Abstract
Background:
Acute venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is traditionally managed with a short course of parenteral anticoagulation followed by 3–6 months of a vitamin-K antagonist. Non-vitamin K oral anticoagulants (NOACs) do not require routine monitoring and dose adjustment, thus potentially provide an alternative treatment option.
Methods and results:
Because of the lack of head-to-head clinical studies, an indirect comparison was conducted of dabigatran etexilate and rivaroxaban based on the respective phase III clinical trial. The derived relative safety and efficacy estimates were used to evaluate the cost-utility of dabigatran compared with rivaroxaban in the treatment and secondary prevention of VTE. The results of the indirect comparison showed no significant difference between dabigatran and rivaroxaban in avoiding recurrent VTE following index PE, index DVT, or DVT/PE combined, in treatment and extended anticoagulation. Dabigatran has significantly less major or clinically relevant bleeds (MCRBE) compared to rivaroxaban in treatment after index DVT and treatment after DVT or PE combined, but was not significantly different from rivaroxaban after index PE or in extended anticoagulation. In cost-utility deterministic analyses, dabigatran was projected dominant in all analyzed settings, given its marginally lower total cost and marginally higher QALYs gained compared to rivaroxaban. Probabilistic analyses results showed a high likelihood of dabigatran being considered good value for money in the UK, in treatment and in secondary prevention of VTE.
Conclusion:
The cost-effectiveness evaluations showed that dabigatran can be considered the dominant treatment strategy compared to rivaroxaban in the patients’ sub-groups considered, given the projected marginally higher clinical benefits and lower treatment costs.
Introduction
Venous thromboembolism (VTE) is associated with a high disease burden, due to mortality risks following acute pulmonary embolism (PE) and life-long complications such as chronic thromboembolic pulmonary hypertension (CTEPH), and post-thrombotic syndrome (PTS)Citation1–3. Traditionally, VTE has been managed with short course heparin, followed by 3–6 months dose-adjusted vitamin K antagonists (VKAs)Citation4. Unlike VKAs, non-vitamin K oral anticoagulants (NOACs) have the benefits of a predictable pharmacokinetic and pharmacodynamic profile, and allow fixed dose administration. Hence, they may provide an opportunity for longer and safer anticoagulation, and cost-savings from reduction in medical resources involved in anticoagulation monitoring. Due to the increasing availability of NOACs in the treatment of acute VTE, it is paramount to understand their comparability in terms of relative safety, efficacy, and cost-effectiveness.
In the absence of head-to-head randomized trials (RCTs) of dabigatran etexilate, hereafter referred to as dabigatran, and rivaroxaban, the most appropriate method to assess their relative efficacy and safety is by means of an indirect comparison (IC). Several ICs of NOACs have already been published in treatment of acute VTECitation5–7, and in extended anticoagulationCitation8,Citation9. Published ICs of dabigatran and rivaroxaban were conducted on efficacy endpoints: recurrent VTE (rVTE) and deathCitation5, rVTE and VTE-related deathCitation7, rVTE, recurrent pulmonary embolism (rPE), and recurrent DVT (rDVT)Citation6. IC were conducted on safety end-points: major bleeding events (MBE)Citation5–7, and major and clinical relevant bleeding events (MCRBE)Citation5,Citation7, as well as on death due to any causeCitation5,Citation7. MBE included intracranial hemorrhage and major extra-cranial hemorrhage, whereas MCBE also included clinically relevant non-major bleeds in addition to MBE.
The objectives of our analyses were to conduct additional ICs in patient’s sub-groups respective of the type of index event: DVT alone, PE with or without DVT, and respective of the duration of active anticoagulation, 6 months treatment with dabigatran vs 6 months treatment with rivaroxaban. Next, we used the IC estimates of rVTE and VTE-related death, and MCRBE to evaluate the cost-utility of dabigatran, as an alternative to rivaroxaban in the treatment and in extended anticoagulation of VTE (DVT and/or PE), from an NHS and PSS perspective in England and Wales.
Materials and methods
The dabigatran phase III clinical development program in treatment and prevention of recurrent VTE included two double blind, double-dummy, head-to-head, non-inferiority studies conducted against warfarin: RE-COVERCitation10 and RE-COVER IICitation11, and two double-blind, randomized control studies of dabigatran 150 mg twice daily compared with warfarin (active-control study) or with placebo (placebo-control study), in patients who completed at least 3 months of anticoagulation prior to randomizationCitation12. The two acute treatment studies were identical in their design: inclusion of patients, definition of end-points, and timing for outcomes assessment. Both compared dabigatran 150 mg twice daily with warfarin, adjusted to maintain an international normalized ratio (INR) of 2.0–3.0 during 6 months ‘double-dummy phase’; prior to initiation of the ‘double-dummy phase’ patients in both arms received parenteral anticoagulation followed by warfarin or matched placebo for at least 5 days and until the INR had been 2.0 or higher. Hence, pooling of results from these two studies was straight-forward. The primary efficacy end-point was recurrent, symptomatic, objectively confirmed VTE and VTE-related deaths. The important safety end-point was MCRBE. We decided to include MCRBE which occurred from the start of any treatment rather than MBE only, because, even though clinically relevant non-major bleeds do not lead to treatment discontinuation, they play an important role in terms of additional costs and quality-of-life decrements.
The rivaroxaban phase III VTE-treatment development program, EINSTEIN, consisted of two acute treatment studies in patients with acute symptomatic PE (EINSTEIN-PE)Citation13, and in acute symptomatic DVT with or without PE (EINSTEIN-DVT)Citation14, and one extension study in patients treated for an additional 6 or 12 months in whom 6–12 months of treatment with anticoagulants had been completed (EINSTEIN-Ext)Citation14. In the acute treatment studies, rixaroxaban was administered twice daily at 15 mg for 3 weeks, followed by 20 mg once daily in the acute treatment studies, and was compared with enoxaparin (for at least 5 days) followed by a VKA (warfarin or acenocoumarol) for 3, 6, or 12 months, at a target INR of 2–3. EINSTEIN-Ext was a double-blind, randomized, event-driven superiority study that compared rivaroxaban alone (20 mg once daily) with placebo.
Across the studies, the characteristics of patients at baseline (average age, proportion of Caucasian-origin, proportion with cancer and baseline, proportion with prior of VTE; ) were comparable. In all studies, warfarin or VKA was titrated to a target INR level of 2–3. Patients in RE-COVER studies received low molecular weight or unfractioned heparin for at least 5 days before randomization, while in EINSTEIN studies patients were excluded if they had received therapeutic doses of low molecular weight heparin, fondaparinux, or unfractionated heparin for more than 48 h before randomization. All of the trials comparing rivaroxaban or dabigatran with VKAs were designed to assess for non-inferiority; efficacy analyses of RE-COVER were conducted on a modified intention-to-treat patients set, representing all randomized patients who took at least one dose of the study drug; in EINSTIN treatment studies, analyses were conducted on the intention-to-treat dataset. A total of 5132 patients were randomized to receive warfarin or dabigatran in the RE-COVER studies, and 8281 patients received rivaroxaban or VKA in the EINSTEIN treatment studies. The primary efficacy end-point in all acute VTE studies was VTE and VTE-related death, however, in EINSTEIN studies, PE was considered the cause of death if there was an objective documentation or if death could not be attributed to a documented cause and PE could not be confidently ruled out. This deviated from the definition of VTE-related death in the RE-COVER studies, where unexplained deaths were excluded from the primary efficacy assessment. All studies had a common safety end-point, MCRBE. In both the RE-COVER and EINSTEIN, a bleeding event was defined as MCRBE if it was clinically overt and associated with a fall in the hemoglobin level of 20 g/l or more, or if it led to transfusion of two or more units of red cells, or if it occurred in a critical site, or contributed to death. The EINSTEIN study definition also includes retroperitoneal and intracranial bleeding explicitly, in addition to critical site bleeding.
Table 1. Acute treatment studies characteristics.
A notable difference between the designs of the four acute treatment studies was the intended duration of treatment, which was 6 months in RE-COVER studies and 3, 6, or 12 months in EINSTEIN treatment studies. Potential bias due to differences in unobserved patient characteristics affecting the assignment to a treatment duration was mitigated by conducting an IC on the 6 months treatment EINSTEIN-PE and 6 months treatment EINSTEIN-DVT groups, vs 6 months of treatment in RE-COVER I, II.
We conducted a series of ICs using the adjusted indirect comparison methodology developed by Bucher et al.Citation15. This method derives the indirect estimates by comparing the effects of each treatment vs the common comparator, and retains the benefits of randomization from the original trial data. This approach is in line with the conclusions from a comprehensive review of published indirect comparisons commissioned by the UK National Health Service (NHS) Health Technology Assessment Program, which highlighted the need to use an adjusted indirect comparison to minimize bias and the drawing of overly precise conclusionsCitation16. ICs were conducted to compare 6 months of treatment with dabigatran in the following groups: index VTE, with rivaroxaban pooled 3, 6, and 12 months treatment, index VTE, with rivaroxaban 6 months treatment group, index DVT, with rivaroxaban pooled 3, 6 and 12 months treatment, or rivaroxaban 6 months treatment group, index PE, with rivaroxaban pooled 3, 6, and 12 months treatment, or with rivaroxaban 6 months treatment group. Efficacy and safety end-points of extended anticoagulation were obtained from published ICs in secondary preventionCitation5–7.
For the analyses of cost-effectiveness, we used a health-economic model in treatment of VTE published for the comparison between dabigatran and warfarinCitation17. Briefly, this was a Markov cohort model with health states defined around the primary composite end-points: rVTE and VTE-related death, and MCRBE. Long-term consequences of VTE, CTEPH, and PTS were considered. Patients entered the model at the age of 55 years following index VTE (DVT/PE) and received 5-days low molecular weight heparin (LMWH) followed by 6 months treatment with dabigatran or 1-day LMWH followed by 6 months treatment with rivaroxaban. At any time, patients may discontinue treatment if experiencing a recurrent VTE, a MBE, or due to other reasons as observed in the clinical studies. After each rVTE, a new treatment course of 5 days LMWH followed by 6 months warfarin is reinstated, irrespective of the initial treatment (). Data on the relative safety and efficacy of rivaroxaban vs dabigatran pertained to the conducted ICs presented herein. The baseline probabilities for rVTE were sourced from the pooled dabigatran arms of RE-COVER studies for the treatment periodCitation10,Citation11, and from the dabigatran arm of RE-SONATECitation12 in extended anticoagulation. The 6 months probability of recurrent VTE and VTE-related death was 2.35% (60/2553) in index VTE, 2.39% (42/1755) in index DVT, and 2.26% (18/795) in PE; after treatment, in extended anticoagulation, the baseline probability applied over 6 months for rVTE and VTE-related death was 0.44% (3/681). The same sources were used to inform baseline probabilities of MCRBE during treatment and in extended anticoagulation. Six-months probabilities were 5.54% (134/2553) in index VTE, 5.02% (84/1757) in index DVT, 6.73% (50/793) in index PE, and 5.26% (36/684) in extended anticoagulation. Beyond active anticoagulation, the probability of recurrent VTE was sourced from the literatureCitation18. Dabigatran clinical trials did not assess the incidence of PTS and CPHT. The probabilities of CPHTCitation2 and PTSCitation3 were, therefore, obtained from the literature and assumed equal between dabigatran and rivaroxaban. Health-state utility values for recurrent events and bleeds were sourced from Euroqual 5 dimensions (EQ-5D) results of RE-COVER. Unit costs of drugs, medical contacts, diagnostic tests, and inpatient stays were sourced from fees published by the NHS in England and Wales, and the Personal Social Services Research Unit (PSSRU). Medical resource use data was obtained from different sources (literature or clinical expert advice) since the data collected in the clinical trials was limited and not representative of real world clinical practice (). Both deterministic and probabilistic analyses were conducted. For the probabilistic analysis, a distribution was assigned to each parameter of the model based on specific characteristics. A Gamma distribution was assigned to costs and to resource use, a Normal distribution was assigned to relative risks and hazard ratios a Beta distribution was assigned to probabilities of events and utility values. The analysis was performed with 1000 Monte Carlo simulations and the results were presented in a CE plane and through a cost-effectiveness acceptability curve, which together quantified the level of confidence that can be placed in the model results.
Figure 1. Model diagram. CRNMBE, clinically relevant non-major bleeds; CTEPH, chronic thromboembolic pulmonary hypertension; ICH, intracranial hemorrhage; iDVT, index DVT; iPE, index PE; iVTE, index VTE; MBE, major bleeds; PTS, post-thrombotic syndrome; rDVT, recurrent DVT; rPE, recurrent PE; rVTE, recurrent VTE.
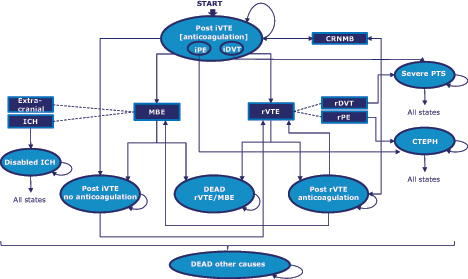
Table 2. Unit costs and resource use inputs, £(2014).
Results
The IC results on the primary efficacy end-point, rVTE and VTE-related death, showed treatment with rivaroxaban to be associated with a lower risk of rVTE, when compared with dabigatran. However, differences were not statistically significant, with relative risk for rivaroxaban vs dabigatran equal to 0.83 (95% CI = 0.46–1.49) in the combined index DVT/PE for the rivaroxaban 3, 6, 12 months treatment set and equal to 0.90 (95% CI = 0.53–1.52) for the 6 months treatment set. In the index DVT sub-group, relative risks for rivaroxaban vs dabigatran were 0.57 (95% CI = 0.31–1.05) and 0.70 (95% CI = 0.35–1.39) for the 3, 6, 12 months, and the 6 months treatment set, respectively. In contrast, the rate of recurrent VTE in the index PE population was higher with rivaroxaban than with dabigatran, in both 3, 6, 12 months (RR = 1.30, 95% CI = 0.62–2.70) and 6 months treatment groups (RR = 1.30, 95% CI = 0.57–2.94) (); however, also here the difference was non-statistically significant. Observed MCRBE rates were higher with rivaroxaban than with dabigatran in the combined DVT/PE treatment group with relative risks of 1.49 (95% CI = 1.16–1.92) and 1.61 (95% CI = 1.18–2.17) for the 3, 6, 12 months, and the 6 months treatment set, respectively. In the index DVT sub-group, the same relative risks were 1.75 (95% CI = 1.25–2.50) and 2.04 (95% CI = 1.39–3.03), respectively. Differences in MCRBE rates were statistically significant in the index DVT group, but were non-statistically significantly different in the index PE group, with relative risks of 1.22 (95% CI = 0.83–1.79) and 1.22 (95% CI = 0.80–1.85) for the 3, 6, 12 months, and the 6 months treatment set, respectively (). In extended anticoagulation, rivaroxaban vs dabigatran relative risk of rVTE was 2.25 (95% CI = 0.52–9.69) and MCRBE relative risk was 1.93 (95% CI = 0.69–5.41), however differences were non-significant.
Table 3. Indirect comparison results—RE-COVER I, II, and EINSTEIN studies.
In all, treatment and secondary prevention settings, cost-effectiveness analyses results showed marginally higher clinical benefits with dabigatran than with rivaroxaban. These resulted from avoidance of bleeding events, except for other major bleeds which were higher in the dabigatran arm for the VTE and for index DVT, and less recurrent non-fatal PEs in all settings, as well as less recurrent all VTE in the PE sub-group. Results were consistent across the two rivaroxaban treatment sets: 3, 6, 12 months treatment () and 6 months treatment (). Marginal cost savings were projected for dabigatran in all settings, dabigatran resulting dominant over treatment with rivaroxaban (higher net health benefits and lower costs). However, in all probabilistic sensitivity analyses conducted (PSA), the cloud of the scatter plots was located on both sides of the 0x and 0y axes, covering all possible cost-effectiveness scenarios: dominant, dominated, lower costs lower outcomes, higher costs higher outcomes (). At a willingness-to-pay threshold of £20,000, the probability that dabigatran would be considered good value for money compared with 3, 6, 12 months rivaroxaban is 61% and 88%, in treatment and extended anticoagulation of VTE, respectively, and 62% and 62% in DVT and PE, respectively.
Figure 2. Probabilistic sensitivity analyses results. (1a, b) CE plane and CEAC VTE (DVT/PE) treatment; (2a, b) CE plane and CEAC VTE extended anticoagulation; (3a, b) CE plane and CEAC index DVT treatment; (4a, b) CE plane and CEAC index PE treatment; CE, cost-effectiveness; CEAC, cost-effectiveness acceptability curve; DVT, deep vein thrombosis; IC, incremental costs; Incr., incremental; PE, pulmonary embolism; QALY, quality-adjusted life years; VTE, venous thromboembolism.
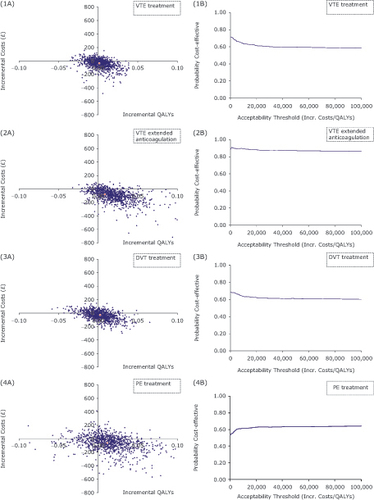
Table 4. Deterministic analyses results: 6 months dabigatran vs 3-6-12 months rivaroxaban.
Table 5. Deterministic analyses results: 6 months dabigatran vs 6 months rivaroxaban.
Discussion
Based on the ICs, conducted dabigatran and rivaroxaban appear to have a comparable efficacy profile in both patients with index VTE, and individually in index PE and index DVT; the risk of recurrent events was higher with dabigatran in index DVT and in combined DVT/PE, but lower in the index PE group, yet differences were not statistically significant in either of the analyzed groups. This was true for both the comparisons with rivaroxaban 3, 6, 12 months, and rivaroxaban 6 months treatment. Dabigatran was associated with a significantly lower risk of MCRBE in index DVT and in combined DVT/PE, both against 6 months rivaroxaban or the 3, 6, 12 months treatment set. Dabigatran had non-significantly lower rates of MCRBE in the index PE group.
Several other ICs of dabigatran and rivaroxaban have been published in treatment and extended anticoagulationCitation5–9. Yet, the analyzed safety and efficacy end-points deviated from the ones needed to populate our cost-effectiveness model. Specifically, Mantha and AnsellCitation5 compared rates of rVTE and death due to any cause in acute treatment studies, differently from VTE and VTE related deaths used in our model. Kang and SobierajCitation6 looked at rVTE, rPE, and rDVT and MBE, whereas we conducted our analysis looking at MCRBE. Hence, our results are not directly comparable with those already reported. Despite inherent limitations of IC methods, these remain the most appropriate means of substantiating relative safety and efficacy in lack of head-to-head trials, and are useful to reimbursement and formulary decision-makers to assist in informing analyses of value for money.
Indeed, our cost-effectiveness evaluations showed that dabigatran can be considered the dominant treatment strategy compared to rivaroxaban in the patients’ sub-groups considered, given the projected marginally higher clinical benefits and lower treatment costs.
The present analyses were intended to offer an indication of the relative clinical and cost benefits of dabigatran compared with rivaroxaban, also available in England and Wales, and did not attempt to compare dabigatran with other NOACs currently in development, such as apixaban and edoxaban. This is a recognized limitation of the present study and analyses will need to be updated as these new molecules will become available to patients and prescribers.
The ICs and cost-effectiveness analyses conducted showed that dabigatran and rivaroxaban are comparable in terms of their net clinical benefits and net costs. In deterministic analyses, dabigatran was projected dominant in all analyzed settings. Probabilistic analyses results showed a high likelihood of dabigatran being considered good value for money in the UK, in treatment, and in extended anticoagulation of VTE.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim International GmbH.
Declaration of financial/other relationships
Boehringer Ingelheim International GmbH employed IMS Health Consulting to conduct the present study and write the manuscript, of which MDF and ML are employees. TS and AU are employees of and VH is a consultant for Boehringer Ingelheim. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors of this paper acknowledge the work of Sorrel E Wolowacz (RTI) and James Brockbank (RTI) who supported the development of the cost-effectiveness model.
References
- Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med 2013;126:832
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64
- Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997;82:423-8
- Perrier A, Konstantinides S, Agnelli G, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315
- Mantha S, Ansell J. Indirect comparison of dabigatran, rivaroxaban, apixaban and edoxaban for the treatment of acute venous thromboembolism. J Thromb Thrombolysis 2015;39:155-65
- Kang N, Sobieraj DM. Indirect treatment comparison of new oral anticoagulants for the treatment of acute venous thromboembolism. Thromb Res 2014;133:1145-51
- Clemens A, Abeysinghe S, Gonschior AK, et al. Comparative efficacy and safety of dabigatran etexilate and rivaroxaban for the treatment of deep vein thrombosis and pulmonary embolism. Blood 2013;122:4807
- Rollins BM, Silva MA, Donovan JL, et al. Evaluation of oral anticoagulants for the extended treatment of venous thromboembolism using a mixed-treatment comparison, meta-analytic approach. Clin Ther 2014;36:1454-64
- Castellucci LA, Cameron C, Le Gal G, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ 2013;347:f5133
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72
- Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709-18
- EINSTEIN–PE investigators, Büller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97
- EINSTEIN investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
- Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134
- Jugrin AM, Ustyugova A, Urbich M, et al. The cost-utility of dabigatran etexilate compared with Warfarin in treatment and extended anticoagulation of acute venous thromboembolism in the UK. Thromb Haemost 2015;114. [Epub ahead of print]. doi: https://doi.org/http://dx.doi.org/10.1160/TH14-12-1027
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007;92:199-205
