Abstract
Objective:
To evaluate the health system costs among patients with hemophilia A and B with and without inhibitors over 5 years.
Methods:
This was a retrospective, observational study utilizing medical and pharmacy electronic medical records and administrative encounters/claims data tracking US patients between 2006–2011. Patients with diagnosis codes for hemophilia A and B were identified. Patients with inhibitors were characterized by utilization of bypassing agents activated prothrombin complex concentrate or factor VIIa on two or more distinct dates. Severity was classified as mild, moderate, or severe based on laboratory tests of clotting factor.
Results:
There were 160 hemophilia A patients and 54 hemophilia B patients identified. From this group, seven were designated as patients with inhibitors (five with hemophilia A and two with hemophilia B). Hemophilia A patients without inhibitors reported 65 (41.9%) as being severe, 19 (12.3%) as moderate, and 71 (45.8%) as mild. Hemophilia B patients without inhibitors reported nine (17.3%) had severe, 13 (25.0%) had moderate, and 30 (57.7%) had mild hemophilia. All patients with inhibitors had been hospitalized in the previous 5 years compared to 64 (41.3%) with hemophilia A without inhibitors and 22 (42.3%) with hemophilia B without inhibitors. The median aggregate cost per year (including factor and health resource use) was $325,780 for patients with inhibitors compared to $98,334 for hemophilia A patients without inhibitors and $23,265 for hemophilia B patients without inhibitors.
Conclusions:
The results suggest that, while the frequency of inhibitors within the hemophilia cohort was low, there was a higher frequency of hospitalizations, and the associated median aggregate costs per year were 3-fold higher than those patients without inhibitors. In contrast, hemophilia B patients experience less severe disease and account for lower aggregate yearly costs compared to either patients with hemophilia A or patients with inhibitors.
Introduction
Hemophilia is a congenital bleeding disorder resulting from clotting factor deficiency, which leads to bleeding complications, joint damage, and requires the use of lifelong clotting factor replacement therapyCitation1. Hemophilia A results from alterations in the gene of clotting factor VIII leading to lowered Factor VIII activity that involves 1 in 5000 male birthsCitation2. Hemophilia B results from lowered Factor IX activity that impacts ∼1 in 30,000 males around the worldCitation2,Citation3. People categorized with severe hemophilia experience spontaneous bleeding into the joints and soft tissue that may be recurrent and have complications including joint damage and severe disabilityCitation4,Citation5. The joint damage may lead to reduced joint mobility, muscle atrophy, and chronic pain that may require orthopedic surgery. Only a few joint bleeds may result in joint damageCitation6,Citation7. Adult patients with hemophilia may have more joint damage and reduced physical functioning compared to pediatric patients with hemophiliaCitation8. The ground-breaking clinical trial conducted by Manco-Johnson et al.Citation9 found that use of Factor VIII prophylaxis in children reduced joint bleeding in boys.
In addition, some patients with hemophilia develop inhibitors (alloantibodies) to replacement clotting factors, which renders the treatment ineffective and, therefore, requires a different treatment strategy. It is estimated that inhibitors occur in 25–30% of severe hemophilia A patients and 3–5% of hemophilia BCitation10. The presence of inhibitors may make standard hemophilia replacement therapy ineffectiveCitation11–14. Patients with inhibitors are often managed by using bypassing agents such as activated prothrombin complex concentrate or factor VIIaCitation10. The activated prothrombin complex concentrate Factor Eight Inhibitor Bypassing Agent (FEIBA) is often administered as 50–100 IU/kg every 8–24 h to decrease the risk of bleeding. The usual dose of factor VIIa ranges from 90–120 mcg/kg. Higher dose regimens of factor VIIa have also been evaluated. Another treatment option is immune tolerance induction which involves using very high doses of clotting factor to sensitize the patient to the novel protein. This strategy may be able to eradicate persistent inhibitors in severe hemophilia A patientsCitation10. When hemophilia patients have inhibitors, the need for bypassing agents and immune tolerance induction further increases the costs for patients and the healthcare organizations responsible for covering these expendituresCitation15. Since hemophilia is an uncommon disease with an even smaller sub-set of patients with inhibitors, there is limited data describing the resources and costs required to care for these patients. Therefore, the purpose of this study was to evaluate the characteristics and health system costs among patients with hemophilia A and B with and without inhibitors over a 5-year period within the US Department of Defense (DOD) database for military servicemen/women and their families.
Methods
This study was a retrospective, observational study utilizing electronic medical records and administrative encounters/claims data tracking US patients between 2006–2011 for up to 5 years. These databases were searched and patients with International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes for hemophilia (ICD-9 code 286.0 for hemophilia A [factor VIII deficiency] and 286.1 for hemophilia B [factor IX deficiency]) were identified. Eligible patients must have received clotting factors during the study period and had at least 6 months of continuous enrolment in the worldwide DOD healthcare system. Once the data were collected, administrative and record review data were stripped of patient identifiers and integrated into the analytical data-set. These research data were derived from an approved Naval Medical Center, Portsmouth, VA IRB protocol (IRB NMCP.2012.0016).
Patients were excluded if they had von Willebrand disease. These persons were categorized as the following: (1) one or more prescriptions/claims for Humate-P or Wilate; (2) young persons (less than 50 years of age) receiving Alphanate or Koate; (3) one or more healthcare encounters with a diagnosis code specific to von Willebrand disease; or (4) older persons (age greater than 50) receiving desmopressin therapy.
Patients with inhibitors were characterized by the utilization of at least one of two bypassing agents: activated prothrombin complex concentrate or factor VIIa, on two or more distinct datesCitation16. Hemophilia severity was classified as mild (5–40% normal factor activity), moderate (1–5% normal factor activity), or severe (<1% normal factor activity). A review of the electronic medical records was conducted by a trained nurse using a standardized data collection instrument. Factor level documented in the electronic medical record was used to categorize the hemophilia severity. Bleeding events were identified by ICD-9 codes. Hospitalizations were identified using the inpatient DOD database. Aggregate costs included factor and health resource use for each patient. Statistical analyses were conducted using Stata 12.1. Due to the limited sample size of patients with inhibitors, statistical analyses were restricted to descriptive analyses.
Results
There were a total of 214 patients that met the study inclusion criteria. Of this group, seven (3.3%) patients were categorized as patients with inhibitors (). Of the hemophilia A patients without inhibitors, 65 (41.9%) had severe, 19 (12.3%) had moderate, and 71 (45.8%) had mild hemophilia. Of the patients with hemophilia B without inhibitors, nine (17.3%) had severe, 13 (25.0%) had moderate, and 30 (57.7%) had mild hemophilia. Patients with inhibitors were children younger (median [range] age = 2.0 [<1 – 11] years) than those patients without inhibitors ().
Table 1. Demographic and baseline characteristics.
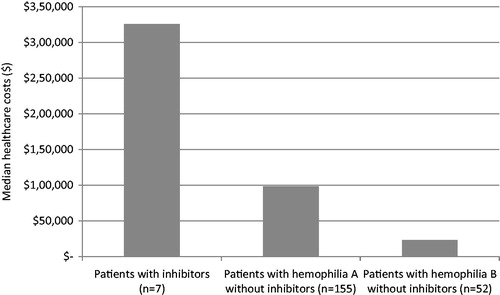
Patients with inhibitors had a median (range) of 1.4 (0–3.8) bleeding events per year compared with 0.3 (0–5.0) for mild, 0.4 (0–4.0) for moderate, and 0.2 (0–3.3) for severe patients without inhibitors. A key finding in this analysis is that all patients with inhibitors had at least one hospitalization in the previous 5 years. In contrast, less than 50% of patients with either hemophilia A or B without inhibitors had a hospitalization within the same time period ().
Figure 1. Percentage of patients with at least one hospitalization within 5 years by inhibitor status.
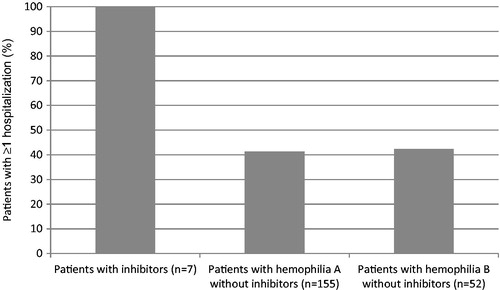
Median aggregate overall healthcare costs per year were substantially higher for patients with inhibitors than patients with hemophilia A or hemophilia B without inhibitors (). For the seven patients identified with inhibitors, the median overall aggregate healthcare cost per year was $325,780. Individuals with hemophilia A without inhibitors had considerably lower yearly total healthcare costs (median = $98,334). Patients with hemophilia B without inhibitors had the lowest total healthcare costs (median = $23,265).
Discussion
This analysis found that a small proportion of patients with hemophilia (3.3% in this study) develop inhibitors to replacement clotting factor products and required treatment with bypassing agents. However, these patients are very important in accounting for additional health resource utilization to a health system secondary to the increased costs associated with their management. The proportion of patients with inhibitors in this study (3.3%) is slightly lower than the proportion identified by Valentino et al.Citation16 (6%). Wight and PaisleyCitation13 found that the prevalence of inhibitors among hemophilia A patients was 5–7%, and that the cumulative incidence may be up to 39%. Thus, the proportion of patients with inhibitors in the present study was slightly below the values noted by Valentino et al.Citation16 and Wight and PaisleyCitation13.
This analysis found that every patient with inhibitors had at least one hospitalization in the previous 5 years compared to less than 50% for other patients with either hemophilia A or B. This suggests that treatment of patients with inhibitors is more challenging as their morbidities can be more severe.
In addition, patients with inhibitors accounted for more than 3-times the annual cost of hemophilia A patients and 6-times the cost of hemophilia B patients. Difficult control and frequent utilization of inpatient and outpatient services lead to higher costs associated with these patients. These results suggest that health systems should devote attention to ensure optimal management to minimize complications and hospitalizations in patients with inhibitors. Patients on prophylaxis or immune tolerance induction would also have higher costs due to increased factor consumption. In addition, it should be kept in mind that these costs and patients are specific to the Department of Defense healthcare system and are not generalizable to the general US hemophilia population.
It should be noted that patients with inhibitors in this study were children and, therefore, the results cannot be extrapolated to the adult hemophilia population. Older, larger patients with inhibitors would be expected to require larger doses and have higher factor costs than the costs identified with these seven children with inhibitors. The age data suggest that health systems work closely with the parents and caregivers of younger patients with inhibitors to optimize care and monitoring.
It is difficult to find other cost data to compare these results. Globe et al.Citation17,Citation18 conducted a medical record review at three California hemophilia treatment centers. These previous studies also found that patients with inhibitors had higher costs than patients with moderate hemophilia. However, the authors were not able to measure actual costs paid for factor and had to impute costs based on average wholesale price. In contrast, this study measured actual costs paid by the DOD.
There are three important limitations to this analysis. The first is that hemophilia is a rare disease and uncommon in many healthcare databases. The large DOD database used in this study found only 214 patients with hemophilia out of more than 9 million beneficiaries. It is possible that this analysis under-estimated the number of patients with inhibitors. However, this does not reduce the importance of the frequent hospitalizations and very high healthcare costs in patients with hemophilia inhibitors. The second limitation is that claims in this analysis were limited to services from a hospital, outpatient, or pharmacy settings, and that other resources consumed in homecare for breakthrough bleeding not requiring a clinic visit were not captured. The third limitation is that this study may have under-estimated the number of patients with inhibitors. Some patients could have been referred to outside Hemophilia Treatment Centers, although it should be kept in mind that the DOD database covers care of DOD members and their families around the world, not just within the US. It is also possible that patients with inhibitors could have been released from military service, but, noting the age of patients in this study, the population appears to largely be children of military members, not the military members themselves. It is also possible that using ICD-9 codes 286.52, 286.53, and 286.59 may have identified additional patients with inhibitors, but these codes are not specific to patients with inhibitors and may also have included patients with other comorbidities as well.
Conclusions
The results of this analysis suggest that, while the frequency of inhibitors within the hemophilia cohort was low, there was a higher frequency of hospitalizations and the associated median aggregate costs per year are 3-fold higher than those patients without inhibitors. Hemophilia B patients experience less severe disease and account for lower aggregate yearly costs compared with either patients with hemophilia A or patients with inhibitors.
Transparency
Declaration of funding
This project was funded by Biogen Idec.
Declaration of interest
EA and DM were consultants to Biogen Idec through Strategic Therapeutics, LLC for this project. SK is an employee of and holds equity in Biogen Idec. MW has no interests which might be perceived as posing a conflict or bias. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
Acknowledgments
EA, DM, SK, and MW were all involved in the design of the research and writing and editing the manuscript. EA and DM were responsible for the analysis of the data.
References
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet 2003;361:1801-9
- Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med 2001;344:1773-9
- Evatt BL. Demographics of hemophilia in developing countries. Sem Thromb Hemost 2005;31:489-94
- Lafeber FP, Miossec P, Valentino LA. Physiopathology of haemophilic arthropathy. Haemophilia 2008;14(4 Suppl):3-9
- Rodriguez-Merchan EC. Management of musculoskeletal complications of hemophilia. Sem Thromb Hemost 2003;29:87-96
- Valentino LA. Secondary prophylaxis therapy: what are the benefits, limitations and unknowns? Haemophilia 2004;10:147-57
- Kilcoyne RF, Nuss R. Radiological assessment of haemophilic arthropathy with emphasis on MRI findings. Haemophilia 2003;9(1 Suppl):57-64
- Zhou ZY, Wu J, Baker J, Curtis R, et al. Haemophilia utilization group study - Part Va (HUGS Va): design, methods and baseline data. Haemophilia 2011;17:729-36
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007;357:535-44
- Franchini M, Mannucci PM. Inhibitors of propagation of coagulation (factors VIII, IX and XI): a review of current therapeutic practice. Br J Clin Pharmacol 2011;72:553-62
- Lusher JM. Inhibitor antibodies to factor VIII and factor IX: management. Sem Thromb Hemost 2000;26:179-88
- Salomon O, Zivelin A, Livnat T, et al. Prevalence, causes, and characterization of factor XI inhibitors in patients with inherited factor XI deficiency. Blood 2003;101:4783-8
- Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia 2003;9:418-35
- Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol 2004;127:379-91
- Gringeri A, Mantovani LG, Scalone L, et al. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood 2003;102:2358-63
- Valentino LA, Pipe SW, Tarantino MD, et al. Healthcare resource utilization among haemophilia A patients in the United States. Haemophilia 2012;18:332-8
- Globe DR, Cunningham WE, Andersen R, et al. The Hemophilia Utilization Group Study (HUGS): determinants of costs of care in persons with haemophilia A. Haemophilia 2003;9:325-31
- Globe DR, Curtis RG, Koerper MA, et al. Utilization of care in haemophilia: a resource-based method for cost analysis from the Haemophilia Utilization Group Study (HUGS). Haemophilia 2004;10(1 Suppl):63-70