Abstract
Objective:
The economic implications from the US Medicare perspective of adopting alternative treatment strategies for acute bacterial skin and skin structure infections (ABSSSIs) are substantial. The objective of this study is to describe a modeling framework that explores the impact of decisions related to both the location of care and switching to different antibiotics at discharge.
Methods:
A discrete event simulation (DES) was developed to model the treatment pathway of each patient through various locations (emergency department [ED], inpatient, and outpatient) and the treatments prescribed (empiric antibiotic, switching to a different antibiotic at discharge, or a second antibiotic). Costs are reported in 2012 USD.
Results:
The mean number of days on antibiotic in a cohort assigned to a full course of vancomycin was 11.2 days, with 64% of the treatment course being administered in the outpatient setting. Mean total costs per patient were $8671, with inpatient care accounting for 58% of the costs accrued. The majority of outpatient costs were associated with parenteral administration rather than drug acquisition or monitoring. Scenarios modifying the treatment pathway to increase the proportion of patients receiving the first dose in the ED, and then managing them in the outpatient setting or prescribing an oral antibiotic at discharge to avoid the cost associated with administering parenteral therapy, therefore have a major impact and lower the typical cost per patient by 11–20%. Since vancomycin is commonly used as empiric therapy in clinical practice, based on these analyses, a shift in treatment practice could result in substantial savings from the Medicare perspective.
Conclusions:
The choice of antibiotic and location of care influence the costs and resource use associated with the management of ABSSSIs. The DES framework presented here can provide insight into the potential economic implications of decisions that modify the treatment pathway.
Introduction
Acute bacterial skin and skin structure infections (ABSSSIs) are a common cause of considerable morbidity in both the community and hospital settings worldwideCitation1–3. They represent one of the most common indications for antibiotic therapy and account for ∼10% of hospital admissions in the USCitation4. The economic burden of ABSSSI treatment is also substantial—in 2010, the cost of treating these infections to US hospitals was >$6 billionCitation5. ABSSSIs are primarily caused by Gram-positive pathogens, with the most predominant one being Staphylococcus aureus (S. aureus), which is often resistant to conventional therapy such as methicillin, with resistance rates as high as 60% observed in many US hospitalsCitation6–8. Infections caused by methicillin resistant S. aureus (MRSA) are associated with increased morbidity, mortality, costs, and resource use compared to infections due to methicillin-susceptible S. aureusCitation9–14.
Outpatient parenteral antimicrobial therapy (OPAT) has been shown to be effective and has become an established part of medical practice in the US for the treatment of ABSSSIs. It is expected that there will continue to be a considerable expansion of ambulatory care services in the coming years, which will allow more patients to avoid hospital admission or reduce their length of stay, since the course of treatment can be completed post-dischargeCitation15,Citation16. This anticipated growth can be attributed to factors such as the need for cost containment, development of antibiotics that can be administered once daily, technological advances in vascular access and infusion devices, as well as the increased acceptance of such therapy by both patients and healthcare providers and the availability of reliable and skilled services in the communityCitation16.
Most prior economic models developed to evaluate the cost implications of treatment strategies for ABSSSIs have made simplifying assumptions and, thus, failed to capture the extensive variations in treatment pathways observed in the real world. The impact of different types of infections, changes to the antibiotic prescribed at discharge from the hospital, as well as decisions related to providing care in the inpatient vs the outpatient setting on cost and resource use was also not explored within their frameworksCitation17–20. Given that real-world treatment strategies have become more multi-faceted and encompass both changes in antibiotics as well as locations of care, it is crucial that a model be flexible enough to capture these nuances in order to analyze their associated costs.
In this study, we describe a discrete event simulation (DES) framework that was developed to support comparisons among alternative treatment pathways. The model has the flexibility to capture the initial empiric antibiotic, switching to a different antibiotic at discharge, a second antibiotic for lack of response or relapse, differential antibiotic course length and route of administration, as well as the various settings in which care can be administered. The analyses presented illustrate using this model to evaluate the potential economic implications from the US Medicare perspective of different treatment strategies for ABSSSIs, including those that involve utilization of OPAT. The impact of decisions related to location of care and switching to different antibiotics was explored.
Methods
Model overview
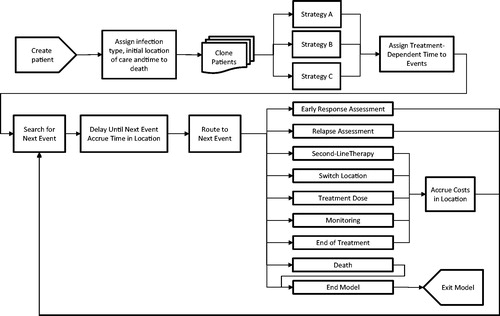
An overview of the model flow is presented in . This individual patient simulation was implemented as a DES using ARENA® software. This approach was chosen as it can accurately capture the real-world complexities associated with the treatment of ABSSSIs, including assignment of empiric antibiotic and any treatment switches. It also allows the time and costs accrued in each location of care to be tracked precisely. This approach allowed the management of patients to be simulated in detail and to capture changes in their care in the emergency department (ED), outpatient and inpatient settings while keeping the model logic transparent. Other commonly used approaches are decision trees or Markov models where patients transition between health states. Whilst the Markov models can also be programmed as individual patient simulations and thereby allow individual characteristics to determine transition probabilities for each patient, this approach requires compartmentalizing the disease into states rather than events. These two alternative approaches have less flexibility to capture treatment switches and changes in location of care as well as the associated outcomes, and cannot capture a patient’s treatment path in as much detail as this DES.
At the start of the simulation, a cohort of individual patients is created and each patient is assigned an infection type (i.e. major cutaneous abscess, cellulitis, traumatic wound infection, or surgical wound infection) and a time to death. The initial location of care (either the ED or inpatient setting) for the patient is assigned, taking into consideration the type of infection. Each patient is then cloned to create two more patients, creating three identical cohorts, in order to conduct comparative analyses between treatment strategies. Cloning patients ensures that the only difference between the simulated cohorts is the assigned strategy.
Each treatment strategy is comprised of empiric therapy, ambulatory continuation therapy (i.e. treatment prescribed to inpatients responding at 72 h to complete the treatment course in the outpatient setting), and an antibiotic prescribed due to failure of empiric therapy at 72 h or to manage a relapse. A single antibiotic or a distribution of antibiotics can be specified for each component of a particular treatment strategy, thus allowing the flexibility to explore the impact of more complex strategies. If initiating treatment in the ED, patients may continue their empiric therapy in either the outpatient or inpatient setting after the first dose.
Treatment-dependent event times (e.g. monitoring test, next treatment dose) are initialized following assignment of a treatment strategy (events shown in ). The model then searches for the event that will occur next for each patient (i.e. the event that has the minimum time) and schedules the event at that time for that patient. When the simulation reaches that time, the relevant costs and health outcomes up to that point are accrued. The patient moves through the model in this fashion. The model captures the patient’s movement through the ED, inpatient, and outpatient locations of care until they die or the model time horizon (30 days) is reached, at which point they exit the model, thus allowing cost and health outcomes to be reported by each location.
Patients can undergo two clinical assessments during their treatment pathway. The early response assessment occurs 72 h from initiation of treatment and is used to determine whether a patient is responding to their empiric therapy. Responders who are currently in the outpatient setting may continue their current therapy there or switch to a different antibiotic (the ambulatory continuation component of their treatment strategy). If currently in the inpatient setting, they can finish their empiric treatment there or switch to an ambulatory continuation therapy (either complete the course with their current antibiotic or switch to a new antibiotic), which will be received in the outpatient setting, thus reducing their hospital length of stay. Non-responders are switched to a different antibiotic.
An assessment for relapse occurs 14 days after initiation of empiric therapy in order to assess clinical cure in patients who were responding at 72 h. Patients who have achieved a clinical cure exit the model after completing any remaining doses. Similar to those who fail the empiric therapy at the early assessment, patients who experience a relapse are switched to a different antibiotic that is assumed to be administered entirely in the inpatient setting, since they represent more complicated cases.
Model inputs and data sources
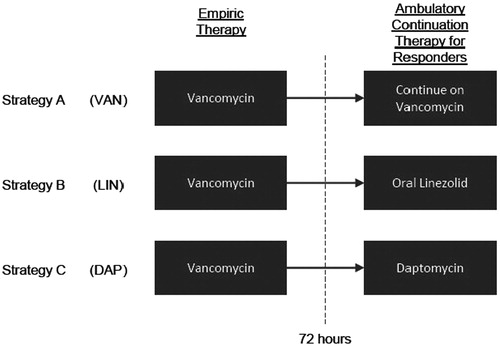
The inputs and their sources are summarized in . Although the framework described above is flexible enough to evaluate many treatment strategies, the analyses presented in this study focused on three specific strategies, as illustrated in . Each treatment strategy is comprised of parenteral vancomycin 1000 mg q12H as empiric therapy and parenteral linezolid 600 mg q12H when patients switch to a second antibiotic for lack of response or relapse. One cohort remained on vancomycin for the entire treatment duration (referred to as the ‘VAN’ cohort). The ambulatory continuation therapy for the other two cohorts was switched to either oral linezolid 600 mg q12H or parenteral daptomycin 325 mg QD if responding to empiric therapy at 72 h (referred to as the ‘LIN’ and ‘DAP’ cohorts, respectively). The following tests were assumed to be required once a week with each of the treatments: vancomycin (renal panel, trough levels), linezolid (complete blood count), and daptomycin (renal panel, hepatic panel, creatine phosphokinase levels).
Table 1. Model inputs and data sources.
Most sources used to inform this model were analyses of real-world US databases. The duration of each antibiotic course is summarized in . The durations for vancomycin as empiric therapy and oral linezolid as ambulatory continuation therapy were obtained from an analysis of a US claims databaseCitation21. The results for the Medicare population (n = 4125) with an index medication of linezolid, vancomycin, or daptomycin were used for this study. The duration assumed for daptomycin as ambulatory continuation was obtained from a literature review citing an analysis of the Cubicin Outcomes Registry and Experience (CORE) program, a retrospective observational study in the USCitation22.
The infection type distributions were obtained by conducting a specific analysis of the Medicare Standard Analytical Files: 41.9% of the patients with ABSSSI present with a major cutaneous abscess, 41.9% with cellulitis, 0.4% with a traumatic wound infection, and 15.8% with a surgical wound infectionCitation23. The analysis included 17,140 patients with an ABSSSI diagnosis between Q4 2009 and Q3 2010.
Inputs such as initial location of care, inpatient length of stay, and mortality risk were derived from an analysis of the Premier hospital database, one of the largest administrative hospital databases in the USCitation5. This analysis examined the characteristics, treatments, costs, and length of hospital stays of 490,368 patients discharged in 2010 with a principal diagnosis of ABSSSI. Overall, 77% of patients with a major cutaneous abscess or cellulitis present at the ED, and 60.4% and 56.3% with a traumatic or surgical wound infection, respectively. All other patients initiated treatment in the inpatient setting. Note that the Premier hospital database does not contain information on the timing of services, and, thus, for this analysis, it was assumed that all patients that present at the ED would receive the initial dose of the antibiotic in the ED as well. The proportion of patients moving to the inpatient setting after the ED was derived based on the Premier analysis and the SOLO I Phase III trial, which was a double-blind, randomized trial that evaluated the safety and efficacy of a single dose of oritavancin vs vancomycin in adults with ABSSSI, including MRSA infectionsCitation5,Citation24.
Clinical response and relapse rates were derived from an analysis of US hospital data (17,786 patients admitted with diagnosis of a cSSSI between 2000 and 2009)Citation25. Equal efficacy between vancomycin, linezolid, and daptomycin was assumed for this model. When patients switched to a second antibiotic for lack of response or relapse, this treatment was assumed to have a 100% response rate.
Costs were assessed from the US Medicare perspective and reported in $US, 2012 values (). Cost components of the ED include visit, drug acquisition, monitoring and laboratory tests, and procedure and doctor fees related to drug administration and insertion of a peripherally inserted central catheter (PICC) (if patient is to be discharged following the first dose and will continue treatment with vancomycin). Per the Medicare reimbursement rules, patients admitted to the inpatient setting accrue the costs associated with their diagnosis-related group (DRG), which depends on their infection type and response to treatment (i.e. patients on a second antibiotic due to lack of response or relapse represent more complicated cases). The average reimbursements for four DRG codes (602 and 603 cellulitis with and without major complication or comorbidity; 862 and 863 post-operative or post-traumatic infections with or without major complication of comorbidity) were obtained from the analysis of the Medicare Public Use Files described above and were inflated to 2012 valuesCitation23. ED costs are also covered under the DRG payment; thus, the same DRG cost will be incurred to all admitted cases regardless of whether the patients had a prior ED visit. OPAT is assumed to take place at a hospital infusion center and administered via a PICC for vancomycin and a peripheral catheter for daptomycin. Cost components of OPAT include drug acquisition, monitoring and laboratory tests, and procedure and doctor fees associated with administration of the drug and the removal of a PICC upon completion of treatment.
Analyses
For each analysis, 500,000 patients were simulated. In addition to the VAN reference case, scenarios were tested to examine the impact of decisions that modify the locations of care along the treatment pathway—inputs related to the initial location of care (ED vs inpatient), subsequent setting of care for those initiating treatment in the ED (inpatient vs outpatient), and proportion of inpatient responders discharged from the hospital at 72 h were varied.
The second set of scenarios focused on the impact of changes to the antibiotic prescribed at discharge for inpatient responders (demonstrated by the LIN and DAP treatment strategies as described above). For each of these strategies, alternative treatment durations, inpatient stay costs, and baseline infection distributions were tested.
An analysis utilizing real-world treatment patterns as observed in a patient journey analysis was also conducted to examine the impact of using a treatment strategy that is a closer representation of real-life practiceCitation26. The patient journey analysis utilized a large US commercial insurer database (∼21.8 million unique members from 2009–2011) to identify the frequently used anti-infective treatment pathways in clinical practice for severe ABSSSI patients moving from inpatient to post-discharge treatment. This treatment strategy was subsequently adjusted to consider a scenario where management practices were modified to allow a greater proportion of responding inpatients at 72 h to switch to oral linezolid at discharge in order to gain insight into the potential implications of this adjustment.
A probabilistic sensitivity analysis (PSA) was conducted for all three treatment strategies. The inputs varied and the assumed distributions are listed in . The mean of each distribution was assumed equal to the reference case and the standard error was assumed to be 20% of the mean. The analysis was run on 10,000 patients for 1000 trials. Given the short time horizon, discounting was not required.
Results
In the VAN cohort, 82% of the patients achieved clinical cure on empiric therapy while 10% achieved cure after switching to a second therapy. The mean total number of days on antibiotic was 11.2 days, with nearly 64% of the treatment course occurring in the outpatient setting (). The estimated average costs per patient were $8671 (). Although only 35% of the treatment course took place in the inpatient setting, inpatient costs accounted for more than half (58%) of total costs, which highlights the high cost burden associated with inpatient treatment. The drug cost per dose of vancomycin is quite low, so the majority of costs in the outpatient setting are associated with the administration of the treatment itself.
Table 2. Mean total number of days on antibiotic and mean costs per patient accrued in each location of care.
Scenario analyses were conducted to test the impact of modifying the location of care pathway for the VAN cohort. illustrates the total costs per patient and proportion of treatment course in the outpatient setting as the subsequent location of care for patients initiating treatment in the ED was varied for the VAN treatment strategy. Total costs per patient decreased as more patients were discharged following the first dose in the ED (i.e. a larger proportion of the treatment course takes place in the outpatient setting). As illustrated, total costs decreased by nearly $1000 per patient when 100% of the patients were discharged following their first dose in the ED compared to 60% in the reference case ($7676 vs $8671).
Figure 3. ED + inpatient and outpatient costs (per patient) and total days on antibiotic in the outpatient setting as a percentage of patients that are admitted following the first dose in the ED is varied [VAN cohort].
![Figure 3. ED + inpatient and outpatient costs (per patient) and total days on antibiotic in the outpatient setting as a percentage of patients that are admitted following the first dose in the ED is varied [VAN cohort].](/cms/asset/b8b1b70d-04c6-4c89-ae32-322d44c142c9/ijme_a_941065_f0003_b.jpg)
Assuming all patients received their first antibiotic dose in the ED decreased the total cost by $268 per patient compared to the reference case, where 74% of patients initiated treatment in the ED. Outpatient and ED costs increased by $228, but these were offset by the $496 reduction in inpatient costs. Conversely, when all patients initiated treatment in the inpatient setting, total costs increased by $663, which is driven by an increase in inpatient costs of $1288 that negates the reduction of $625 in the ED and outpatient costs. Alternative rates of discharge from the inpatient setting for responders at 72 h (as 100% was applied in the reference case) were also tested. Total costs per patient were lower by $1054 and $1576 when 50% and 25% of inpatient responders were discharged at 72 h, respectively. However, total time spent in the inpatient setting increased to 6.1 and 7.2 days with the 50% and 25% discharge rate scenarios, respectively (compared to 3.9 days under the reference case), which increases the burden for hospitals and reduces their capacity to accommodate other patients that are also in need of medical services.
Given the equal efficacy assumption across treatments, health outcomes in the LIN and DAP treatment strategies were identical to the VAN strategy. However, mean total number of days on antibiotic increased by 2.6 [LIN] and 5.1 [DAP] days compared to the VAN strategy. A large proportion of the treatment course, 69% and 71% in LIN and DAP, respectively, occurred in the outpatient setting (). ED and inpatient costs were similar since patients in all three treatment strategies were on vancomycin until 72 h. Mean total costs per patient decreased by $1163 with the LIN strategy, but increased by $929 with the DAP strategy compared to the VAN strategy (). The higher costs for VAN and DAP were primarily due to the required repeat visits to an infusion center for OPAT, while the oral administration of linezolid allowed patients in the LIN strategy to take the medication at home and avoid the infusion-related costs.
presents the total number of days on antibiotic and total costs per patient from scenario analyses. When the lower bounds of treatment durations were tested, total costs decreased by $611 and $1345 in the LIN and VAN cohorts, respectively. Conversely, when the upper bounds were utilized, total costs increased by $311 and $1148 in the DAP and VAN cohorts, respectively. Although oral linezolid had the widest range in treatment duration that was tested in the sensitivity analyses, the LIN strategy resulted in the smallest variation in total costs (−8% with the lower bound and +9% with the upper bound compared to the LIN base case).
Table 3. Mean total number of days on antibiotic and mean costs per patient—results from scenario analyses for VAN, LIN, and DAP.
Total costs are also greatly impacted when the population is comprised solely of wound (traumatic and surgical) infections, resulting in an increase of ∼$2500 per patient in all three treatment strategies. This is primarily due to a greater proportion of patients with wound infections initiating treatment in the inpatient setting and the higher costs associated with wound infections. Using median values of the inpatient DRG costs instead of the mean values had only minimal impact to the results in which total costs on all cohorts were decreased by less than $700.
In a scenario where daptomycin was administered by injection over a 2-min period instead of being administered intravenously via a peripheral catheter over a 30-min period at an outpatient infusion center in the base case, mean total per patient costs of the DAP cohort were decreased to $8635.
Among the treatments being considered in this study, only vancomycin is typically utilized as empiric therapy in US clinical practice. The patient journey analysis concluded that 50% of patients completed the course of vancomycin, while 44% and 6% switched to oral linezolid and daptomycin, respectivelyCitation26. Using this as the basis for a treatment scenario, the model predicted mean total time on antibiotic of 12.6 days (4.2 days in the ED and inpatient, 8.4 days in outpatient) and mean total costs per patient of $8221 ($352 in ED, $5072 in inpatient, $2797 in outpatient). An alternative scenario was tested where more patients switched to oral linezolid at discharge (60%) compared to vancomycin (36%) and daptomycin (4%). Mean time on antibiotic increased by 0.3 days due to additional time in the outpatient setting, given the longer duration of treatment with oral linezolid. However, mean total costs were reduced by $209 per patient, primarily due to a reduction in outpatient costs (savings of $201).
illustrates the results from the PSA for the three cohorts, which are consistent with the findings from the deterministic analyses. The 95% CI for total days on antibiotic was (7.9, 15.2), (10.6, 17.5), and (12.4, 21.1) for the VAN, LIN, and DAP cohorts, respectively, while the 95% CI for total costs was ($7036, $10,737), ($6528, $8779), and ($8209, $11,664) for the VAN, LIN, and DAP cohorts, respectively. Among the three cohorts, LIN had the lowest cost in 88% of the trials.
Discussion
The current model framework improves upon prior models, which typically do not account for treatment in the outpatient setting or assignment of a different antibiotic at discharge from the hospitalCitation17–20. This model is also capable of capturing costs and time spent in each location of care, which allows for a comparison of alternative antibiotic treatment strategies and the pathways through the various locations in which treatment can be administered. Furthermore, the framework is flexible enough to model complex real-world treatment patterns and simulate the potential implications of modifying clinical practice. The type of infection is also explicitly modeled, which allows the additional cost and resource use associated with wound infections to be accounted for, which is important since these can represent anywhere from 16–20% of ABSSSIsCitation5,Citation23.
In addition to the strengths associated with the model framework, many of the data sources that have been utilized ensure the inputs reflect current management practices. Real-world databases were analyzed to inform the inputs related to initial location of care, infection type, inpatient DRG costs, treatment durations, inpatient length of stay, and utilization of specific antibiotics in the treatment pathway. Vancomycin remains the most frequently used empiric therapy for inpatient management of ABSSSICitation5. Linezolid and daptomycin are also recommended for MRSA and are typically used as ambulatory continuation therapy following initial treatment with vancomycinCitation27. Although other antibiotics with MRSA coverage can be used in clinical practice, these were not considered in this analysis, which focused on the potential impact of more specific management decisions. The analyses presented in this study demonstrate that the majority of the costs accrued are associated with inpatient care, although a greater proportion of the treatment course is administered in the outpatient setting for patients that are managed with a full course of vancomycin therapy. The principal component of outpatient costs is related to intravenous (IV) administration of vancomycin. Modifying the location of care in which the patient is treated can have a major impact on costs. Initiating treatment in the ED vs the inpatient setting leads to cost savings, since a larger proportion of patients avoid the inpatient stay and the associated costs. Following the first dose in the ED, total costs decrease as the proportion discharged to complete their course in the outpatient setting increases. When appropriate, the entire management pathway may take place in the ambulatory setting, and a recent study has shown that comparable clinical outcomes can be achievedCitation24.
Decisions related to switching antibiotics for patients at the early response assessment also have an impact on costs. If responding inpatients are discharged, total costs are lowest if a switch is made to an oral antibiotic (such as linezolid) rather than parenteral therapy, since the patient does not require frequent visits to an outpatient infusion center to complete their course of treatment. Furthermore, the impact of prescribing variable treatment durations on total costs is reduced with oral medications because parenteral therapies also accrue the costs associated with administration. Given the widespread use of VAN as empiric therapy (54% according to the PREMIER analysis)Citation5, a shift in management practices to increase utilization of an oral antibiotic as ambulatory continuation therapy will lower costs substantially. For example, projecting the patient numbers from the PREMIER analysis to a national level, ∼292,500 Medicare patientsCitation5 were hospitalized with an ABSSSI in the US in 2010. Increasing the number of patients that are switched to oral linezolid at discharge, per the real-world treatment patterns scenario presented in this study, could result in savings from the Medicare perspective.
The presented analyses have some limitations. Response and cure rates were assumed to be equal across all locations of care, although it is possible that there may be differential selection of the patients who are suitable candidates for outpatient care. Furthermore, resistance patterns or compliance may differ in the outpatient setting, which may impact outcomes. For example, a US-based retrospective claims analysis showed that nearly 25% of Medicare Advantage Prescription Drug patients prescribed oral linezolid at discharge did not fill the prescription due to the high out-of-pocket expensesCitation28. These patients had higher medical costs (excluding drug costs) by nearly $4000 (per patient) in the 60 days following discharge compared to those that filled the prescription. This illustrates the impact of changes in the setting of care on the out-of-pocket costs accrued by the patient and the potential for this to influence subsequent clinical outcomes.
Even though there may be cost savings from the Medicare perspective if an inpatient remains in the hospital rather than being discharged (i.e. DRG cost is applied once the patient is admitted), this model does not capture the potential health risks such as nosocomial infections and subsequent cost implications by extending inpatient length of stayCitation29,Citation30. From the overall healthcare resource allocation viewpoint, reducing inpatient length of stay increases the capacity of the hospital to accommodate other patients. Furthermore, inpatient DRGs from the Medicare perspective do not vary according to the treatment that is used by the patient; rather, these are based on the severity of the infection (a complication such as lack of response or relapse results in a higher DRG cost). The costs associated with the specific treatment would be of importance to a provider, but are not captured under the current perspective. Furthermore, given that the patent for linezolid will expire in 2015, the potential cost savings will be even greater once the drug becomes generic.
Furthermore, in these analyses, daptomycin was assumed to be administered intravenously via a peripheral catheter over a 30-min period at an outpatient infusion center, while an injection over a 2-min period could be another potential mode of administration. The results from scenario analysis indicated a decrease in a mean total per patient costs ($8635 vs $9600) when a 2-min period injection of daptomycin was assumed. The lower costs in this scenario, relative to the DAP base case, are primarily driven by the lower procedure costs associated with the injection compared to the infusion. Given the lack of data to determine the mode of administration utilized in real-world clinical practice for daptomycin, the assumption in this study is conservative. The cost associated with a physician’s time for conducting the cure assessment at 14 days in the outpatient setting was also not considered in this study. This simulation could be enhanced by broadening the perspective to consider the cost shifting between payers, providers and patients; however, appropriate data sources with this level of detail would need to be identified.
Despite these limitations, the model framework presented here is able to capture many of the complexities associated with the treatment of ABSSSIs, which helps provide insight into the consequences associated with adopting alternative treatment strategies or modifying a patient’s journey through the various locations of care.
Conclusions
The choice of antibiotic and location of care in the management of ABSSSIs have a substantial impact on resource use and costs. A significant proportion of the antibiotic treatment course is managed in the outpatient setting; however, costs are reduced when visits to a hospital infusion center are not needed. The health economic implications of new treatment options currently in development such as long-acting lipoglycopeptide IV antibiotics (e.g. oritavancin and dalbavancin), which avoid repeat infusions and are anticipated to allow providers to shift more care to the ambulatory setting, can be assessed using a modeling framework such as the one presented here.
Transparency
Declaration of funding
Funding for this project was provided by The Medicines Company.
Declaration of financial/other relationships
Nikhil Revankar, Alexandra J. Ward, Christopher G. Pelligra, and Thitima Kongnakorn are employees of Evidera, who were paid consultants to The Medicines Company in connection with the development of the manuscript at the time of study conduct. Weihong Fan and Kenneth LaPensee are employees of The Medicines Company. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Dykhuizen RS, Trent RJ, Pacitti DP, et al. An analysis of 900 consecutive admissions to a regional infection unit. J Infect 1994;29:189-93
- Nathwani D, Moitra S, Dunbar J, et al. Skin and soft tissue infections: development of a collaborative management plan between community and hospital care. Int J Clin Pract 1998;52:456-60
- Tice AD, Poretz D, Cook F, et al. Medicare coverage of outpatient ambulatory intravenous antibiotic therapy: a program that pays for itself. Clin Infect Dis 1998;27:1415-21
- Centers for Disease Control and Prevention. Soft tissue infections among injection drug users—San Francisco, California, 1996–2000. MMWR 2001;50:381-4
- LaPensee K, Fan W. Economic burden of hospitalization with antibiotic treatment for ABSSSI in the US: an analysis of the Premier Hospital Database. Poster code: PIN222012. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 17th Annual International Meeting. Washington, DC; 2012
- Frazee BW, Lynn J, Charlebois ED, et al. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med 2005;45:311-20
- Lee SY, Kuti JL, Nicolau DP. Antimicrobial management of complicated skin and skin structure infections in the era of emerging resistance. Surg Infect (Larchmt) 2005;6:283-95
- Swartz MN. Hospital-acquired infections: diseases with increasingly limited therapies. Proc Natl Acad Sci U S A 1994;91:2420-7
- Abramson MA, Sexton DJ. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect Control Hosp Epidemiol 1999;20:408-11
- Cosgrove SE, Qi Y, Kaye KS, et al. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 2005;26:166-74
- Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003;36:53-9
- Lodise TP, Jr McKinnon PS. Burden of methicillin-resistant Staphylococcus aureus: focus on clinical and economic outcomes. Pharmacotherapy 2007;27:1001-12
- Reed SD, Friedman JY, Engemann JJ, et al. Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2005;26:175-83
- Shurland S, Zhan M, Bradham DD, et al. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol 2007;28:273-9
- Chapman AL, Seaton RA, Cooper MA, et al. Good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults in the UK: a consensus statement. J Antimicrob Chemother 2012;67:1053-62
- Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004;38:1651-72
- Bounthavong M, Zargarzadeh A, Hsu DI, et al. Cost-effectiveness analysis of linezolid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus: complicated skin and skin structure infection using Bayesian methods for evidence synthesis. Value Health 2011;14:631-9
- De Cock E, Sorensen S, Levrat F, et al. Cost-effectiveness of linezolid versus vancomycin for hospitalized patients with complicated skin and soft-tissue infections in France. Med Mal Infect 2009;39:330-40
- Shah NP, Reddy P, Paladino JA, et al. Direct medical costs associated with using vancomycin in methicillin-resistant Staphylococcus aureus infections: an economic model. Curr Med Res Opin 2004;20:779-90
- Stephens JM, Gao X, Patel DA, et al. Economic burden of inpatient and outpatient antibiotic treatment for methicillin-resistant Staphylococcus aureus complicated skin and soft-tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res 2013;5:447-57
- Fan W, Mao J, Iorga S, et al. Care pathway and health care cost for acute bacterial skin and skin structure infection subjects with antibiotic treatment in the US: analysis of a real world database. Poster code: PRM742013. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 18th Annual International Meeting. New Orleans, LA; 2013
- White B, Seaton RA. Complicated skin and soft tissue infections: literature review of evidence for and experience with daptomycin. Infect Drug Resist 2011;4:115-27
- Medicare Standard Analytical Files – 5% Sample. Baltimore, MD: Centers for Medicare and Medicaid Services, 2009–2010. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/IdentifiableDataFiles/StandardAnalyticalFiles.html. Accessed, 2013
- LaPensee K, Lodise TP, Fan W, et al. Comparison of efficacy and adverse events between oritavancin versus vancomycin among patients treated in the ambulatory setting in SOLO I. Poster Code: 2303. 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Denver, CO; 2013
- Berger A, Oster G, Edelsberg J, et al. Failure of initial antibiotic therapy in complicated skin and skin structure infections (cSSSI) in US hospitals, 2000–2009. Infectious Diseases Society of America 48th Annual Meeting. Vancouver, Canada; 2010
- Fan W, LaPensee K, Mao J, et al. Direct medical costs and healthcare resource utilization associated with selected antibiotic treatment pathways in acute bacterial skin and skin structure infections in US. International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 16th Annual European Congress. Dublin, Ireland; 2013
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011;52:e18-55 (Erratum in: Clin Infect Dis 2011;53:319)
- Ball AT, Xu Y, Sanchez RJ, et al. Nonadherence to oral linezolid after hospitalization: a retrospective claims analysis of the incidence and consequence of claim reversals. Clin Ther 2010;32:2246-55
- Klevens RM, Edwards JR, Richards J, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160-6
- Roberts RR, Scott RD 2nd, Hota B, et al. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care 2010;48:1026-35
- Physicians’ Desk Reference® PDR-3D: Digital Drug Database. Montvale, NJ: PDR Network LLC, 2013. http://www.pdr3d.com/. Accessed 2013
- Centers for Medicare & Medicaid Services (CMS). Clinical Laboratory Fee Schedule. 2012. Baltimore, MD: Centers for Medicare & Medicaid Services, 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed 2012
- American Academy of Pediatrics Committee on Infectious Diseases. Red Book® Online. Elk Grove IL: American Academy of Pediatrics, 2012. Available at: http://aapredbook.aappublications.org/ [Last accessed May 30, 2014]
- Centers for Medicare & Medicaid Services (CMS). Physician Fee Schedule National Payment Amount File. Baltimore, MD: Centers for Medicare & Medicaid Services, 2012. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-National-Payment-Amount-File.html. Accessed 2012
- Centers for Medicare & Medicaid Services (CMS). Hospital Outpatient Prospective Payment System. Baltimore, MD: Centers for Medicare & Medicaid Services, 2013. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/index.html. Accessed 2013