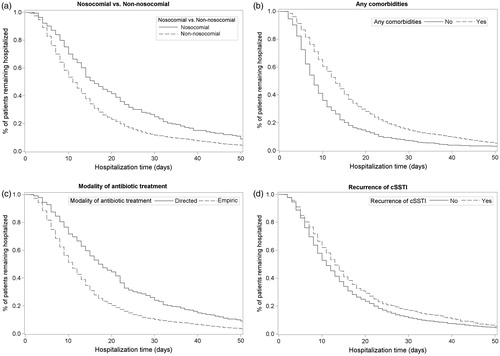
Table 3. Multivariate analysis to identify factors that influence the length of hospital stay in cSSTI.
Some key post-baseline variables (e.g. diabetes, surgery in the 3 months prior to index visit and age group) were expected to have an effect on the length of hospital stay (initial treatment modification and ICU admission) and so any potential interaction with the factors identified from the multivariate analysis were explored. Two significant interactions were observed: initial treatment modification produced an interaction whereby the effect of age on increased length of hospital stay was significantly less pronounced in patients with vs without initial treatment modification (p = 0.0003; data not shown). Similarly, admission to the ICU interacted with the effect of diabetes on length of hospital stay, so that the latter was increased in diabetic patients not admitted to the ICU, whereas it was decreased in diabetic patients admitted to the ICU (p = 0.0132).
Outcomes and resource use across the different participating countries
Patient demographics and characteristics were similar in all participating countries. Outcomes for each of the participating countries are detailed in . Initial antibiotic treatment modification was lowest in the Netherlands, at 26.3%, with Italy having the highest rate, at 47.5%. The total mean length of hospital stay, including hospitalizations for patients with recurrent infections, varied from 11.8 days in the Netherlands to 30.0 days in Germany, where there was additionally the highest percentage of patients admitted to the ICU (22.0%). Belgium also had a high number of patients admitted to the ICU (16.9%), although these patients did not remain there for very long (mean = 6.5 days) compared with Germany and the Netherlands, where patients admitted to the ICU remained for a mean duration of 17.1 and 15.0 days, respectively. The UK had the lowest proportion of patients admitted to the ICU (1.3%) and also had one of the shortest mean times to clinical stability of all the countries, at 5.7 days, with Turkey having the longest, at 16.6 days.
Table 4. Outcome variables and resource use in each participating country.
Comparison of patterns of resource use in the participating countries is shown in . Germany, the Netherlands, and France had notably higher numbers of patients requiring isolation (12.2%, 13.8%, and 10.8%, respectively) compared with other countries. In Germany, 87.8% of patients underwent surgery after cSSTI diagnosis. This is considerably higher than in other countries, where the incidence of surgery ranged from 47.5% in the Netherlands to 23.4% in the UK.
The shorter length of hospital stay in some countries may be balanced with a higher number of patients receiving home-based care once the infection has stabilized. The data suggest this might be the case in the Netherlands and in the UK, where 28.8% and 23.4% of patients, respectively, received home-based care, compared with Germany, where only 14.6% of patients required home-based care.
shows that the estimated cost of the median length of hospitalization for a patient with cSSTI (as determined in REACH for each country) was variable across countries, with the highest estimated costs for Italy and the lowest costs for Turkey, both in secondary-level or tertiary-level hospitals.
Table 5. Unit cost estimates for hospital stay, and estimated cost of mean length of stay in REACH study, in each of the participating countries.
Discussion
Patients hospitalized with cSSTI tended to have hospital stays of almost 3 weeks, and a high requirement (40%) for initial antibiotic treatment modification, suggesting often inadequate initial therapy. This is associated with increased use of hospital resources compared with patients responding to initial antibiotic treatment, as reflected by longer hospital stays (mean difference = 10.9 days) and higher rates of admittance to, and longer stays in, the ICU (mean difference = 7.0 days). Patients with initial antibiotic treatment modification also had increased requirements for surgical intervention, blood pressure support, and isolation, and were more likely to develop septic shock during hospitalization. Initial treatment modification was higher in patients with comorbidities, older patients (≥65 years), patients with recurrent or nosocomial infections, patients with an MRSA infection, and patients with septic shock, suggesting that resource use may be even greater in these groups.
An exploratory multivariate analysis was conducted to identify factors showing a trend towards an increased use of resources in cSSTI. The results showed that, although older age (≥65 years) is a predictor of longer hospital stay, the association between these two parameters is not very strong (p = 0.0924). On the other hand, the presence of comorbidities was found to be a strong predictor of longer hospital stay (p < 0.0001), which may be expected due to the more complex clinical management required. Taken together, these data suggest that the accumulation of comorbidities with age may be more important in determining length of hospital stay than age itself. The observed interactions with post-baseline variables also further demonstrate a trend towards more complicated treatment requirements on the length of hospital stay: when an initial treatment modification was required, the chances of having a lengthy hospital stay were high, even in younger patients.
Interestingly, directed treatment, as opposed to empiric treatment, showed a trend towards longer hospital stay. This might appear surprising; however, a possible explanation for this observation is that patients who had a microbiological diagnosis at the time of treatment initiation may have been a selected population, with a more complicated course of disease.
The increased mean length of hospital stay in patients requiring initial treatment modification observed in REACH was higher than that found in other studies (5.4 daysCitation6; 7 daysCitation16). However, the first of these studies was conducted in the US, where the criteria for hospitalization and discharge of patients with cSSTI may differ from those in Europe. The second study, from Italy, however, was comparable to the increase in hospital length of stay that we found for Italy in the REACH study (mean = 9.7 days, median = 7.0 days)Citation17. The economic burden of initial treatment failure has been reported to be in excess of $1 billion annually (2004) in the USCitation6. Using cost data obtained from the WHO-CHOICE databaseCitation16 (), it is evident that the increased length of hospital stay observed in patients with initial antibiotic treatment modification in our study will have an impact on costs. For example, using the costs for each country shown in , the increased cost in the Netherlands—where bed/day costs are the highest of the countries participating in the REACH study, but increased median length of stay is low (2.0 days), would be US$1877 (€1276) in a tertiary-level/teaching hospital. In France, where the increased median length of stay was 7.5 days and bed/day costs are relatively high, the additional cost would be US$5648 (€3841). In Turkey, where bed/day costs are lowest, the cost incurred by the additional 11.0 days’ hospital stay for initial antibiotic treatment modification would cost US$1606 (Turkish Lira [TL]2090).
We found that patients diagnosed with an MRSA infection required a median extra 6.5 days’ hospitalization compared with patients with an MSSA infection (mean length of stay for patients with MRSA was 27.7 days compared with 18.4 days for patients with MSSA). This is a longer length of stay than that reported in the study by Nathwani et al.Citation11 (mean length of stay of 20.6 [SD = 17.4] days for patients with MRSA), and would result in estimated increased costs in tertiary-level hospitals ranging between US$948.7 (TL1235.0) in Turkey and US$6098.4 (€4147.0) in the Netherlands. Patients with MRSA required less surgical intervention than those with MSSA, but more frequently required isolation (29.4% vs 2.9%), which is likely to impact significantly on the overall cost of treatment. Increased length of hospital stay has previously been observed in patients with mixed-pathogen infections and MRSA infectionsCitation13. The cost of treating cSSTI MRSA infections is reported to be substantial and to vary widely, dependent on initial treatment failure rates, patient comorbidities, and antibiotic therapyCitation7. In previous studies, the mean cost of treating a cSSTI MRSA infection was ∼US$17,000, with the additional cost associated with S. aureus cSSTI estimated at US$3396Citation7. Another study found that mean total costs for treating patients hospitalized with cSSTI ranged from US$6009 to US$7329, depending on treatment givenCitation18. Indeed, one study of the nationally representative hospital database in the US found that patients with S. aureus infections had significantly longer hospital stays (14.3 vs 4.5 days, respectively) incurring 3-times higher costs of treatment (US$48,824 vs US$14,141) compared with hospitalized patients without an S. aureus infectionCitation19.
Patients with obesity, diabetes or a history of MRSA infection are at significantly increased risk for recurrent soft tissue infectionCitation20, and so are likely to require greater use of hospital resources than patients without. REACH identified 676 (33.9%) patients with diabetes and 102 with MRSA infections.
Patients using non-steroidal anti-inflammatory drugs (NSAIDs) were found to have a higher requirement for surgical intervention than patients not receiving these medications, while all other measures of resource use were similar between the two populations. A strong association between the use of NSAIDs and presence of severe necrotizing soft-tissue infection has been noted in previous studiesCitation21. The greater requirement for surgery in patients receiving NSAIDs than in those not, may support this finding. The poorer outcomes and increased resource use seen with elderly patients are as may be expected, since patients in this age group are more likely to have multiple comorbidities that compromise their immunity and increase the likelihood of severe infection.
National studies on treatment strategies for, and economic burden of, cSSTI are non-existent. However, studies comparing the economic impact of one antibiotic treatment with another have been reported for GermanyCitation22 and FranceCitation23. In Germany, costs ranging from €8232–€9206 were reported. A similar study in France reported costs ranging from €7778–€8777, and the mean length of hospital stay was 10.7–13.3 days. In Italy, the full direct hospitalization costs for patients with cSSTI in seven Italian hospitals were reported to be €5530 (2008), and initial antibiotic treatment failure was reported in 12.3% of patients, leading to an increased hospital stay of 7 days, increasing costs by €2850Citation16. Resource use in the different participating countries in REACH varied widely, which may be a reflection of different treatment strategies for cSSTI employed in each country or of the differing economic pressures imposed on hospitals in the individual countries.
This descriptive study is intended to provide a snapshot comparison of the impact of treatment modification on resource use by hospitalized patients with cSSTI. A limitation of REACH is the variation in numbers of patients enrolled in different countries and, in some countries, the number of patients is too low to enable conclusions to be drawn. For example, Germany, Portugal, and the Netherlands enrolled only 41, 55, and 80 patients, respectively. Specific hospitalization costs from each country were not gathered in this study, necessitating use of the WHO-CHOICE cost data, which are not sufficiently specific but do allow comparisons of cost by country. However, such costs may be analyzed in future. In addition, the latest WHO-CHOICE estimates are from 2007 and 2008, and our estimated costs of hospital stay do not allow for inflation since then. Other limitations include possible inter-country differences in treatment strategies for cSSTI. For example, criteria for hospitalization and isolation may differ between countries, along with discharge policies and definitions of what constitutes intensive care. It should also be noted that measuring length of stay has inherent limitationsCitation24,Citation25. Other analyses, describing differences in resource use by type of cSSTI, the impact of differences in country sample size on key variables (such as initial antibiotic treatment), and an investigation of predictors on a country-by-country basis, are not included in this publication.
Successful management of cSSTI involves prompt recognition of infection, timely surgical intervention, and appropriate antibiotic therapyCitation1. In addition, the choice of initial antibiotic therapy may have an impact on patient outcomes and cost. In REACH, it has been demonstrated that treatment of patients with particular characteristics (such as comorbidities, those with a recurrent or nosocomial infection, patients requiring surgical intervention, those taking NSAIDs, or patients with more severe infections), and those requiring initial antibiotic treatment modification has an impact on resource use in hospitals and consequently on resource use of these complicated infections. The results of REACH ratify this conclusion and indicate that adaptations in clinical decision-making are required in order to make improvements in clinical outcomes and reduce the resource use of patients with cSSTI, particularly in certain patient groups.
This study provides data about the use of healthcare resources in cSSTI and particularly about factors associated with increased length of stay, a key determinant of costs. Taken together, they represent both a nucleus and a scaffold for future studies on the economic impact of cSSTI in Europe and worldwide. They should guide clinical decision-making to improve outcomes and reduce costs in cSSTI patients, and prompt further comparative research on patterns of use of healthcare resources between countries.
Transparency
Declaration of funding
This study was supported and funded by AstraZeneca.
Declaration of financial/other relationships
JM and EP are employees of AstraZeneca. HO has received research grants, speaking invitations, and conference invitations from Astellas, AstraZeneca, Gilead, MSD, Pfizer, and TEVA, and consultancy fees from AstraZeneca, Gilead, MSD, and TEVA. FB has received research grants from GSK, Chiesi, Zambon, and Pfizer, congress lecture fees from GSK, Chiesi, Pfizer, and Abbott, and consultancy fees from AstraZeneca, GSK, and Pfizer. KMB has received consultancy fees from Celgene Corporation, AstraZeneca, Worldwide Clinical Trials, Integrium LLC, Cypress Pharmaceuticals, Sigma-Tau Pharmaceuticals, Outcomes Research (now owned by Quintiles), Multiple Myeloma Research Foundation, MedImmune, ACT Oncology, and BioSoteria. JG has received research grants, speaking invitations, and conference invitations from Bayer, GSK, AstraZeneca, Novartis, Vifor Pharma, Pfizer, and Astellas, and has recent or ongoing consultancies with GSK, Bayer, Pfizer, Novartis, Vifor Pharma, Janssen Cilag, AstraZeneca, Astellas, Theravance, and Durata. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors thank Dr Manda Gent from MediTech Media Ltd for medical writing support, funded by AstraZeneca.
References
- Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother 2010;65(3 Suppl):iii35-44
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005;41:1373-406
- Nathwani D. New antibiotics for the management of complicated skin and soft tissue infections: are they any better? Int J Antimicrob Agents 2009;34(1 Suppl):S24-9
- Cainzos M. Review of the guidelines for complicated skin and soft tissue infections and intra-abdominal infections–are they applicable today? Clin Microbiol Infect 2008;14(6 Suppl):9-18
- Merlino JI, Malangoni MA. Complicated skin and soft-tissue infections: diagnostic approach and empiric treatment options. Cleve Clin J Med 2007;74(4 Suppl):S21-8
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol 2008;29:160-9
- Marton JP, Jackel JL, Carson RT, et al. Costs of skin and skin structure infections due to Staphylococcus aureus: an analysis of managed-care claims. Curr Med Res Opin 2008;24:2821-8
- Davey PG, Marwick C. Appropriate vs. inappropriate antimicrobial therapy. Clin Microbiol Infect 2008;14(3 Suppl):15-21
- Lipsky BA, Napolitano LM, Moran GJ, et al. Economic outcomes of inappropriate initial antibiotic treatment for complicated skin and soft tissue infections: a multicenter prospective observational study. Diagn Microbiol Infect Dis 2014;79:266-72
- Zervos MJ, Freeman K, Vo L, et al. Epidemiology and outcomes of complicated skin and soft tissue infections in hospitalized patients. J Clin Microbiol 2012;50:238-45
- Nathwani D, Eckmann C, Lawson W, et al. Pan-European early switch/early discharge opportunities exist for hospitalized patients with methicillin-resistant Staphylococcus aureus complicated skin and soft tissue infections. Clin Microbiol Infect 2014. doi: 10.1111/1469-0691.12632. [Epub ahead of print]
- Menzin J, Marton JP, Meyers JL, et al. Inpatient treatment patterns, outcomes, and costs of skin and skin structure infections because of Staphylococcus aureus. Am J Infect Control 2010;38:44-9
- Itani KM, Merchant S, Lin SJ, et al. Outcomes and management costs in patients hospitalized for skin and skin-structure infections. Am J Infect Control 2011;39:42-9
- Garau J, Ostermann H, Medina J, et al. Current management of patients hospitalized with complicated skin and soft tissue infections across Europe (2010–2011): assessment of clinical practice patterns and real-life effectiveness of antibiotics from the REACH study. Clin Microbiol Infect 2013;19:E377-85
- Goetghebeur M, Landry PA, Han D, et al. Methicillin-resistant Staphylococcus aureus: a public health issue with economic consequences. Can J Infect Dis Med Microbiol 2007;18:27-34
- World Health Organization. CHOosing Interventions that are Cost Effective (WHO-CHOICE). Country-specific unit costs. http://www.who.int/choice/country/country_specific/en/. Accessed April, 2013
- Tarricone R, Aguzzi G, Capone A, et al. How complicated skin and soft tissue infections are treated in Italy: economic evaluation of inpatient intravenous antibiotic treatment in seven hospitals. J Med Econ 2008;11:265-79
- McCollum M, Sorensen SV, Liu LZ. A comparison of costs and hospital length of stay associated with intravenous/oral linezolid or intravenous vancomycin treatment of complicated skin and soft-tissue infections caused by suspected or confirmed methicillin-resistant Staphylococcus aureus in elderly US patients. Clin Ther 2007;29:469-77
- Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 2005;165:1756-61
- Sreeramoju P, Porbandarwalla NS, Arango J, et al. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg 2011;201:216-20
- Souyri C, Olivier P, Grolleau S, et al. French Network of Pharmacovigilance Centres: severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol 2008;33:249-55
- Schurmann D, Sorensen SV, De Cock E, et al. Cost-effectiveness of linezolid versus vancomycin for hospitalised patients with complicated skin and soft-tissue infections in Germany. Eur J Health Econ 2009;10:65-79
- De Cock E, Sorensen S, Levrat F, et al. Cost-effectiveness of linezolid versus vancomycin for hospitalized patients with complicated skin and soft-tissue infections in France. Med Mal Infect 2009;39:330-40
- De Angelis G, Murthy A, Beyermann J, et al. Estimating the impact of healthcare-associated infections on length of stay and costs. Clin Microbiol Infect 2010;16:1729-35
- Shardell M, Harris AD, El-Kamary SS, et al. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis 2007;45:901-7
