Abstract
Aims:
The aim of this analysis was to assess the cost-effectiveness of switching from biphasic human insulin 30 (BHI), insulin glargine (IGlar), or neutral protamine Hagedorn (NPH) insulin (all ± oral glucose-lowering drugs [OGLDs]) to biphasic insulin aspart 30 (BIAsp 30) in people with type 2 diabetes in India, Indonesia, and Saudi Arabia.
Methods:
The IMS CORE Diabetes Model was used to determine the clinical outcome, costs, and cost-effectiveness of switching from treatment with BHI, IGlar, or NPH to BIAsp 30 over a 30-year time horizon. A 1-year analysis was also performed based on quality-of-life data and treatment costs. Incremental cost-effectiveness ratios (ICERs) were expressed as a fraction of gross domestic product (GDP) per capita, and cost-effectiveness was defined as ICER <3-times GDP per capita.
Results:
Switching treatment from BHI, IGlar, or NPH to BIAsp 30 was associated with an increase in life expectancy of >0.7 years, reduction in all diabetes-related complications, and was considered as cost-effective or highly cost-effective in India, Indonesia, and Saudi Arabia (BHI to BIAsp 30, 0.26 in India, 1.25 in Indonesia, 0.01 in Saudi Arabia; IGlar to BIAsp 30, −0.68 in India, −0.21 in Saudi Arabia; NPH to BIAsp 30, 0.15 in India, −0.07 in Saudi Arabia; GDP per head per annum/quality-adjusted life-year). Cost-effectiveness was maintained in the 1-year analyses.
Conclusions:
Switching from treatment with BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs) was found to be cost-effective in India, Indonesia, and Saudi Arabia, both in the long and short term.
Introduction
The International Diabetes Federation estimates that, globally, 382 million people currently have diabetes, and this is projected to increase over the coming years, resulting in 592 million people having diabetes by 2035Citation1. The long-term health consequences of diabetes caused by poor glycemic control include complications such as amputations, renal disease, blindness, blood pressure problems, kidney problems, and cardiovascular disease, which may lead to a premature deathCitation2. Currently, glycemic targets are not being met worldwideCitation3–6, leading to an increased incidence of diabetes-related complications, which translates into greater overall diabetes management costsCitation2.
The total global health expenditure on treating people with diabetes was USD 548 billion in 2013Citation1. Type 2 diabetes has been growing at a particularly high rate in low- and middle-income countries, where a disproportionally small amount of the global expenditure spent on treatment can be expectedCitation1,Citation2,Citation7. The estimated health expenditure on diabetes is predicted to more than double from 2010 to 2030, from USD 2.8 million to USD 4.8 million in India, from USD 287 million to USD 502 million in Indonesia, and from USD 1.4 billion to USD 3 billion in Saudi Arabia, respectivelyCitation7.
Biphasic insulin aspart 30 (BIAsp 30) is a simple-to-use insulin regimen that provides postprandial glucose regulation in addition to basal insulin coverageCitation8,Citation9. Randomized controlled trials have demonstrated the efficacy and safety of switching from biphasic human insulin 30 (BHI) to BIAsp 30Citation10–12, switching from neutral protamine Hagedorn (NPH) insulin to BIAsp 30Citation13, and greater efficacy of starting therapy with BIAsp 30 vs insulin glargine (IGlar)Citation8,Citation14. In addition, observational studies complement randomized controlled trials and provide a more representative profile of complications as they account for broader populations and allow for longer follow-upCitation15. A number of observational studies have reported positive efficacy and safety outcomes associated with switching from BHI to treatment with BIAsp 30Citation6,Citation16,Citation17.
A1chieve was a global observational study designed to evaluate the safety and clinical effectiveness of insulin analogs in routine clinical practice in people with type 2 diabetes. The aim of this analysis was to assess the cost-effectiveness of switching from BHI ± (with or without) oral glucose-lowering drugs (OGLDs), IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs, in countries in different economic circumstances, based on clinical outcomes and health-related quality-of-life (HRQoL) values from the A1chieve study.
Methods
A1chieve study
The A1chieve study was a 24-week observational study in 28 countries across Asia, Africa, Eastern Europe, and Latin America. The study was designed to evaluate the safety and clinical effectiveness of starting treatment with BIAsp 30, insulin detemir, or insulin aspart (all Novo Nordisk, Copenhagen, Denmark) with or without OGLDs in 66,726 people (44,872 insulin-naïve and 21,854 insulin-experienced) with type 2 diabetes in routine clinical practice. Details of the study design and primary data have been presented previouslyCitation5,Citation18. Briefly, the study showed that, in routine clinical practice in all of the regions studied, people starting treatment with BIAsp 30, insulin detemir, or insulin aspart experienced clinically useful improvements in blood glucose without clinically significant problems associated with hypoglycemia or weight gain, as well as improved HRQoLCitation19.
Simulation cohort and assumptions
The cost-effectiveness analysis was performed using data from insulin-experienced people in the A1chieve study who switched from treatment with BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs). Baseline characteristics and the clinical changes associated with switching to BIAsp 30 are shown in ; data include change in HbA1c, body mass index (BMI), EQ-5D-based HRQoL, systolic blood pressure, lipid content, and hypoglycemia. Study populations for switching from BHI ± OGLDs to BIAsp 30 ± OGLDs were from India (n = 866), Indonesia (n = 175), and Saudi Arabia (n = 401); switching from IGlar ± OGLDs to BIAsp 30 ± OGLDs were from India (n = 191) and Saudi Arabia (n = 103); switching from NPH ± OGLDs to BIAsp 30 ± OGLDs were also from India (n = 113) and Saudi Arabia (n = 176). Data were only included in the cost-effectiveness analysis if HbA1c measurements at baseline and at week 24 were available. Data were collected on the costs associated with the management of diabetes, such as annual costs for medications and screening tests, in all of the individual countries. In addition, the costs of treating relevant co-morbidities, such as cardiovascular and renal complications, eye disease, foot ulcers, and neuropathy, were collected for each country (specific data on costs were collected independently of the investigators for the analysis model)Citation20–22. For the base-case scenario, the clinical and economic impact of switching from BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs) in each of the countries was projected over a 30-year time horizon.
Table 1. Baseline demographics and change in clinical outcomes after 24 weeks of treatment from the A1chieve study in people switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs in people with type 2 diabetes.
An analysis of the incremental cost of treatment and the incremental change in HRQoL was conducted for the first year after the switch from BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs) assessing the cost per quality-adjusted life-year (QALY) in the short term. The direct effect of change in clinical measures, such as HbA1c, BMI, and hypoglycaemia, on costs was excluded from the 1-year analysis. However, the change in clinical measures would indirectly be reflected in the change in HRQoL.
CORE diabetes model
The Centre for Outcomes Research (CORE) Diabetes Model is used to determine the long-term clinical outcome, costs, and cost-effectiveness of clinical interventions for the treatment of type 1 and type 2 diabetes. In this analysis, the CORE model was used to determine the long-term health and cost outcomes of switching from treatment with BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs). The interactive computer model is based on a number of Markov sub-models that use published sources to incorporate time, state, time-in state, and diabetes type-dependent probabilities. These sub-models are used to simulate the complications experienced by patients with diabetes (including eye disease, cardiovascular disease, neuropathy, nephropathy, foot ulcers, stroke, amputation, lactic acidosis, ketoacidosis, and mortality)Citation23. Analyses can be performed on groups of people with type 1 or type 2 diabetes based on specific pre-existing baseline complications, known risk factors, age, or gender. The economic and clinical data used in the model are editable, which enables the inclusion of new data as they become available and the ability to perform long-term analyses on a country-specific basis. The current analysis of the A1chieve study-specific change in EQ-5D HRQoL scores from the participating countriesCitation19 replaced the default CORE values in the current analysis.
Statistics
Non-parametric bootstrapping was used in each simulation. The incremental cost-effectiveness ratio (ICER) is expressed in both USD and local currency (exchange rates as of November 2013 unless stated) as cost per QALY. The ICER is also presented for each country in the analysis as a fraction of the gross domestic product (GDP) per capita. GDP data were taken from the World Bank for 2011Citation24. The relative cost-effectiveness of an intervention was based on the World Health Organization (WHO) CHOosing Interventions that are Cost Effective (CHOICE) program, which recommends a threshold based on GDP per capitaCitation25. An intervention is considered as non-cost-effective if the cost of an additional health benefit gained (i.e. QALY) is >3-times GDP per capita, cost-effective at 1–3-times GDP per capita, highly cost-effective at <1-times GDP per capita, or cost-saving (more effective and less costly, i.e. dominant) if estimated cost per QALY gained is below zero.
A series of sensitivity analyses were conducted to assess the robustness of the results, including an extension of the time horizon to 50 years. The impact of treatment-associated change was assessed by assuming that there was no deterioration in HbA1c over time compared with deterioration by −0.15% (−1.6 mmol/mol) each year (excluding the first year) in the base-case scenario. In addition, the median HbA1c treatment effect was used instead of the mean effect and the first quartile distribution of the HbA1c treatment effect (i.e. HbA1c change in the lowest 25% of the study population) was used instead of the mean effect.
In addition, in an analysis of the switch from IGlar to BIAsp 30, the incremental treatment costs assumed the following: (1) that two self-measured plasma glucose (SMPG) strips were used per day vs the one strip that was used in the base case (it was assumed that BHI, NPH, and BIAsp 30 would all use the same amount of strips per day and, therefore, the switch from BHI or NPH to BIAsp 30 was not included in the analysis); (2) that in the first year after the switch to BIAsp 30, an additional four visits to a healthcare practitioner were assumed to be required (based on public sector prices with one specialist consultation and three subsequent consultations with a general practitioner [GP]; the highest price of a GP/specialist visit for each country was used in order to be conservative); (3) two visits would be required to a GP every year after the switch to BIAsp 30 (based on public sector prices to a non-specialist); (4) the average overall switch specific EQ-5D change could be used from the A1chieve study population (BHI to BIAsp 30 [ ± OGLDs, 0.143; + OGLDs, 0.182]; IGlar to BIAsp 30, 0.123; NPH to BIAsp 30, 0.117), rather than individual EQ-5D scores specific for each country.
A short-term analysis (1 year) was performed where sensitivity analyses were conducted for the costs of additional SMPG strips (IGlar to BIAsp 30 only) and four medical consultations with GPs after the switch to BIAsp 30.
An analysis was also conducted to project the maximum total costs (assuming the same clinical outcome) where BIAsp 30 would still be considered cost-effective as defined by an ICER of 3-times GDP per capita for each country to allow for potential costs not included in the cost-effectiveness analyses.
Results
Life expectancy, diabetes-related complications, costs, and cost-effectiveness
The change in clinical outcomes after 24 weeks of treatment reported in the A1chieve study for patients switching from BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs) are shown in .
Switching from BHI, IGlar, or NPH to BIAsp 30 (all ± OGLDs) was consistently associated with a projected increase in life expectancy and an estimated reduction in all diabetes-related complications over 30 years, compared with remaining on BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs, in India, Indonesia, and Saudi Arabia (). Overall, the simulated risk reduction in myocardial infarction over 30 years from switching to BIAsp 30 ± OGLDs ranged between 12–24%; switching from BHI ± OGLDs to BIAsp 30 ± OGLDs resulted in a 15% (India), 12% (Indonesia), and 17% (Saudi Arabia) reduction; switching from IGlar ± OGLDs to BIAsp 30 ± OGLDs resulted in a 14% (India) and 17% (Saudi Arabia) reduction; switching from NPH ± OGLDs to BIAsp 30 ± OGLDs resulted in a 14% (India) and 24% (Saudi Arabia) reduction.
Table 2. Life expectancy, incidence of selected complications (percentage of people) and estimated time alive and free of complications (years), and QALY gains over 30 years after switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs compared with not switching insulin in people with type 2 diabetes.
The direct total costs of diabetes care, including treatment costs, management costs, and complication costs, are shown in . The total costs of switching to BIAsp 30 ± OGLDs or remaining on BHI ± OGLDs were similar in Saudi Arabia (USD 53,575 and USD 53,128, respectively). However, in India and Indonesia, the cost of switching from BHI ± OGLDs to BIAsp 30 ± OGLDs was greater than remaining on BHI ± OGLDs (USD 6970 and USD 6094, respectively, in India; USD 30,676 and USD 26,856, respectively, in Indonesia), but still well below the cost-effectiveness threshold. The total cost of switching from IGlar ± OGLDs to BIAsp 30 ± OGLDs was less than remaining on IGlar treatment in India (USD 7571 and USD 10,114, respectively) and Saudi Arabia (USD 52,849 and USD 61,569, respectively). The total costs associated with remaining on NPH ± OGLDs or switching to BIAsp 30 ± OGLDs were USD 6705 and USD 6174 in India and USD 47,865 and USD 50,199 in Saudi Arabia, respectively (). In addition, the long-term ICERs per QALY gained from the base-case analysis (calculated from the incremental cost and the incremental QALY) were projected over 30 years and presented as a fraction of GDP per QALY. In Indonesia, switching from BHI ± OGLDs to BIAsp 30 ± OGLDs was cost-effective with GDP per capita of 1.25, whereas, in India and Saudi Arabia, the switch was highly cost-effective (0.26 and 0.01, respectively). Switching from IGlar ± OGLDs to BIAsp 30 ± OGLDs was cost-neutral or potentially cost-saving in both India (−0.68) and Saudia Arabia (−0.21), and switching from NPH ± OGLDs was highly cost-effective in India (0.15) and cost-neutral or potentially cost-saving in Saudi Arabia (−0.07) ().
Table 3. Direct costs of diabetes care are simulated over 30 years with switching to BIAsp 30 ± OGLDs or remaining on BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs in people with type 2 diabetes.
Table 4. Long-term and short-term cost-effectiveness of switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs in people with type 2 diabetes.
In the short-term analysis, when the 1-year ICER was expressed as GDP fractional cost per QALY gained, switching to BIAsp 30 ± OGLDs was highly cost-effective (≤ 1 GDP per capita) in all countries, with the exception of switching from BHI ± OGLDs to BIAsp 30 ± OGLDs in Indonesia, which was determined as being cost-effective (≥ 1 and ≤ 3 GDP per capita) ().
Sensitivity analysis
The sensitivity analysis showed that the ICERs did not change greatly by extending the analysis over a 50-year time horizon, or having no deterioration in HbA1c compared with the base case, where switching from BHI ± OGLDs to BIAsp 30 ± OGLDs in people with type 2 diabetes remained cost-effective (Indonesia) or highly cost-effective (India and Saudi Arabia). In addition, switching from IGlar ± OGLDs or NPH ± OGLDs to BIAsp 30 ± OGLDs remained highly cost-effective, regardless of the modified outcome in India and Saudi Arabia (). The addition of two clinic visits every year after the switch and the cost of SMPG strips affected ICER but did not change the cost-effectiveness of switching insulin treatment to BIAsp 30 ().
Figure 1. Sensitivity analysis of switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs in people with type 2 diabetes. Results presented as a fraction of GDP per capita per QALY gained. BHI, biphasic human insulin 30; BIAsp 30, biphasic insulin aspart 30; GP, general practitioner; IGlar, insulin glargine; NPH, neutral protamine Hagedorn insulin; SMBG, self-measured blood glucose.
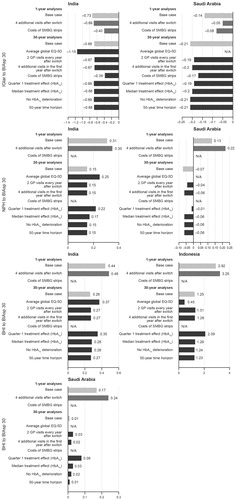
The increase in total current costs that would still deliver an ICER of 3.0 GDP/capita/QALY gained (i.e. which would be on the cost-effectiveness limit) was estimated to be 131% for India, 17% for Indonesia, and 229% for Saudi Arabia when switching from BHI ± OGLDs to BIAsp 30 ± OGLDs; 182% for India and 257% for Saudi Arabia when switching from IGlar ± OGLDs to BIAsp 30 ± OGLDs; 155% for India and 226% for Saudi Arabia when switching from NPH ± OGLDs to BIAsp 30 ± OGLDs in Indonesia.
Discussion
In this analysis, switching therapy from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs in patients with type 2 diabetes was found to be highly cost-effective or cost-effective over a 30-year time horizon in India, Indonesia, and Saudi Arabia using the CORE Diabetes Model. In each case, local baseline clinical characteristics and costs for the treatment and management of diabetes were used for the analysis to ensure local validity.
The long-term analysis showed a projected reduction in the incidence of serious diabetes-related complications by switching from BHI, IGlar, or NPH ± OGLDs to BIAsp 30 ± OGLDs over a 30-year time horizon. In all countries analyzed (India, Indonesia, and Saudi Arabia), there was an expected reduction in the incidence of severe vision loss, end-stage renal disease, myocardial infarction, and ulcer when treatment was switched to BIAsp 30, resulting in HbA1c levels closer to the recommended targets. In addition, as would be expected with a reduction in the incidence of serious complications, there was an increase in life expectancy in patients who switched to BIAsp 30 ± OGLDs in all countries.
The 30-year time horizon was judged to be a suitable length of time for the analysis, as the majority of patients would be expected to have died within that period of time, given the mean age range at baseline (). The analysis was actually extended over a 50-year time horizon and the increase in duration of treatment was shown to have very little effect on the overall cost-effectiveness in any of the countries in this analysis. In addition, in the 1-year analysis, the cost-effectiveness of switching to BIAsp 30 was similar, but not as great as in the 30-year analysis (long term), where switching from BHI, IGlar, or NPH ± OGLDs to BIAsp 30 ± OGLDs remained highly cost-effective or cost-effective in India, Indonesia, or Saudi Arabia. This is to be expected as the 30-year analysis provides cost-saving information on the long-term benefits and reduction in costs of treating diabetes-related complications, whereas the 1-year analysis only provides information on the short-term costs, which relate only to direct-treatment costs. These analyses demonstrating the cost-effectiveness of switching from BHI, IGlar, or NPH ± OGLDs to BIAsp 30 ± OGLDs complement the finding that switching to BIAsp 30 improves glycemic control in patients with type 2 diabetesCitation17,Citation26–30.
With the predicted increase in the number of people with diabetes, and therefore diabetes-related complications, over the next 20 years, the long- and short-term cost-effectiveness of different treatments for type 2 diabetes will become increasingly important. Currently, policy-makers regard randomized controlled trials as the ‘gold standard’ for evidenceCitation2, whereas the data presented in this analysis are generated from the observational A1chieve study. Observational studies have the benefit of providing information that is representative of what actually happens in routine clinical practice in the real worldCitation31.
Cost-effectiveness analyses are inherently comparative, evaluating one treatment type vs another. In this analysis, it is assumed that the comparator group remained on BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs indefinitely and did not switch to another type of therapy at any stage during the projected 30-year time horizon. As diabetes is a progressive disease, the majority of patients would switch insulin or increase their dose and dosing frequency of insulin during the 30-year time horizon. In an effort to address this potential limitation of the study, we conducted several sensitivity analyses using median and first quartile distribution of HbA1c treatment effect as well as assuming no deterioration in HbA1c over time, which showed no significant effect on the cost-effectiveness measure. A further limitation of the study is the lack of published cost studies that are specific to the Indian, Indonesian, and Saudi Arabian populations. However, estimates were obtained from three local experts in each country where data were lackingCitation20–22. The findings of high cost-effectiveness in all three countries included in the analyses were robust in the sensitivity analyses, including the threshold analyses of insulin cost, and so the variance in cost would not appear to be an issue in this instance.
An advantage of the current study is that specific country and treatment QoL data were used for each of the countries included in the analysis. Results of the cost-effectiveness of switching from BHI, IGlar, or NPH to BIAsp 30 are not transferable between regions. However, a previous study has demonstrated the cost-effectiveness of switching to BIAsp 30 ± OGLDs in other countriesCitation32. In addition, in South Korea, switching to BIAsp 30 in patients with poorly controlled type 2 diabetes with NPH improved life expectancy and quality-adjusted life expectancy, and was a cost-effective treatment optionCitation15. In the US, switching to BIAsp 30 from human pre-mixed insulin in patients with poor glycemic control or hypoglycemia was shown to be a cost-effective treatment optionCitation33. In addition, switching from BHI to BIAsp 30 in China was projected to substantially improve clinical outcomes in patients with type 2 diabetes while increasing lifetime medical costs. However, BIAsp 30 would be considered a cost-effective treatment option in China in patients with type 2 diabetes poorly controlled with BHICitation34.
Direct treatment costs with IGlar are higher than with BIAsp 30, so switching from IGlar to BIAsp 30 provides a direct cost saving from the time of the switch. However, many of the healthcare costs associated with diabetes relate to the treatment of diabetes-related complications rather than treatment of the disease itself. The present analysis demonstrates that, even if the initial direct cost of switching the treatment is higher than remaining on the current insulin (as in the case of switching from BHI to BIAsp 30), the treatment can still be cost-effective over a longer period of time when the effect of treatment on long-term complications is taken into account (30-year time horizon). If a patient has poorly controlled type 2 diabetes with a basal insulin ± OGLDs, switching to BIAsp 30 ± OGLDs can offset the cost of treatment with the reduction in the cost of management of serious diabetes-related complications. However, a problem for funders and policy-makers is justifying the increased upfront costs of diabetes treatments to achieve savings in the future. This analysis shows BIAsp 30 to be good value for money by demonstrating that, in India, Indonesia, and Saudi Arabia, switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs was cost-effective over the short-term, with a 1-year time horizon, where only cost of medication was considered and costs saved on reduction in diabetes-related complications were not included.
In conclusion, switching from BHI ± OGLDs, IGlar ± OGLDs, or NPH ± OGLDs to BIAsp 30 ± OGLDs was found to be cost-effective, based on data from the A1chieve study, in India, Indonesia, and Saudi Arabia, both in the long- and short-term.
Transparency
Declaration of funding
Funded by Novo Nordisk.
Declaration of financial/other relationships
VG, AS, and RB, for themselves or institutions with which they are associated, receive funding from all major international pharmaceutical companies for their advisory, lecturing, and research activities, including from Novo Nordisk. EH and AN are employees of Novo Nordisk.
Acknowledgments
We thank all the participants and investigators who helped with the A1chieve study. The authors take full responsibility for the data and analysis supporting this article, and the results and discussion presented, but acknowledge editorial assistance from Kirsty Ratcliffe of Watermeadow Medical and Last Mile P/S for assistance with analyses.
ClinicalTrials.gov trial identifier: NCT00869908.
References
- International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium: International Diabetes Federation, 2013 Update. http://www.idf.org/diabetesatlas. Accessed September 17, 2013
- International Diabetes Federation. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation. 2012. www.idf.org. Accessed October 1, 2013
- Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract 2007;57:455–60
- Del Prato S, Felton AM, Munro N, et al; Global Partnership for Effective Diabetes Management. Improving glucose management: ten stops to get more patients with type 2 diabetes to glycaemic goal. Recommendations from the Global Partnership for Effective Diabetes Management. Int J Clin Pract 2007;157:47–57
- Home P, Naggar NE, Khamseh M, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1cheive study. Diabetes Res Clin Pract 2011;94:352–63
- Valensi P, Benroubi M, Borzi V, et al; IMPROVE Study Group Expert Panel. Initiating insulin therapy with, or switching existing insulin therapy to, biphasic insulin aspart 30/70 (NovoMix 30) in routine care: safety and effectiveness in patients with type 2 diabetes in the IMPROVE observational study. Int J Clin Pract 2009;63:522–31
- Zhang P, Zhang X, Brown J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:293–301
- Raskin P, Allen E, Hollander P, et al; INITIATE Study Group. Initiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260–5
- Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab 2006;8:58–66
- McSorley PT, Bell PM, Jacobsen LV, et al. Twice-daily biphasic insulin aspart 30 versus biphasic human insulin 30: a double-blind crossover study in adults with type 2 diabetes mellitus. Clin Ther 2002;24:530–9
- Boehm BO, Home PD, Behrend C, et al. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in type 1 and type 2 diabetic patients. Diabet Med 2002;19:393–9
- Hermansen K, Colombo M, Storgaard H, et al. Improved postprandial glycemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care 2002;25:883–8
- Christiansen JS1, Vaz JA, Metelko Z, et al. Twice daily biphasic insulin aspart improves postprandial glycaemic control more effectively than twice daily NPH insulin, with low risk of hypoglycaemia, in patients with type 2 diabetes. Diabetes Obes Metab 2003;5:446–54
- Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006;114:527–32
- Lee KH, Seo SJ, Smith-Palmer J, et al. Cost-effectiveness of switching to biphasic insulin aspart 30 from human insulin in patients with poorly controlled type 2 diabetes in south korea. Value in Health 2009;12(Suppl 3):S55–61
- Gumprecht J, Benroubi M, Borzi V, et al; IMPROVE Study Group Expert Panel. Intensification to biphasic insulin aspart 30/70 (BIAsp 30, NovoMix 30) can improve glycaemic control in patients treated with basal insulins: a subgroup analysis of the IMPROVE observational study. Int J Clin Pract 2009;63:966–72
- Shah S, Benroubi M, Borzi V, et al; IMPROVE Study Group Expert Panel. Safety and effectiveness of biphasic insulin aspart 30/70 (NovoMix 30) when switching from human premix insulin in patients with type 2 diabetes: subgroup analysis from the 6-month IMPROVE observational study. Int J Clin Pract 2009;63:574–82
- Shah SN, Litwak L, Haddad J, et al. The A1chieve study: a 60 000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract 2010;88(Suppl 1):S11–6
- Shah S, Zilov A, Malek R, et al. Improvements in quality of life associated with insulin analogue therapies in people with type 2 diabetes: results from the A1chieve observational study. Diabetes Res Clin Pract 2011;94:364–70
- Hnoosh A, Vega-Hernández G, Jugrin A, et al. PDB45 Direct medical costs of diabetes-related complications in Saudi Arabia. Value Health 2012;15:A178
- Todorova L, Hnoosh A, Bloomfield E, et al. PDB20 Estimating the direct medical costs associated with diabetes-related complications in Indonesia. Value Health 2012;15:A662
- Todorova L, Hnoosh A, Korde G, et al. PDB21 Direct medical costs of diabetes-related complications in India. Value Health 2012;15:A662
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20:S5–26
- The World Bank. World Bank Open Data. USA: The World Bank. 2012. http://data.worldbank.org/. Accessed January 2, 2014
- World Health Organisation. World Health Organisation Choice Programme. http://www.who.int/choice/costs/CER_thresholds/en/index.html. Switzerland: World Health Organization. Accessed October 1, 2013
- El Naggar NK, Soewondo P, Khamseh ME, et al. Switching from biphasic human insulin 30 to biphasic insulin aspart 30 in type 2 diabetes is associated with improved glycaemic control and a positive safety profile: results from the A1chieve study. Diabetes Res Clin Pract 2012;98:408–13
- Soewondo P, Lindarto D, Wibisono S, et al. Clinical safety and effectiveness of biphasic insulin aspart 30 in type 2 diabetes patients switched from biphasic human insulin 30: results from the Indonesian cohort of the A1chieve study. Diabetes Res Clin Pract 2013;100(Suppl 1):S41–6
- Shestakova M, Sharma SK, Almustafa M, et al. Transferring type 2 diabetes patients with uncontrolled glycaemia from biphasic human insulin to biphasic insulin aspart 30: experiences from the PRESENT study. Curr Med Res Opin 2007;23:3209–14
- Ohira M, Endo K, Oyama T, et al. Improvement of postprandial hyperglycemia and arterial stiffness upon switching from premixed human insulin 30/70 to biphasic insulin aspart 30/70. Metabolism 2011;60:78–85
- Mäkelä JK, Schmüser C, Askonen K, et al. Starting or switching to biphasic insulin aspart 30 (BIAsp 30) in type 2 diabetes: a multicenter, observational, primary care study conducted in Finland. Diabetes Res Clin Pract 2012;95:10–18
- Dreyer NA, Tunis SR, Berger M, et al. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood) 2010;29:1818–25
- Valentine WJ, Pollock RF, Plun-Favreau J, et al. Systematic review of the cost-effectiveness of biphasic insulin aspart 30 in type 2 diabetes. Curr Med Res Opin 2010;26:1399–412
- Palmer JL, Knudsen MS, Aagren M, et al. Cost-effectiveness of switching to biphasic insulin aspart from human premix insulin in a US setting. J Med Econ 2010;13:212–20
- Palmer JL, Gibbs M, Scheijbeler HW, et al. Cost-effectiveness of switching to biphasic insulin aspart in poorly-controlled type 2 diabetes patients in China. Adv Ther 2008;25:752–74
