Abstract
Objective:
To assess the real-world medical services utilization and associated costs of Medicare patients with diabetic foot ulcers (DFUs) treated with Apligraf (bioengineered living cellular construct (BLCC)) or Dermagraft (human fibroblast-derived dermal substitute (HFDS)) compared with those receiving conventional care (CC).
Methods:
DFU patients were selected from Medicare de-identified administrative claims using ICD-9-CM codes. The analysis followed an ‘intent-to-treat’ design, with cohorts assigned based on use of (1) BLCC, (2) HFDS, or (3) CC (i.e., ≥1 claim for a DFU-related treatment procedure or podiatrist visit and no evidence of skin substitute use) for treatment of DFU in 2006–2012. Propensity score models were used to separately match BLCC and HFDS patients to CC patients with similar baseline demographics, wound severity, and physician experience measures. Medical resource use, lower-limb amputation rates, and total healthcare costs (2012 USD; from payer perspective) during the 18 months following treatment initiation were compared among the resulting matched samples.
Results:
Data for 502 matched BLCC-CC patient pairs and 222 matched HFDS-CC patient pairs were analyzed. Increased costs associated with outpatient service utilization relative to matched CC patients were offset by lower amputation rates (−27.6% BLCC, −22.2% HFDS), fewer days hospitalized (−33.3% BLCC, −42.4% HFDS), and fewer emergency department visits (−32.3% BLCC, −25.7% HFDS) among BLCC/HFDS patients. Consequently, BLCC and HFDS patients had per-patient average healthcare costs during the 18-month follow-up period that were lower than their respective matched CC counterparts (−$5253 BLCC, −$6991 HFDS).
Limitations:
Findings relied on accuracy of diagnosis and procedure codes contained in the claims data, and did not account for outcomes and costs beyond 18 months after treatment initiation.
Conclusion:
These findings suggest that use of BLCC and HFDS for treatment of DFU may lower overall medical costs through reduced utilization of costly healthcare services.
Introduction
Diabetic foot ulcers (DFUs), a common and costly complication of diabetes, have been estimated to affect ∼1–6% of diabetes patients annually, and as many as 25% of diabetes patients over their lifetimeCitation1–4. DFUs often require extensive healing time and are associated with severe and costly outcomes such as infection and lower-limb amputationsCitation5. For example, prior studies have identified DFU duration as an independent risk factor for the development of a foot infection, and that, the risk of hospitalization was 55.7-times greater and the risk of amputation was 154.5-times greater than those who did not develop foot infectionsCitation6. Approximately 5% of diabetes patients with foot ulceration require a lower-limb amputation within the year following a DFU diagnosis compared to almost no amputations among those without DFUsCitation7. Further, foot ulceration is one of the major sources of hospitalizations among patients with diabetes and has been estimated to precede more than four out of every five lower-limb amputations in these patientsCitation4,Citation8. The 3- and 5-year mortality rates following amputation in patients with diabetes have been reported to be 50% and 60%, respectively, which are higher than some common cancersCitation5,Citation9. In terms of economic impact, the annual payer (i.e. private insurance and Medicare) costs of treating DFU in the US, above and beyond the costs of treating diabetes itself, have been estimated to range from $9.1 billion to $13.2 billionCitation7. These statistics highlight the importance of identifying and aggressively implementing the appropriate treatment, which may reduce the risk of complications and costs as well as improve patient care.
Typical treatment of DFUs includes ‘conventional’ wound management procedures such as debridement, infection control, moist dressings, and offloading areas of high pressure or friction. However, conventional care alone is often unsuccessful in healing DFUs, with estimates suggesting that ∼70% of DFUs remain unhealed after 20 weeks of conventional wound managementCitation10. Among the advanced adjuncts to conventional wound management are bioengineered cellular technologies such as Apligraf (bioengineered living cellular construct (BLCC), Organogenesis Inc., Canton, MA) and Dermagraft (human fibroblast-derived dermal substitute (HFDS), Organogenesis Inc., Canton, MA). These products are living cellular constructs and are the only skin substitutes that are FDA approved for the treatment of full-thickness, uninfected DFUsCitation11,Citation12. Both in large prospective pivotal randomized controlled trials and in large real-world comparative effectiveness analyses, BLCC and HFDS have been shown to heal significantly more patients in significantly less timeCitation13–15. Additionally, the rates of amputation and resection were found to be significantly lower in BLCC and HFDS treated patients compared to the conventional care control groupsCitation13,Citation16. Despite having been shown to reduce complications and healing time among DFU patients, questions remain regarding the cost effectiveness of these treatments, and specifically the extent to which the potential benefits of reduced healing time (e.g. reduced costly outcomes) are sufficient to offset the application costs.
Prior economic research suggests that the improved efficacy of BLCC or HFDS may result in cost offsets relative to use of conventional wound management aloneCitation17,Citation18. However, these results are based on clinical trial data and simulation modeling techniques. As a result, the extent to which these results apply to current real-world outcomes is unknown, especially given recent changes in standards of care and costs.
The objective of this study was to expand and improve on previous research to compare the real-world medical services utilization, rates of lower-limb amputations, and costs for Medicare DFU patients who were treated with BLCC or HFDS vs those treated with conventional care. This analysis used administrative claims data to separately examine the differences between DFU patients treated with BLCC or HFDS during a recent time-period (January 2006–December 2012) and matched control DFU patients treated with conventional care alone for a Medicare population.
Patients and methods
Data sources
This study used de-identified administrative claims data from the Standard Analytical Files for a 5% random sample of Medicare beneficiaries (n ∼ 3.6 million). The data span the period 2000–2012 (although only patients receiving treatment for DFUs from 2006–2012 were included for this analysis). The data include information on patient demographics (age, gender, and state of residence), enrollment history, medical diagnoses received, procedures performed, dates of service, place of service (e.g. hospitalizations, physician office visits, emergency department (ED) visits), and payment amounts. Medicare claims for prescription drugs were not available.
Sample selection
displays the summary of the selection criteria used to identify the final analytic samples. Patients with at least two distinct claims with a diabetes diagnosis and at least one claim with a foot ulcer diagnosis on or after the first diabetes diagnosis (with the foot ulcer diagnosis date considered as DFU diagnosis date) were selected from the period between January 2000 and December 2012. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes used to identify diabetes included 249.xx and 250.xx, and ICD-9-CM codes used to identify foot ulcers included 707.10, 707.14, 707.15, 707.19, 707.8x, and 707.9x. All DFU patients with at least one claim for BLCC (identified using Healthcare Common Procedure Coding System (HCPCS) codes C1305, J7340, Q0185, and Q4101), or at least one claim for HFDS (HCPCS codes C9201, J7342, and Q4106) within 12 months following a DFU diagnosis were considered for inclusion in the respective BLCC and HFDS cohorts. To be included in the sample, patients were required to have had ‘treatment initiation’ with BLCC or HFDS, defined as having no claim for commonly used skin substitutes in the 12 months prior to their first BLCC/HFDS claim following a DFU diagnosis. Furthermore, because patients qualifying for Medicare under age 65 tend to have very different cost and comorbidity profiles (e.g. diagnosed with end-stage renal disease, disabled and receiving Social Security Disability Insurance for at least 24 months) we have focused this analysis on patients aged 65+. Finally, to ensure complete visibility of relevant diagnosis and cost information, patients were required to have continuous non-health maintenance organization enrollment during the 12 months prior to (baseline period) and during the 18 months following initiation of treatment with BLCC/HFDS (follow-up period). The index dates for the BLCC/HFDS cohorts were selected as the dates of first treatment initiation with either product for a DFU episode in 2006–2012.
Figure 1. Selection of BLCC/HFDS and CC patients. BLCC: bioengineered living cellular construct; CC: conventional care; DFU: diabetic foot ulcer; HBO: hyperbaric oxygen; HFDS: human fibroblast-derived dermal substitute; HMO: health maintenance organization. *Total patient population includes all Medicare beneficiaries in the 5% Standard Analytic File with at least one medical claim in 2000–2012. †Includes patients with at least two distinct claims with a diabetes diagnosis (ICD-9-CM: 249.xx, 250.xx) and at least one claim with a foot ulcer diagnosis (ICD-9-CM: 707.10, 707.14 707.15, 707.19, 707.8, 707.9) on or after the first diabetes diagnosis at any time in 2000–2012 (the date of foot ulcer diagnosis was considered as the DFU diagnosis date). ‡Patients were required to have at least one claim for the specified skin substitute within 12 months following a DFU diagnosis (as defined above). Patients were not permitted to have a claim for the specified treatment during the 12 months preceding the treatment. §Patients were required to have no claims for skin substitutes at any time in 2000–2012 and were required to have at least one claim with either a DFU diagnosis, DFU-related procedure (debridement, drainage and incision, offloading, negative pressure wound therapy, HBO procedure), or a podiatrist visit. ||The study period was defined as the 12 months prior to and 18 months following the start of new treatment (index date) for the BLCC/HFDS patients, and following a random DFU-related claim (as defined above) meeting all sample selection criteria for the conventional care patients.
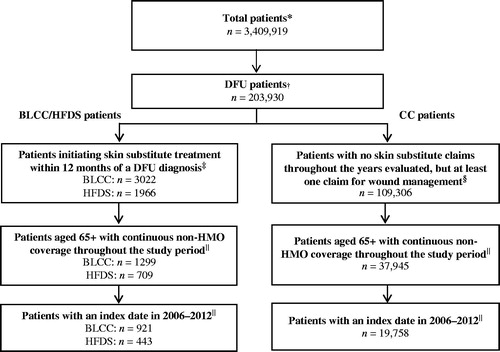
All DFU patients without a claim for commonly used skin substitute treatments but with at least one claim with either a DFU diagnosis code or DFU-related procedure (debridement, drainage and incision, offloading, negative pressure wound therapy, or hyperbaric oxygen (HBO) therapy), or podiatrist visit in the 12 months following a DFU diagnosis were considered as conventional care (CC) patients. CC patients were required to have the same age and continuous data visibility requirements as the BLCC/HFDS cohorts. The index date for the CC cohort was selected as the date of a random medical claim (meeting all of the sample selection criteria) associated with conventional care for treatment of a DFU episode during 2006 or later.
These selection criteria resulted in a final analytic sample of 21,122 total patients, with 921 (4.4%) identified as BLCC patients, 443 (2.1%) identified as HFDS patients, and the remaining 19,758 comprising the control population of CC patients ().
Propensity score matching
BLCC and HFDS patients were separately matched one-to-one to the CC patients (using a greedy matching method)Citation19 based on year of index, age (±2 years), and the likelihood of being treated with BLCC/HFDS (±¼ SD) as determined by propensity scores, which have been used extensively in prior wound care evaluationsCitation7,Citation20–23. Propensity scores were estimated using separate logistic regression models that accounted for differences in baseline characteristics across treatment groups, including patient-level demographics (gender and state), comorbidities, and wound severity, as well as physician-level experience measures (see and ). Wound severity measures included variables denoting unique ulcer types at index (using 5-digit ICD-9-CM codes), calendar quarters of active ulceration (i.e. quarters between index date and the date of the earliest DFU diagnosis during the baseline period), prior DFU-related infections (i.e. cellulitis, osteomyelitis, periostitis, gangrene, and other infections of lower extremity bone), prior lower-limb amputation(s), and DFU-related medical costs (i.e. payments to providers by Medicare for claims with either DFU or DFU-related infection diagnosis, an amputation procedure, a DFU-related procedure, or podiatrist visit). Finally, physician-level measures were calculated to account for potentially important differences in each physician’s contemporaneous level of experience in treating DFUs and/or treatment preferences. Specifically, the following physician-level variables were included in the analysis, based on the treating physician’s (identified using physician ID on the index claim) experience with treatment of DFU in the calendar year corresponding to the index date: number of claims with foot ulcer diagnoses, number of claims for HBO procedures, and number of patients treated with skin substitutes. To account for potential underlying differences that remain after matching, we also estimated total medical cost differentials for the two comparisons using multivariable regression models that controlled for baseline characteristics that remained statistically significant (i.e. p < 0.05) after matching.
Table 1. Patient characteristics during the 12 months prior to the index date: BLCC vs CC.
Table 2. Patient characteristics during the 12 months prior to the index date: HFDS vs CC.
Note that because separate propensity score models were estimated for the two comparisons (i.e. BLCC vs CC; HFDS vs CC) it is possible that certain CC patients are separately matched to both a BLCC and an HFDS patient. As such, the findings from one comparison should be considered independently from the findings for the other comparison.
Outcomes
Rates of lower-limb amputations, stratified by type of amputation (i.e. above knee, below knee, and foot), were compared between BLCC/HFDS patients and their respective matched CC counterparts. In addition, all-cause medical resource utilization and costs from the payer perspective (i.e. payments by Medicare to providers) in the 18-month follow-up period were compared between BLCC-CC and HFDS-CC matched pairs. Resource utilization and costs were further categorized by place of service (i.e. inpatient, outpatient/physician office, home health, ED, other (e.g. durable medical equipment, skilled nursing facilities, daycare)) in order to identify sources of utilization that comprise the cost differential. Because prescription drug claims were unavailable, no pharmacy drug costs were included in the analyses. Additionally, in light of the recent and ongoing changes in Medicare reimbursement policies for skin substitute productsCitation24, we calculated the total medical costs excluding costs associated with the product and application of BLCC and HFDS in those cohorts. All costs were inflated to 2012 US dollars using the medical care component of the Consumer Price IndexCitation25.
Statistical analyses
For categorical variables, statistical significance was assessed using chi-squared tests for comparisons between pre-match BLCC/HFDS patients and conventional care controls, and McNemar tests for the matched cohorts. For continuous variables, statistical significance was assessed using Wilcoxon rank-sum tests (pre-match) and Wilcoxon signed-rank tests (post-match). Statistical analyses were performed separately for the BLCC vs CC and HFDS vs CC comparisons, using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
BLCC-CC baseline comparisons
Prior to matching, BLCC and CC patients differed across a number of different characteristics examined (). Specifically, BLCC patients had higher rates of comorbidities, including diabetes with chronic complications (70.4% vs 49.7%), peripheral vascular disease (66.3% vs 38.6%), and atherosclerosis (42.8% vs 21.0%). In addition, compared with CC patients, BLCC patients had more severe wounds in the baseline period; as evidenced by longer duration of active ulceration (2.1 quarters vs 1.4), a greater number of foot ulcer diagnoses (3.2 distinct ICD-9-CM codes vs 1.2), higher rates of DFU-related infections (60.3% vs 30.9%), higher rates of prior lower-limb amputations (8.0% vs 3.2%), and higher DFU-related medical costs ($17,406 vs $4612) prior to the index date. Finally, physicians treating BLCC patients had more experience with treatment of DFU, particularly using skin substitutes and HBO therapy (). All the above comparisons were statistically significant at p < 0.01.
HFDS-CC baseline comparisons
As with the BLCC cohort described above, HFDS patients differed from CC patients across nearly all baseline characteristics examined (). Specifically, relative to the CC cohort, HFDS patients were younger, more likely to be male, and had higher rates of most comorbidities evaluated. Additionally, HFDS patients had higher rates of lower-limb amputations (15.8% vs 3.2%), and higher DFU-related medical costs ($18,412 vs $4612) prior to the index date. Finally, as in the BLCC-CC comparison above, physicians treating HFDS patients had more experience treating DFUs as well as use of skin substitutes and HBO therapy (). All the above comparisons were statistically significant at p < 0.01.
The matching process resulted in identification of 502 matched pairs of BLCC and CC patients and 222 matched pairs of HFDS and CC patients ( and ). The baseline characteristics were well balanced across the matched cohorts in the two comparisons.
Amputation rates and resource use during the follow-up period
BLCC-CC comparisons
During the 18-month follow-up period, the rates of lower-limb amputations among BLCC patients were 28% lower than their matched CC counterparts (11.8% vs 16.3%, p = 0.04) (). The proportion of BLCC patients with below knee amputations during the follow-up period was approximately half that of matched CC patients (3.0% vs 5.8%, p = 0.03). In addition, the proportion of BLCC patients receiving above knee amputations was 64% lower than that of matched CC patients (2.0% vs 5.6%, p < 0.01). Foot amputation rates were reduced by 11% (p = 0.57).
Table 3. Healthcare resource use and costs during the 18-month follow-up period among BLCC/HFDS patients vs respective matched CC patients.
In terms of overall medical resource use, compared with the matched CC patients, BLCC patients had more outpatient/physician office visits (+17.2%) during the 18-month follow-up period. However, consistent with the lower amputation rates described above, BLCC patients had fewer inpatient days and ED visits (−33.3% and −32.3%, respectively) compared with their matched CC counterparts (). All the above comparisons were statistically significant at p < 0.01.
HFDS-CC comparisons
As with the BLCC-CC comparison above, fewer HFDS patients had above knee amputation during the follow-up period, with the rates being 72% lower than their matched CC counterparts (1.4% vs 5.0%, p = 0.03). Further, rates of below knee and foot amputations were reduced by 34% (p = 0.32) and 3% (p = 0.90), respectively ().
The results of comparisons of resource use metrics were similar to those seen with BLCC-CC comparisons: HFDS patients had more outpatient/physician office visits (+16.1%, p < 0.01), but fewer inpatient days and ED visits (−42.4%, p < 0.01 and −25.7%, p < 0.01, respectively) during the follow-up period than matched CC patients.
Costs during the follow-up period
BLCC-CC comparisons
Compared with their matched CC counterparts, BLCC patients had lower total medical costs in the 18-month follow-up period (). In terms of costs by place of service, BLCC patients, on average incurred $7100 more for outpatient/physician office visits than matched CC patients (p < 0.01). However, these increased costs were offset by reductions in costs associated with inpatient visits ($8528 lower than CC patients, p < 0.01) and other types of medical services (e.g. ED visits, home healthcare; ). Overall, these cost offsets resulted in BLCC patients having average per-patient total medical costs that were $5253 lower than matched CC patients ($62,809 vs $68,062, p = 0.49). Excluding costs associated with the acquisition and administration of BLCC, the average all-cause medical costs among BLCC patients were $9037 lower than their matched CC counterparts (p = 0.71).
HFDS-CC comparisons
As with the BLCC-CC comparison, HFDS patients had average costs for outpatient/physician office visits that were $11,947 higher than their matched CC counterparts in the 18-month follow-up period (p < 0.01). Also as above, however, these costs were offset by lower inpatient costs ($14,484 lower than matched CC controls, p < 0.01) and other places of service, which led to average total medical costs for HFDS patients that were $6991 lower than the matched CC patients ($66,063 vs $73,054; p = 0.84). Average per-patient medical costs excluding costs associated with HFDS and its application were $17,101 lower for HFDS patients compared with matched CC patients (p = 0.08) ().
The observed cost differentials for both BLCC-CC and HFDS-CC comparisons were similar when accounting for baseline characteristics that remained statistically significantly (i.e. p < 0.05) different after matching (using multivariable regression models; results not reported).
Discussion
This study assessed the real-world medical resource utilization and costs (from the payer perspective) associated with the use of BLCC and HFDS relative to CC in DFU patients. Of the patients included in the final analytic sample (prior to matching), 6.5% used bioengineered skin substitutes for treatment of a DFU episode in 2006–2012 (4.4% used BLCC and 2.1% used HFDS), with the remaining patients receiving conventional wound management without the use of skin substitutes. Prior to matching, patients using BLCC or HFDS for treatment of a recent DFU episode were generally more complex, with statistically significant (i.e. p < 0.01) differences vs CC patients across nearly all patient and physician-level characteristics evaluated. This resulted in BLCC and HFDS patients having statistically significantly (i.e. p < 0.01) higher DFU-related costs prior to initiating treatment with skin substitutes. Despite these differences in the overall patient populations, the matching process resulted in BLCC-CC and HFDS-CC patient pairs that had similar characteristics.
Among the matched samples, BLCC and HFDS patients had greater intensity of outpatient/physician office services and incurred greater costs of direct treatment compared with their respective matched CC cohorts. However, the costs of more intensive treatment in the physician office or outpatient setting were offset by reductions in lower-limb amputations and other resource use, particularly inpatient services, during the follow-up period. This finding may partly be attributable to increased effectiveness of BLCC and HFDS for DFU treatment, in terms of higher healing rates and reduced healing timesCitation13–15. As a result, in spite of the higher cost associated with these products and their application, the average total medical costs among patients treated with BLCC or HFDS in this study were directionally lower compared with the matched CC controls.
While this is the first study to estimate the clinical and economic outcomes of using different treatments for treating DFUs using real-world claims data and accounting for a broad array of differences in patient, wound, and physician-level characteristics, aspects of our findings are consistent with prior research. For instance, despite differences in study populations and methodology, our finding that patients treated with skin substitutes are less likely to receive lower-limb amputations and use fewer medical resources is similar to the conclusions of studies by Redekop et al.Citation17 and the Lewin GroupCitation18.
Study findings, however, need to be interpreted in light of a number of limitations, primarily those inherent to any claims-based analysis. First, this analysis relied on the accuracy of diagnosis and procedure codes to identify patients with DFU and different treatments, as well as to evaluate the characteristics and outcomes. Any miscoding along these lines would affect our results, although we have no reason to believe that inaccuracies in the data may have affected the various treatment groups differently. In addition, while the study controlled for various proxies for wound severity (e.g. duration of active ulceration, amputations), clinical measures (e.g. wound size and depth) were not directly observable in the database. Second, the matching process resulted in an analytic sample that included only a sub-set of the overall patients included in each cohort. Assessments regarding the extent to which these results apply to patients with different underlying characteristics would be an important line of future research. Third, the study examined medical resource use and costs from the payer perspective for 18 months after treatment initiation with one of the two specific skin substitute products as adjuncts to CC. As such, further research is needed to understand the long-term costs (e.g. stemming from physical therapy, developing additional ulcers) or costs directly borne by the patient, as well as the cost effectiveness compared with other treatment alternatives. Fourth, the findings are based on analysis of Medicare beneficiaries aged 65+ and the generalizability of these results to other patient populations (e.g. private insurance, Medicaid) is unknown. Finally, although this study suggests potential pathways by which the costs of BLCC and HFDS are offset (e.g. through reductions in hospitalizations and amputations, indirect measures of effectiveness), future research is warranted to understand these and other potential mechanisms more fully.
Conclusions
Notwithstanding the aforementioned limitations, this study provides an up-to-date estimate of the relative resource use and costs associated with use of BLCC and HFDS in the treatment of DFUs using a large administrative claims database, employing rigorous statistical methodologies and controlling for a broad array of underlying differences between patients receiving each treatment. These comparative effectiveness findings suggest that use of BLCC and HFDS for treatment of DFU reduces utilization of costly healthcare services without increasing costs. Such findings have potentially important implications regarding policies surrounding optimal DFU treatment and suggest that more widespread use of bioengineered cellular technologies can improve patient outcomes without incremental financial impact for payers.
Transparency
Declaration of funding
This study was funded by Organogenesis, Inc., Canton, MA.
Declaration of financial relationship
MS and NBP are employees of Organogenesis, Inc., which provided research funding to Analysis Group (employer of JBR, UD, LR, AKGC, and HGB) for this project. DJM is a Professor at the Perelman School of Medicine, University of Pennsylvania, and has consulted to Organogenesis. The authors report no other conflict of interest.
References
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217-28
- Margolis D, Malay DS, Hoffstad OJ, et al. Incidence of diabetic foot ulcer and lower extremity amputation among Medicare beneficiaries, 2006 to 2008. Data Points #2. AHRQ Publication No. 10(11)-EHC009-1-EF [article online]. Rockville, MD: Agency for Healthcare Research and Quality (AHRQ); 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/287/627/Data-points_2_Diabetic-Foot-Ulcer_Report_02-2011.pdf. Accessed November 4, 2014
- Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999;22:382-7
- Cook JJ, Simonson DC. Epidemiology and health care cost of diabetic foot problems. In: Veves A, Giurini JM, LoGerfo FW, eds. The diabetic foot. New York City: Springer, pp. 17-32. http://link.springer.com/book/10.1007%2F978-1-61779-791-0. Accessed February 24, 2015
- Frykberg RG, Zgonis T, Armstrong DG, et al; American College at Foot and Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline. J Foot Ankle Surg 2006;45:52-66
- Lavery LA, Armstrong DG, Wunderlich RP, et al. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29:1288-93
- Rice JB, Desai U, Cummings AK, et al. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care 2014;37:651-8
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513-21
- Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J 2007;4:286-7
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment: a meta-analysis. Diabetes Care 1999;22:692-5
- Apligraf prescribing information [online]. Organogenesis, Canton, MA. 2010. http://www.apligraf.com/professional/pdf/prescribing_information.pdf. Accessed November 4, 2014
- Dermagraft prescribing information [online]. Organogenesis, Canton, MA. 2014. http://www.dermagraft.com/wp-content/uploads/sites/1/Dermagraft_Directions_For_Use1.pdf. Accessed November 4, 2014
- Veves A, Falanga V, Armstrong DG, et al. Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care 2001;24:290-5
- Marston WA, Hanft J, Norwood P, et al; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 2003;26:1701-5
- Kirsner RS, Sabolinski ML, Parsons NB, et al. A retrospective, comparative effectiveness analysis of bioengineered living cellular technologies and an acellular porcine collagen wound dressing for the treatment of diabetic foot ulcers in a real world setting. Poster presentation #CR 016, Symposium on Advanced Wound Care, October 17, 2014. Las Vegas, NV
- Frykberg RG, Marston WA, Cardinal M. The incidence of lower-extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast-derived dermal substitute. Adv Skin Wound Care 2015;28:17-20
- Redekop WK, McDonnell J, Verboom P, et al. The cost effectiveness of Apligraf treatment of diabetic foot ulcers. Pharmacoeconomics 2003;21:1171-83
- Zhang Y, Hogan P. Cost effectiveness of a human fibroblast-derived dermal substitute for the treatment of diabetic foot ulcers in Medicare and commercially insured populations. Diabetes 2011;60(1A Suppl):LB15-16
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424
- Margolis DJ, Bartus C, Hoffstad O, et al. Effectiveness of recombinant human platelet-derived growth factor for the treatment of diabetic neuropathic foot ulcers. Wound Repair Regen 2005;13:531-6
- Margolis DJ, Gupta J, Hoffstad O, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care 2013;36:1961-6
- Margolis DJ, Kantor J, Santanna J, et al. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001;24:483-8
- Rice JB, Desai U, Cummings AK, et al. Burden of venous leg ulcers in the United States. J Med Econ 2014;17:347-56
- Centers for Medicare and Medicaid Services. MLN Matters Article MM8572 [online]. Baltimore, MD. 2013. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM8572.pdf. Accessed November 4, 2014
- U.S. Bureau of Labor Statistics. Consumer Price Index [online]. Washington, DC. 2013. http://www.bls.gov/cpi/home.htm. Accessed November 4, 2014
