Abstract
Background:
Traditional pathology techniques alone can be insufficient to reliably distinguish between malignant melanoma, dysplastic nevi, and benign nevi in biopsies of suspicious pigmented lesions. Numerous studies have shown high rates of ambiguity when assessing such samples. A novel gene expression assay has been developed to objectively differentiate malignant melanoma from benign nevi.
Objective:
The purpose of this study was to quantify the economic impact of the gene expression assay on a US commercial health plan.
Methods:
The clinical paradigm of care was modeled for a hypothetical cohort of patients with suspicious pigmented lesions that are difficult-to-diagnose. Costs were assigned to each unit of care provided based on 2013 Medicare fee-for-service rates. Patients were followed for 10 years and were modeled to progress according to the natural history of their disease. The total cost of care was calculated for two scenarios: a Reference Scenario, representing current clinical practice, and a Test Scenario, in which each lesion was tested with the gene expression assay and diagnosed. Total cost of care was compared between the two scenarios to determine overall budget impact. Sensitivity analyses were performed to test the robustness of the model.
Results:
The gene expression assay reduces costs by $1268 per patient tested over 10 years, a decrease of 8.3%, after accounting for the cost of the assay. For a health plan with 10 million members, this would translate to over $8 million in savings. The largest portion of this saving comes from reducing the number of missed melanomas, which would otherwise progress to advanced disease. In sensitivity analyses, no single model input changed within a reasonable range of values caused the model to show that the assay was not cost-saving.
Conclusion:
In addition to improving the diagnosis of melanoma, this gene expression assay would likely reduce costs for health plans that choose to cover it.
Introduction
Melanoma affects over 76,000 people per year in the US and nearly 10,000 will die of their diseaseCitation1. Early detection is crucial; over 98% of patients with localized melanoma survive 5 years after their diagnosis, compared to only 16% of patients with metastatic diseaseCitation2. Although the treatment of metastatic melanoma has improved significantly in recent years due to the approval of several novel therapies, the majority of patients with metastatic disease still typically progress within 10 monthsCitation3–5.
Early detection of melanoma relies on patients or physicians identifying a pigmented lesion that displays worrisome characteristicsCitation6. Once identified, these ‘suspicious pigmented lesions’ (SPLs) are biopsied and sent to a pathologist for analysis. Approximately 2 million SPL biopsies occur per year in the US, less than 5% of which are actually diagnosed as melanomaCitation7,Citation8. Although this illustrates the frequency of potentially unnecessary biopsies of non-malignant lesions, the more troubling aspect of melanoma diagnosis is the uncertainty pathologists often encounter when attempting to distinguish melanoma from non-melanoma.
Numerous studies have illustrated that pathologists arrive at different diagnoses for the same samples in a small percentage of cases. For example, on 478 consecutive cases referred to the Massachusetts General Hospital Pigmented Lesion Clinic, expert pathologists documented a change from the original pathologist’s diagnosis in 35% of cases, nearly two-fifths of which led to a change in recommended treatmentCitation9. Another study showed that, in 143 difficult-to-diagnose cases, two Columbia University pathologists agreed only 55% of the time; in 36% of cases, one pathologist called the lesion definite or probable melanoma, while the other called it definite or probable benign nevusCitation10. Piepkorn et al.Citation11 showed that concordance was only 56% between six dermatopathologists, while Farmer et al.Citation12 showed that 38% of tested samples had at least two discordant interpretations. Alarmingly, Brochez et al.Citation13 found that pathologists on average missed 13% of melanomas, and 25% of samples diagnosed as melanomas were actually false-positives. Numerous other studies show similar levels of discordanceCitation7,Citation14–20. Although certain factors pre-dispose a sample to misdiagnosis—such as originating from a punch biopsy or being a dysplastic nevus or a Spitz nevus—misdiagnosis is found across all lesion typesCitation13,Citation14,Citation16,Citation18.
The over-diagnosis of melanoma is concerning because it not only causes patients to undergo unnecessary procedures and years of close clinical follow-up, but can also cause significant psychological and emotional stress for patientsCitation21–23. Missed melanomas, on the other hand, can be clinically and financially devastating because untreated melanomas are likely to progress to more advanced—and potentially incurable—disease. In both cases, physicians risk litigation; one notable analysis found that misdiagnosis of melanoma was the second-most common cause of pathology malpractice claimsCitation24.
It is clear from this evidence that pathologists would benefit from improved tools to clearly distinguish melanoma from non-melanoma. Many pathologists commonly use immunohistochemical stains for markers such as Ki-67 to attempt to make a more informed diagnosis, but misdiagnosis persistsCitation25–27. Some pathologists have begun using various types of genetic investigations such as fluorescence in situ hybridization (FISH)Citation28–31 or array comparative genomic hybridization (aCGH)Citation32. However, these assays are largely investigational and require time and particular expertise to develop, perform, and interpretCitation33–35. Therefore, pathologists still have an unmet need for an objective melanoma diagnostic that is broadly accessible and provides a clear, actionable result.
Myriad myPath™ Melanoma is a gene expression assay that was developed to address this unmet need. Using formalin-fixed, paraffin-embedded (FFPE) SPL biopsy tissue as a sample, this real time PCR-based assay measures the expression levels of 23 genes from various independent biological pathways and combines it into a diagnostic ‘score’ that is used to distinguish melanoma from non-melanomaCitation36. The assay was developed on a training cohort of 595 samples and validated on an independent cohort of 571 samples from four leading US institutionsCitation36. In the validation cohort, the assay was able to identify melanoma with 90% sensitivity and 91% specificityCitation36. In addition, early data on the assay’s clinical utility suggests that it improves concordance between pathologists and causes pathologists to change their treatment recommendations in some casesCitation37.
However, in order to impact patient care in real-world clinical practice, the test must be covered by healthcare payers such as commercial insurance companies and government health plans. These payers are increasingly concerned with the cost of molecular tests due to the growing number of high-cost molecular diagnostic and prognostic tests currently being developed and launchedCitation38,Citation39. In particular, the economic impact of this melanoma diagnostic test has not yet been evaluated.
The objective of this study was to model the economic impact of Myriad myPath Melanoma (‘the assay’) on a hypothetical US commercial payer.
Methods
Model design and modeled population
A deterministic, decision-analytic model was developed to project the cost of using the gene expression assay compared to standard clinicopathologic evaluation. The assay is intended for use in ambiguous, difficult-to-diagnose SPL biopsy samples. Accordingly, the model included only patients with difficult-to-diagnose SPLs. The model followed a single cohort of patients, each with a difficult-to-diagnose SPL biopsy in the first year of the model. The clinical care given to these patients was modeled over 10 years, including natural progression to more advanced stages of melanoma as appropriate. Costs were assigned to each unit of care according to the reimbursement rates paid by a typical US commercial health plan. The clinical paradigm and total costs were calculated for two parallel scenarios: the Reference Scenario, designed to reflect current clinical practice in the absence of the assay, and the Test Scenario, in which all of the patients were assumed to receive the assay in the first year of the model and the subsequent clinical paradigm was adjusted accordingly. Costs were compared between the Reference Scenario and the Test Scenario in order to determine the assay’s economic impact.
Within the modeled population, patients were followed in separate cohorts according to their diagnosis: conventional nevus, dysplastic/atypical nevus, or malignant melanoma. The distribution of samples in the Reference Scenario was determined by combining the current distribution of all SPLs among these three cohorts with the varying rates of ambiguity in each (Supplemental Table 1)Citation7,Citation9–13,Citation17–20. Within the dysplastic cohort, mild, moderate, and severe dysplasia were modeled separately due to differences in recommended treatment for eachCitation40,Citation41. Although severe dysplasia is more rare than mild/moderate dysplasia among all SPLs, it accounts for a larger portion of the modeled population due to its significantly higher rate of ambiguityCitation13,Citation18. Within the malignant melanoma cohort, localized, regional, and distant melanoma were modeled separately due to their significantly different treatment and prognosis. The majority of melanoma diagnoses in the model were of localized disease, since there is significantly less ambiguity in diagnosis of regional or distant melanoma.
Cost inputs
displays the unit costs employed in the model. Unless otherwise specified, costs were based on 2013 Medicare fee-for-service ratesCitation42. To determine which Current Procedural Terminology (CPT) codes were most commonly used in real-world practice, interviews were conducted with professional coders in the fields of dermatology, dermatopathology, and surgical oncology. CPT codes were then mapped to national payment rates using 2013 Medicare fee schedules. For each code, the total Medicare reimbursement (combining both professional fees and facility fees, as appropriate) was calculated for four separate Place of Service settings: physician office, ambulatory surgical center, hospital outpatient, and hospital inpatient. The payment amounts for each setting were then combined in a weighted average according to the number of times the relevant CPT code was billed to Medicare from each of the four settings in 2012. This data was sourced from the 2012 Physician/Supplier Procedure Summary (PSPS) database, which contains data on all fee-for-service claims billed to Medicare Part B.
Table 1. Unit costs.
For certain types of care, a variety of CPT codes are used to describe similar services; in these cases, a single payment amount was calculated by performing a weighted average of the different codes according to their real-world billing frequency using the PSPS database. Finally, based on interviews with several commercial health plans, all Medicare payment rates were inflated by 25% to better reflect the rates paid by commercial insurers (with the exception of payment rates for pharmaceuticals).
If Medicare costs were unavailable for a certain type of care, its cost was determined from an alternative source. The cost of aCGH ($1650) was sourced from commercial laboratories’ advertised pricesCitation43,Citation44. The cost of the gene expression assay ($1500) was sourced from the assay manufacturer. Costs for oral pharmaceuticals were sourced from published Wholesaler Acquisition Cost (WAC) pricesCitation45.
For pharmaceuticals, the cost per milligram was translated into the cost of a full course of treatment according to the dosing schedule specified in the drug’s FDA label, clinical guidelines, or the most relevant clinical trialsCitation46–53. For dosing schedules dependent on body weight or surface area, an average body weight of 70 kg and body surface area of 1.73 m2 were used.
Clinical paradigm
In order to calculate the total cost incurred by a payer in the Reference Scenario, a clinical paradigm was mapped that specified exactly what units of care were received by each patient. This paradigm was determined using clinical guidelines, clinical literature, and interviews with expert physicians. Fourteen physician interviews were conducted, comprising four dermatologists, eight dermatopathologists, and two surgical oncologists. Within each specialty, interviewees were geographically varied and from a mix of community and academic practices. The clinical paradigm in the Reference Scenario, along with associated costs, is displayed in .
Figure 1. Clinical paradigm and per-patient costs. Percentages indicate the proportion of patients within each diagnosis who receive the unit of care. Dollar values indicate the average per-patient cost of all the listed units of care in each cell. Costs in column (A) are incurred in the first year of the model only. Costs in column (B) are incurred only in the year the patient receives the diagnosis. Costs in column (C) are incurred in every year after the diagnosis, as long as the patient does not progress to more advanced disease. Where WLE, SLNB, or CLND are indicated, the cost of associated pathology services was included.
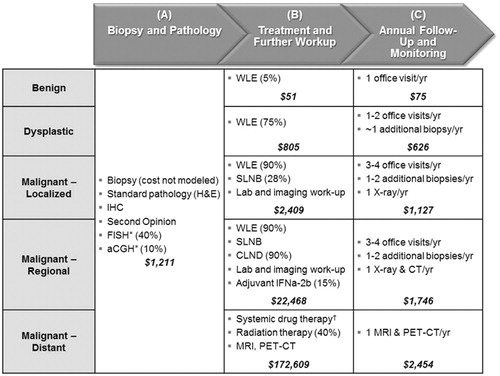
The clinical paradigm and costs were modeled according to each patient’s diagnosis and were separated into three distinct categories: diagnostic pathology costs, to be incurred in the first year of the model (), initial treatment costs, to be incurred in the year that the patient is diagnosed (), and follow-up and monitoring costs, to be incurred each year after the patient is diagnosed ().
Diagnostic pathology costs did not vary by diagnosis, since they are incurred before a diagnosis is made. All patients were assumed to receive the ‘standard pathology’ evaluation (i.e., H&E stain and visual sample examination). Within the modeled population (which consists only of difficult-to-diagnose samples), all samples received immunohistochemistry and a second opinion. In addition, half received some form of advanced molecular testing in the Reference Scenario (40% FISH, 10% aCGH). In the Test Scenario, the gene expression assay replaced any use of FISH and aCGH.
Initial treatment costs varied significantly by diagnosis. Few benign lesions receive wide local excision (WLE), although most dysplastic and malignant lesions doCitation40,Citation54,Citation55. In the model, a high percentage of dysplastic lesions received WLE because over 75% of the modeled dysplastic population is composed of lesions with severe dysplasia due to their greater ambiguity (Supplemental Table 1). Not all malignant lesions receive WLE, because some are sufficiently excised with the original biopsyCitation54.
After WLE, patients with Breslow thickness >1 mm are recommended to receive a sentinel lymph node biopsy (SLNB) to test for regional diseaseCitation46,Citation55–57. Approximately 60–70% of melanoma lesions have a depth of ≤1 mm, and therefore do not get a SLNBCitation54,Citation58–60. Of those who do receive a SLNB, 15–25% have detectable disease in the sentinel node and are, therefore, upstaged to regional diseaseCitation61,Citation62. Patients with regional disease receive a complete lymph node dissection (CLND)Citation54,Citation58, and a minority (∼15%) also receive adjuvant therapy with high-dose interferon alpha-2bCitation63,Citation64.
Finally, all patients who progress to metastatic melanoma were assumed to receive systemic drug therapy, while ∼40% receive radiation therapy as wellCitation64. The cost of systemic drug therapy was calculated using a hypothetical sequential drug regimen based on clinical guidelines and physician expert opinionCitation46,Citation65. For each drug in the regimen, the cost of a full course of therapy was pro-rated according to the drug’s published progression-free survival. These pro-rated costs were summed to determine the overall cost of systemic drug therapy (see ).
Follow-up and Monitoring protocols were based on published literature and guidelinesCitation46,Citation64,Citation66. Only incremental costs directly attributable to the original SPL were modeled. Patients with dysplastic or malignant lesions received incrementally more SPL biopsies in subsequent years since their original lesion would cause them to be considered higher-risk. These subsequent biopsies of unrelated lesions were all assumed to be unambiguously benign, and therefore incurred no costs beyond the biopsy itself and initial pathology. Patients with melanoma received annual imaging exams, increasing from X-Rays for localized melanoma to CT or PET/CT for regional or distant melanoma.
Disease progression
Over the 10-year duration of the model, patients were shifted between diagnosis categories according to the natural progression rate of their disease. For benign and dysplastic lesions, no lesions progressed to melanoma except ‘False Negative’ lesions (i.e., missed melanomas). The percentage of false negative lesions that progressed to melanoma varied according to the diagnosis the patient actually received, since a missed melanoma can be cured by WLE (Supplemental Figure 1B)Citation17,Citation67. Therefore, false negatives had a greater chance of progressing to melanoma if they were initially diagnosed as benign (which do not typically receive WLE) vs dysplastic (which commonly receive further excision)Citation14. All disease progression was modeled according to the rate specified by published Kaplan-Meier curves for similar diseaseCitation67,Citation68. When missed melanomas that progressed were eventually detected, they were split between progression to localized disease (20%), regional disease (50%), or metastatic disease (30%)Citation69,Citation70.
For patients with melanoma, progression to later stages of disease was based on published survival statistics (Supplemental Figure 1A)Citation4,Citation5,Citation67–69.
Test impact
In the Test Scenario, modeled samples were tested with the gene expression assay. To determine its impact on patients’ diagnoses, the assay’s sensitivity (90%) and specificity (91%) were applied to the modeled population so that it correctly identified 90% of Condition Positives (i.e., true positives + false negatives) and 91% of Condition Negatives (i.e., true negative + false positives). The rates of misdiagnosis (i.e., false positives and false negatives) in the Reference Scenario were calculated by dividing the overall misdiagnosis rate in the whole SPL population by the percentage of samples that are ambiguous (and therefore modeled) ().
Table 2. False result rate.
Cost savings and sensitivity analysis
To determine the economic impact of the assay on a hypothetical commercial payer, total and per-patient costs were compared between the Test Scenario and the Reference Scenario. One-way sensitivity analyses were conducted for each major assumption to test the model’s robustness. For this analysis, the assay’s cost impact was re-assessed after changing each assumption to its minimum and maximum plausible value.
Results
Impact on diagnostic accuracy
The net effect of the assay was to increase diagnostic accuracy from 87% of tested samples to 91% (). More specifically, the assay drastically reduced the rate of missed melanomas in the modeled population from 11% to 2%, while increasing the false positive rate from 3% to 8%.
Table 3. Assay impact on diagnostic accuracy in modeled population.
The cumulative 10-year cost of managing patients with inaccurate diagnoses is much higher than the cost if they had been diagnosed accurately (). Benign lesions cost less than $2800 over 10 years if they are diagnosed accurately, but cost ∼$14,600 if they are misdiagnosed as melanoma. Melanomas cost, on average, ∼$43,000 over 10 years if diagnosed accurately, but cost anywhere from $58,000 to $104,000 if they are misdiagnosed as benign or dysplastic, respectively, due to progression to more advanced disease. On the whole, the assay re-allocates patients from the more expensive missed melanoma categories to less expensive categories.
Overall savings
The cumulative 10-year cost per patient in the Reference Scenario was $15,329. In the Test Scenario, the cumulative 10-year cost per patient (including the assay) was $14,061, generating a savings of $1268 (8.3%) per patient tested over 10 years ().
Table 4. Assay economic impact.
Assuming a payer has a nationally representative patient population, this savings translates to $0.067 per member per month. A commercial health plan of 10 million members would see ∼64,000 SPL biopsies per year, ∼6400 of which will be difficult-to-diagnose and therefore tested. For these 6400 assays, 10-year cumulative savings would be over $8 million. For a commercial health plan of 5 million members, 10-year cumulative savings would be over $4 million from ∼3200 assays.
Source of savings
The largest portion of the savings came from a reduction in treatment for advanced disease (due to reducing the number of missed melanomas) and from a reduction in the use of other advanced molecular pathology methods such as FISH and aCGH. shows the assay’s cost impact for each type of care.
Figure 2. Per-patient economic impact by Reference Scenario diagnosis and type of care. Negative numbers indicate cost savings; positive numbers indicate cost increases. The assay is cost-saving in all diagnostic categories. In lesions originally diagnosed as benign or dysplastic, the assay identifies a number of melanomas that would otherwise be missed, which increases initial treatment and follow-up costs while significantly decreasing the costs incurred treating advanced disease. In lesions originally diagnosed as Melanoma, the assay identifies a number of false positives, which reduces initial treatment and follow-up costs.
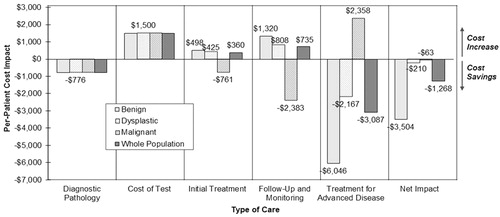
The assay reduces missed melanomas from 10% of the benign tested population and 13% of the dysplastic tested population to 1% of each (). This leads to an increase in spending on initial treatment and annual follow-up, since these missed melanomas are now accurately diagnosed as melanoma. However, this increase is offset by significant savings on downstream treatment for advanced disease, since many of these lesions would have progressed to regional or distant disease if left untreated. Combined with the savings on other molecular pathology tools and the cost of the assay itself, net per-patient savings over 10 years was $3504 for patients originally diagnosed as benign and $210 for patients originally diagnosed as dysplastic ().
For the lesions in the tested population that were originally diagnosed as melanoma, the assay reduces over-diagnosis of melanoma from 33% of this sample of highly ambiguous lesions to only 3% (). This downgrading leads to savings on initial treatment and annual follow-up, since non-melanomas incur fewer costs than melanomas. The net per-patient savings over 10-years for these samples was $63 (). The spending on advanced treatment in this cohort increases due to the assay’s imperfect sensitivity, which leads to some of these melanomas being missed and progressing to advanced disease.
Sensitivity analysis
To assess the model’s sensitivity to changes in specific inputs, each input was modified within its range of plausible values and the overall cost savings were re-calculated. Each tested input was changed in a way that lowered the cost savings (‘Conservative’) and in a way that increased it (‘Aggressive’).
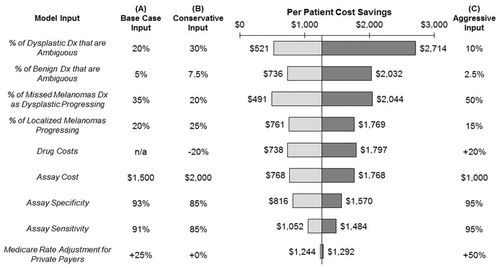
No single input, when changed within a reasonable range of values, caused the model to show that the assay was no longer cost-saving (). Of the 21 inputs tested, 13 changed the per-patient cost savings by less than 30% in either direction. The eight inputs to which the model was most sensitive are shown in . The model was most sensitive to the percentage of samples that are ambiguous and, therefore, sent for testing with the gene expression assay; poor selection of appropriate samples by referring dermatopathologists would lead to reduced cost-effectiveness. The model was also sensitive to the rates of disease progression for localized melanomas and missed melanomas diagnosed as dysplastic lesions. If only 20% of missed melanomas diagnosed as dysplastic lesions progress (vs 35% in the model’s base case), cost savings would decrease by 64%, to $491 per patient. More plausibly, if 50% of missed melanomas diagnosed as dysplastic lesions progress, cost savings would increase by 61% to over $2000 per patient.
Figure 3. Sensitivity analysis. To determine the model’s sensitivity to individual inputs, inputs were modified from the base case (A) to either a Conservative value (B) or an Aggressive value (C). No input, when modified within a reasonable range, caused the model to show the assay as no longer cost-saving. The model was most sensitive to the percentage of samples that are ambiguous (and therefore tested) and to the rates of disease progression for localized melanomas and missed melanomas diagnosed as dysplastic lesions.
Discussion
The diagnosis of malignant melanoma is imperfect. Although routine pathology examination is sufficient to diagnose the majority of suspicious pigmented lesions, discordance rates between different pathologists examining ambiguous samples are alarmingly high. This discordance leads to misdiagnosis in both directions: some nevi are misdiagnosed as melanoma and some melanomas are misdiagnosed as benign or atypica/dysplastic nevi. In both cases, patients suffer, physicians become vulnerable to litigation, and healthcare costs are needlessly increased.
Myriad myPath Melanoma is a gene expression assay designed to serve as an objective, unambiguous tool to differentiate melanoma from non-melanoma. It has been shown to be highly accurate and has demonstrated strong potential for clinical utility. To further assess the assay’s value to the healthcare system, this study modeled its impact on costs incurred by a hypothetical third-party payer.
The analysis demonstrated that this assay could generate significant cost savings for commercial health plans. Even after accounting for the cost of the assay, 10-year cumulative costs decreased by $1268 per patient tested, a drop of over 8%. For a health plan with 10 million covered lives, this would translate to over $8 million in savings ($0.067 per member per month).
The largest contributor to the assay’s economic benefit was a reduction in the incidence of missed melanomas, which are otherwise likely to progress to advanced disease and require expensive interventions such as more extensive surgery, lymph node dissections and systemic drug therapy. However, when used on lesions currently diagnosed as melanoma, the assay would also re-classify many of the false-positive melanoma diagnoses.
The assay’s health-economic benefit depends on it being ordered selectively for the ∼10% of suspicious pigmented lesion biopsies that are ambiguous and difficult-to-diagnose. If used on all SPL biopsies, the assay would no longer be cost-saving. Health plans could, therefore, consider coverage criteria that would ensure that the assay is ordered for the appropriate population.
Sensitivity analyses demonstrated that the model was not overly sensitive to any one input or assumption, indicating a reasonable degree of confidence in the results. No single input, when modified within a reasonable range, caused the assay to no longer be cost-saving.
The quality and reliability of any budget impact model are directly related to the quality and reliability of the data used to generate it. If the clinical paradigm is not reflective of real-world clinical practice, the modeled cost savings may not be realized. Similarly, if the model’s cost inputs are not reflective of true costs, the model will be inaccurate. In this analysis, the extensively sourced clinical paradigm and robustly calculated cost inputs help to instill further confidence in the results. However, this study is not without limitations.
Limitations
Medicare rates may have changed since this analysis was conducted due to changes in policy (e.g., bundling laboratory tests into OPPS rates) or in individual payment rates (e.g., cuts to pathology rates). In addition, although patients typically owe some out-of-pocket cost-sharing for medical care, this was not taken into account due to its high variation between health plans.
Patients were followed for the duration of the model without regard to turnover in health plan membership, which may affect the proportion of the savings realized by any individual health plan over the model’s time horizon.
Importantly, the model assumes that the treatment patients receive aligns with their gene expression assay result. In the real world, however, it is possible that physicians could disregard the assay result after ordering it.
Clinical practice patterns and actual costs may vary by region or by health plan, which would modify the modeled cost savings. Further study using real-world practice and costs is warranted to validate these findings.
Conclusion
This budget impact model shows that use of a novel gene expression assay for the diagnosis of melanoma has the potential to be cost-saving to payers, as patients are diagnosed more accurately and treated more appropriately. Further study is required to validate these findings, particularly in a real-world setting.
Supplementary Material
Download PDF (115 KB)Transparency
Declaration of funding
Myriad Genetic Laboratories, Inc.
Declaration of financial/other relationships
David Cassarino has served on an advisory board and received an honorarium from Myriad Genetic Laboratories, Inc. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER Stat Fact Sheets: Melanoma of the Skin. http://seer.cancer.gov/statfacts/html/melan.html. Accessed January 28, 2014
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600–Mutant advanced melanoma treated with Vemurafenib. N Engl J Med 2012;366:707-14
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23
- Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin 2010;60:301-16
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol 2010;62:751-6
- Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol 2012;67:54-9
- Hawryluk EB, Sober AJ, Piris A, et al. Histologically challenging melanocytic tumors referred to a tertiary care pigmented lesion clinic. J Am Acad Dermatol 2012;67:727-35
- Lodha S, Saggar S, Celebi JT, et al. Discordance in the histopathologic diagnosis of difficult melanocytic neoplasms in the clinical setting. J Cutan Pathol 2008;35:349-52
- Piepkorn MW, Barnhill RL, Cannon-Albright LA, et al. A multiobserver, population-based analysis of histologic dysplasia in melanocytic nevi. J Am Acad Dermatol 1994;30:707-14
- Farmer ER, Gonin R, Hanna MP. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528-31
- Brochez L, Verhaeghe E, Grosshans E, et al. Inter-observer variation in the histopathological diagnosis of clinically suspicious pigmented skin lesions. J Pathol 2002;196:459-66
- Santillan AA, Messina JL, Marzban SS, et al. Pathology review of thin melanoma and melanoma in situ in a multidisciplinary melanoma clinic: impact on treatment decisions. J Clin Oncol 2010;28:481-6
- Ng JC, Swain S, Dowling JP, et al. the impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma. Arch Dermatol 2010;146:234-9
- Armour K, Mann S, Lee S. Dysplastic naevi: to shave, or not to shave? Australas J Dermatol 2005;46:70-5
- McGinnis KS, Lessin SR, Elder DE, et al. Pathology review of cases presenting to a multidisciplinary pigmented lesion clinic. Arch Dermatol 2002;138:617-21
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology. J Pathol 1997;182:266-72
- Weinstock MA, Barnhill RL, Rhodes AR, et al. Reliability of the histopathologic diagnosis of melanocytic dysplasia. Arch Dermatol 1997;133:953-8
- Corona R, Mele A, Amini M, et al. Interobserver variability on the histopathologic diagnosis of cutaneous melanoma and other pigmented skin lesions. J Clin Oncol 1996;14:1218-23
- Cornish D, Holterhues C, van de Poll-Franse LV, et al. A systematic review of health-related quality of life in cutaneous melanoma. Ann Oncol 2009;20(6 Suppl):vi51-8
- Atkinson TM, Noce NS, Hay J, et al. Illness-related distress in women with clinically localized cutaneous melanoma. Ann Surg Oncol 2013;20:675-9
- Morton RL, Rychetnik L, McCaffery K, et al. Patients' perspectives of long-term follow-up for localised cutaneous melanoma. Eur J Surg Oncol 2013;39:297-303
- Troxel DB. Pitfalls in the diagnosis of malignant melanoma: findings of a risk management panel study. Am J Surg Pathol 2003;27:1278-83
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol 2008;35:433-44
- Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med 2011;135:853-9
- Zhang G, Li G. Novel multiple markers to distinguish melanoma from dysplastic nevi. PLoS One 2012;7:e45037
- Gerami P, Jewell SS, Morrison LE, et al. Fluorescence in Situ Hybridization (FISH) as an ancillary diagnostic tool in the diagnosis of melanoma. Am J Surg Pathol 2009;33:1146-56
- Gerami P, Zembowicz A. Update on Fluorescence in Situ Hybridization in melanoma: state of the art. Arch Pathol Lab Med 2011;135:830-7
- Gerami P, Li G, Pouryazdanparast P, et al. A highly specific and discriminatory FISH assay for distinguishing between benign and malignant melanocytic neoplasms. Am J Surg Pathol 2012;36:808-17
- Moore MW, Gasparini R. FISH as an effective diagnostic tool for the management of challenging melanocytic lesions. Diagn Pathol 2011;6:76-81
- Vanison C, Tanna N, Murthy AS. Comparative genomic hybridization for the diagnosis of melanoma. Eur J Plast Surg 2010;33:45-8
- North JP, Vemula SS, Bastian BC. Chromosomal copy number analysis in melanoma diagnostics. Methods Mol Biol 2014;1102:199-226
- Wang L, Rao M, Fang Y, et al. A genome-wide high-resolution array-CGH analysis of cutaneous melanoma and comparison of array-CGH to FISH in diagnostic evaluation. J Mol Diagn 2013;15:581-91
- Dadras SS. Molecular diagnostics in melanoma: current status and perspectives. Arch Pathol Lab Med 2011;135:860-9
- Rock C, Clarke LE, Warf MB, et al. Development and validation of a gene expression signature to distinguish malignant melanoma from benign nevi. ASCO Annual Meeting 2014
- Clarke LE, Berking C, Tahan S, et al. The clinical impact of a gene expression signature that differentiates benign nevi from malignant melanoma. SMR International Congress 2013
- Carlson B. Payers try new approaches to manage molecular diagnostics. Biotechnol Healthc 2010;7:26-30
- United Health Center for Health Reform & Modernization. Personalized medicine: trends and prospects for the new science of genetic testing and molecular diagnostics. Monnetonka, MN: 2012
- Reddy KK, Farber MJ, Bhawan J, et al. Atypical (Dysplastic) Nevi: outcomes of surgical excision and association with melanoma. JAMA Dermatol 2013;149:928-34
- Goodson AG, Florell SR, Boucher KM, et al. Low rates of clinical recurrence following biopsy of benign to moderately atypical dysplastic melanocytic nevi. J Am Acad Dermatol 2010;62:591-6
- Centers for Medicare & Medicaid Services. Clinical Laboratory and Physician Fee Schedules. Atlanta, GA; American Medical Association: 2013.
- Emory Genetics Laboratory. Informed consent for array CGH Analysis. Decatur, GA: 2010
- Greenwood Genetic Center. Diagnostic Laboratory Tests, Charges, & CPT Codes 2013
- Truven Healthcare Micromedex Solutions. Red Book Drug References 2013
- National Comprehensive Cancer Network. Melanoma (Version 2.2013)
- U.S. Food and Drug Administration. Full Prescribing Information: YERVOY (ipilimumab). BLA no. 125377 2013.
- U.S. Food and Drug Administration. Full Prescribing Information: ZELBORAF (vemurafenib). NDA no. 202429 2013.
- U.S. Food and Drug Administration. Full Prescribing Information: TAFINLAR (dabrafenib). NDA no. 202806 2013.
- U.S. Food and Drug Administration. Full Prescribing Information: MEKINIST (trametinib). NDA no. 204114 2013.
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66
- Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 2013;31:373-9
- Okuyama S, Gonzalez R, Lewis KD. Pegylated interferon alpha-2b as adjuvant treatment of Stage III malignant melanoma: an evidence-based review. Core Evid 2010;5:39-48
- Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol 2005;23:6054-62
- Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol 2011;65:1032-47
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206
- Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol 2012;30:2912-8
- Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer Melanoma Staging System. J Clin Oncol 2001;19:3622-34
- Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol 2009;129:1666-74
- Martinez SR, Shah DR, Maverakis E, et al. Geographic variation in utilization of sentinel lymph node biopsy for intermediate thickness cutaneous melanoma. J Surg Oncol 2012;106:807-10
- McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol 2001;8:192-7
- Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma. Ann Surg 2005;242:302-11
- Fox MC, Lao CD, Schwartz JL, et al. Management options for metastatic melanoma in the era of novel therapies: a primer for the practicing dermatologist. Part I: management of stage III disease. J Am Acad Dermatol 2013;68:1.e1-9
- Alexandrescu DT. Melanoma costs: a dynamic model comparing estimated overall costs of various clinical stages. Dermatol Online J 2009;15:1
- Fox MC, Lao CD, Schwartz JL, et al. Management options for metastatic melanoma in the era of novel therapies: a primer for the practicing dermatologist. Part II: management of stage IV disease. J Am Acad Dermatol 2013;68:13.e1-13
- Cromwell KD, Ross MI, Xing Y, et al. Variability in melanoma post-treatment surveillance practices by country and physician specialty: a systematic review. Melanoma Res 2012;22:376-85
- Kansal AR, Shaul AJ, Stern S, et al. Cost-effectiveness of a FISH assay for the diagnosis of melanoma in the USA. Expert Rev Pharmacoecon Outcomes Res 2013;13:371-80
- Salama AK, de Rosa N, Scheri RP, et al. Hazard-rate analysis and patterns of recurrence in early stage melanoma: moving towards a rationally designed surveillance strategy. PLoS One 2013;8:e57665
- Soong SJ, Harrison RA, McCarthy WH, et al. Factors affecting survival following local, regional, or distant recurrence from localized melanoma. J Surg Oncol 1998;67:228-33
- Karakousis CP, Balch CM, Urist MM, et al. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann Surg Oncol 1996;3:446-52
