Abstract
Objective:
This retrospective cohort study compared exacerbations, health services utilization, and costs among chronic obstructive pulmonary disease (COPD) patients who received nebulized arformoterol or nebulized formoterol therapy.
Methods:
Using PharMetrics Plus health plan claims, 417 nebulized long-acting β2-agonist (LABA) users meeting the study inclusion criteria were identified: had ≥2 fills of nebulized arformoterol or nebulized formoterol from January 1, 2009, to December 31, 2011, adhered to using their index drug ≥60% of the days during 1 year post-index, were ≥35 years old and continuously enrolled 180 days pre- and 1 year post-index, and did not use a nebulized LABA or have an asthma diagnosis during the pre-index period. Descriptive and multivariate analyses were performed.
Results:
A total of 274 nebulized arformoterol users and 143 nebulized formoterol users were identified with comparable demographic characteristics. However, significant differences were observed between the two groups in some clinical characteristics at index including comorbidities and use of antibiotics. At 1 year post-index, a lower proportion of nebulized arformoterol users had ≥1 exacerbation compared to nebulized formoterol users (70.4% vs 80.4%; p = 0.028). Among patients with ≥1 hospital admission, COPD-related costs per inpatient stay were significantly lower for nebulized arformoterol users than nebulized formoterol users (median = $9542 vs $14,025; p = 0.009). After controlling for confounders, nebulized arformoterol users had 19% marginally lower risk of exacerbations than nebulized formoterol users (hazard ratio = 0.81, 95% confidence interval = 0.64–1.03; p < 0.084) and 14.4% marginally lower COPD-related total costs at 1 year post-index (p = 0.062), primarily related to fewer hospital readmissions (7.6% vs 12.2%) and lower average costs per readmission stay (median = $7392 vs $18 081; p = 0.006).
Conclusions:
This study suggests that the choice of nebulized LABA may influence COPD-related exacerbation occurrence and costs. Future studies with larger and more closely matched nebulized arformoterol and nebulized formoterol users are needed to confirm these findings.
Introduction
Chronic obstructive pulmonary disease (COPD) is a serious public health challenge that adversely affects the lives of 12.7 million people in the US over 18 years of ageCitation1. Among the highest-risk Americans, those between the ages of 40–79 years, an estimated 14% have COPDCitation2. Since COPD is typically slowly progressive and takes years before symptoms are clinically evidentCitation3,Citation4, the Centers for Disease Control estimates its true prevalence to be close to 24 million US adultsCitation5.
COPD is now the third leading cause of death in AmericaCitation6. As a progressive condition, COPD significantly reduces health-related quality-of-life, and this negative association increases with disease severityCitation7–11. The disease also results in substantial and increasing economic burden over timeCitation12–16. Disease-specific costs for COPD in the US have been estimated at $49.9 billion annually, including $29.5 billion in direct healthcare expenditures and $20.4 billion in indirect costsCitation1. The majority of the cost burden associated with COPD is a consequence of increased health services utilization, most often related to hospitalizations and emergency department (ED) use following an exacerbation, an acute worsening of respiratory symptoms that is associated with poor outcomesCitation12,Citation17,Citation18. Annual healthcare costs are 10-fold greater for COPD patients with acute exacerbations than for COPD patients without exacerbationsCitation18. Lowering the frequency of exacerbations is, therefore, a major focus of COPD managementCitation18,Citation19.
Medications used to treat COPD include maintenance therapies (e.g., long-acting β2-agonists [LABAs], long-acting muscarinic antagonists [LAMAs], inhaled corticosteroids, and phosphodiesterase-4 inhibitors) and rescue therapies (e.g., short-acting β2-agonists [SABAs], short-acting muscarinic antagonists [SAMAs], and short-course systemic corticosteroids)Citation19,Citation20. The appropriate use of maintenance therapies has been shown to reduce the incidence of exacerbations and, consequently, reduce overall health services utilization and costs associated with the management of COPDCitation18,Citation21–24.
Critical to the proper management of COPD is the effective delivery of inhaled medications to the lungs. LABAs can be delivered via metered-dose inhaler (MDI), dry powder inhaler (DPI), or nebulizer. Despite the perceived simplicity of handheld inhaler devices, including pressurized MDIs and DPIs, incorrect use of these inhalers is common, resulting in increased healthcare resource utilization due to poor disease controlCitation25. In practice, effective inhaler use depends on a number of factors, such as manual dexterity, cognitive function, and hand strength, all of which are challenging for COPD patients with functional impairmentCitation26. In contrast, nebulizers are well received by COPD patients because they require minimal coordination and only tidal breathing, thereby facilitating medication deliveryCitation18,Citation26–28.
Current Food and Drug Administration–approved nebulizer formulations of LABAs in the US are arformoterol (BrovanaFootnote†) and formoterol (PerforomistFootnote‡). Arformoterol is the (R,R) enantiomer of racemic formoterol with full agonist activity at the beta-2 adrenergic receptor causing bronchial smooth muscle relaxation and inhibiting the release of inflammatory mediatorsCitation29,Citation30. Comparative safety and efficacy studies have been conducted between the two therapeutic agents for COPDCitation31–36 and, although their potential use in the clinical management of COPD patients has been summarizedCitation37, no studies to date have investigated the effects of nebulized arformoterol and nebulized formoterol on health and economic outcomes. Given this dearth in the literature, the purpose of this study was to use real-world evidence to compare the relative effects of nebulized formulations of arformoterol and formoterol on COPD-related exacerbations, health services utilization, and costs.
Methods
Data source
This retrospective cohort study used the PharMetrics Plus health plan claims database comprising adjudicated medical and pharmacy claims data for >150 million unique enrollees across the US. The data are longitudinal, with ∼22 million patients having 4 or more years of continuous enrollment in their health plan. The database is nationally representative of the commercially insured US population in terms of age and gender, with broad geographic coverage, including patients in each three-digit zip code area of the US and data from 90% of US hospitals, 80% of all US doctors, and 85% of employees of Fortune 100 companies.
The database contains comprehensive health-related information including inpatient and outpatient diagnoses recorded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; inpatient and outpatient procedures; dates of service; retail and mail-order prescription records; information on pharmacy and medical benefits (copayment/coinsurance amount, deductible, and in-network vs out-of-network); inpatient stay (diagnosis-specific admissions, admission type and source, and discharge status); and provider specifications (specialty, zip code, identification of attending, referring, rendering, prescribing, and primary care providers). Cost information includes amounts charged by providers, and amounts allowed and claims paid by health plans for all services rendered. Other data elements include demographic variables (e.g., patient age, gender, and geographic region), product type (e.g., health maintenance organization [HMO] or preferred provider organization [PPO]), payer type (e.g., commercial, self-pay), and start and stop dates of health-plan enrollment. Additional details regarding the content of the database are provided elsewhereCitation38.
Study design
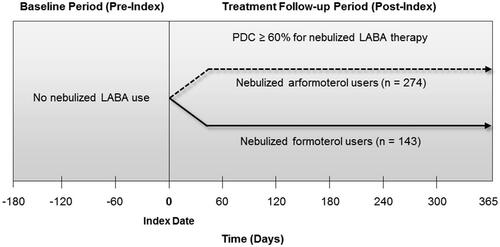
To assess the differential effects of nebulized arformoterol vs nebulized formoterol therapy on outcomes, COPD patients were retrospectively stratified based on their level of adherence to their LABA treatmentCitation39. The proportion of days covered (PDC) by prescription fills within 1 year post-index was used to differentiate patients who were adherent or partially adherent (PDC ≥ 60%) to their index drug from patients who were less adherent or non-adherent. Each patient was then followed for an 18-month period that was divided into two time segments, a 6-month pre-index or baseline period and a 12-month post-index or treatment follow-up period (see ). During the pre-index period, patients had no nebulized LABA use. The index date indicated the first use of a nebulized LABA. The impact of nebulized LABA therapy on outcomes was assessed 12 months after the index date.
Selection of study population
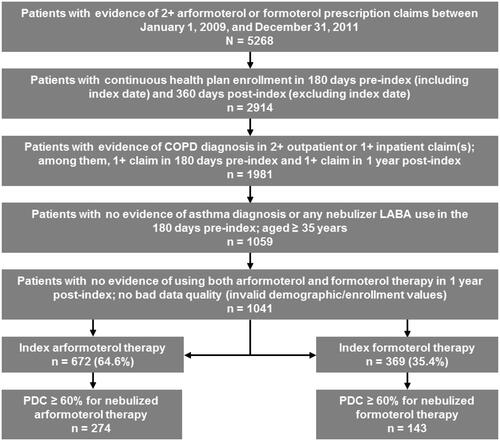
The study population was identified using the following inclusion criteria: (a) had ≥2 fills of nebulized arformoterol or nebulized formoterol from January 1, 2009 to December 31, 2011 (selection period for first nebulized LABA as index); (b) was continuously enrolled 180 days pre- and 360 days post-index; (c) had a COPD diagnosis (ICD-9-CM 491.x, 492.x, or 496.x) on ≥2 outpatient or ≥1 inpatient claims; and (d) was ≥35 years of age. The following exclusion criteria were also applied: (a) any use of nebulized LABA or asthma diagnosis during the 180-day pre-index period; (b) evidence of using both nebulized arformoterol and nebulized formoterol at any time in the 1-year post-index period; and (c) inconsistent or missing data specific to age and gender. The selection flow chart by eligibility criteria is shown in .
Measures and definitions
Three time periods were used to evaluate measures: at index, 180 days pre-index, and 1 year post-index. At index, the patients’ demographic characteristics, payer information, and health provider information were examined, including age, gender, geographic region, health plan type, and the prescribing physician's specialty. At 180 days pre-index, the patients’ clinical characteristics were examined including use of oral antibiotics; anticholinergic bronchodilator; steroid inhalant therapy; oral corticosteroids; non-nebulized LABA therapy and non-nebulized or nebulized short-acting bronchodilator therapy; spirometry use; the number and type of comorbidities using ICD-9 diagnosis codes for abnormal sputum (786.4), congestive heart failure (428.xx), cough (786.2), deficiency anemia (280.x, 281.x, 282.3, and 285.9), depression (296.2x, 296.3x, 296.9x, 298.0x, 300.4x, 301.12, 309.0, 309.1x, and 311.xx), diabetes (250.x), dyspnea and respiratory abnormalities (786), hemoptysis (786.3), hyperlipidemia (272, and 272.0 to 272.4), hypertension (401.xx to 405.xx), hypothyroidism (243.x and 244.x), and valvular disease (394.xx to 396.xx, 746.xx); and the Charlson Comorbidity Index scoreCitation40,Citation41.
At 1 year post-index, the following three outcome measures were evaluated: (1) COPD-related exacerbations defined based on the patient experiencing one or more of the following three types of qualifying events identified by using service claims with ICD-9 codes for COPD in the primary or secondary diagnosis fields: (a) a COPD-related hospitalization, (b) a COPD-related emergency department (ED) encounter, and/or (c) a COPD-related office encounter accompanied by antibiotics and/or corticosteroid prescription fills within 30 days after a physician office encounterCitation42,Citation43; (2) COPD-related health services utilization patterns including the number of outpatient pharmacy prescriptions, inpatient admissions, average annual inpatient days, average length in days of an inpatient stay, ED and office encounters, lab tests, and other services; and (3) COPD-related costs as measured by allowable amounts reported by payers adjusted to 2012 US dollars using the medical care component of the US Consumer Price Index.
The following approach was applied to identify COPD-related health services use and associated costs: (a) medical claims with a COPD diagnosis code (primary or secondary); (b) outpatient pharmacy claims for COPD-related medications (e.g., LABAs, LAMAs, phosphodiesterase-4 inhibitors, SABAs, and SAMAs); and (c) medical claims for COPD-related services (e.g., inhaled corticosteroids, spirometry).
Multiple exacerbation events with overlap in service dates were collapsed as one single event. Adherence to nebulized LABA therapy was defined as the time from index date of initiating nebulized LABA to the first medication gap of ≥60 consecutive days (i.e., without any drug supply of the index nebulized LABA) during the 1 year following initiation, or the end of study, whichever came first.
Statistical analyses
Comparisons between nebulized arformoterol and nebulized formoterol users were conducted using chi-square or Fisher’s exact test for categorical variables, and Student’s t-test or Wilcoxon rank-sum test for continuous or count variables. Descriptive information for categorical measures was examined as counts and proportions. Continuous variables were evaluated using the mean, standard deviation (SD), and median. A Cox proportional hazards model was employed to evaluate the risk of exacerbations as a function of index nebulized LABA and various demographic and clinical characteristics. Generalized linear models with negative binomial (for COPD-related health services utilization) and gamma (for cost) distributions were used to compare the two groups of nebulized LABA users in the 1-year post-index period, controlling for baseline demographic and clinical characteristics and pre-index COPD-related exacerbations. Variable selection for inclusion in models was based on prior subject matter knowledge, empirical observations, and sensitivity analyses. A p value <0.05 denoted statistical significance. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
Description of study population
Of the 1041 patients who met the study inclusion/exclusion criteria, 672 (64.6%) were nebulized arformoterol users and 369 (35.4%) were nebulized formoterol users (). Among these patients, 417 had a PDC ≥ 60%, of whom 274 (40.7%) were nebulized arformoterol users and 143 (38.8%) were nebulized formoterol users.
Demographic and clinical characteristics of the sudy population at index
At index, nebulized arformoterol users and nebulized formoterol users were comparable in demographic characteristics (). However, there were differences in several pre-index clinical characteristics. As compared to nebulized formoterol users, a significantly lower proportion of nebulized arformoterol users were taking an oral antibiotic (72.7% vs 61.6%, p = 0.024), using a nebulized SABA (46.8% vs 33.2%, p = 0.006), or had a COPD-related exacerbation (68.5% vs 56.9%, p = 0.021). However, nebulized arformoterol users had a significantly higher Charlson Comorbidity Index score on average relative to nebulized formoterol users (2.6 ± 1.84 vs 2.3 ± 1.80; p = 0.040). In addition, crude (unadjusted) total COPD-related costs were marginally lower for nebulized arformoterol users as compared to nebulized formoterol users (median = $2497 vs $2957, respectively; p = 0.054).
Table 1. Demographic and clinical characteristics of patients.
Occurrence of COPD exacerbation events 1 year post-index
Crude (unadjusted) results revealed that, compared to nebulized formoterol users, a significantly lower proportion of nebulized arformoterol users had ≥1 post-index exacerbation (70.4% vs 80.4%; p = 0.028). Among COPD patients who experienced an exacerbation post-index (n = 308), the majority in both groups (52.5% of nebulized arformoterol users and 56.6% of nebulized formoterol users) had at least two events annually, resulting in the use of >2 qualifying service types or office visits accompanied by antibiotics/corticosteroids treatment. No differences were observed in hospital admission rates associated with an exacerbation event between the two groups (8.4% admitted to the hospital in each group).
After adjusting for potential confounding factors, nebulized arformoterol users were found to have a 19% marginally lower risk of exacerbations than nebulized formoterol users (hazard ratio [HR] = 0.81, 95% confidence interval [CI] = 0.64–1.03; p < 0.10) (). Other covariates that significantly impacted post-index exacerbation risk included pre-index use of oral corticosteroids, which increased exacerbation risk by 107% (HR = 2.07, 95% CI = 1.59–2.69; p < 0.05), pre-index history of depression, which increased exacerbation risk by 120% (HR = 2.20, 95% CI = 1.48–3.27; p < 0.05), and being a non-PPO beneficiary (i.e., covered by indemnity, HMO, or other plans), which lowered exacerbation risk by 30% (HR = 0.70, 95% CI = 0.51–0.96; p < 0.05).
Table 2. Cox proportional hazard model of the risk of exacerbation 1 year post-index among adherent or partially adherent patients (n = 417).
Comparisons of COPD-related health services utilization patterns 1 year post-index
As shown in , both groups had similar COPD-related health services utilization patterns at 1 year post-index, with the exception of antibiotic use and ancillary services. On average, compared to nebulized formoterol users, nebulized arformoterol users had fewer antibiotics prescribed (3.8 ± 4.3 vs 3.0 ± 3.6 prescriptions; p = 0.035), but greater use of ancillary services (14.4 ± 10.8 vs 21.2 ± 24.7 visits; p = 0.006). COPD-related hospital admissions were not significantly different between the two groups, although a lower proportion of nebulized arformoterol users (7.6%) than nebulized formoterol users (12.2%) had hospital re-admissions within 30 days of being discharged. Adjusted results revealed no statistically significant differences in health services utilization patterns between the two groups.
Table 3. COPD-related health services utilization at 1-year post-index.
Comparisons of COPD-related healthcare costs 1 year post-index
No differences were found in unadjusted costs associated with pharmacy, outpatient, or inpatient services between nebulized arformoterol and nebulized formoterol users (). Total COPD-related medical care costs were also comparable between the two groups. However, among COPD patients with ≥1 hospital admission, COPD-related costs per inpatient stay were significantly lower for nebulized arformoterol users than for nebulized formoterol users (median = $9542 vs $14,025; p = 0.009). This trend was also observed among the few COPD patients (n = 11) who were re-admitted to the hospital within 30 days of being discharged, with nebulized formoterol users incurring more than twice the costs of nebulized arformoterol users per inpatient stay (median = $7392 vs $18,081; p = 0.006) and per inpatient day (median = $1357 vs $3094; p = 0.018). After controlling for confounding factors, nebulized arformoterol users had 14.4% marginally lower COPD-related costs at 1 year post-index than nebulized formoterol users (p = 0.062) ().
Table 4. COPD-related medical care costs at 1 year post-index.
Table 5. Generalized linear model (GLM) estimates of mean COPD-related total costs at 1 year post-index.
Discussion
To the best of our knowledge, this is the first study to comparatively examine the relative impact of nebulized arformoterol and nebulized formoterol on COPD-related exacerbations, health services utilization, and costs in a real-world setting. The study findings reveal that, although post-index exacerbations occurred in both groups of nebulized LABA users, exacerbation occurrence was 10% higher among patients on nebulized formoterol therapy than nebulized arformoterol therapy.
Other retrospective claims analyses have also reported COPD-related exacerbations to be high among patients on maintenance therapy. Pasquale et al.Citation44 found that 49.8% of COPD patients from a population predominantly on Medicare experienced an exacerbation, 13.9% had a severe exacerbation (defined as a COPD-related hospitalization or death within 7 days of a COPD diagnosis), 29.1% had a moderate exacerbation (defined as oral or parenteral corticosteroid use on the same day or within 7 days of a COPD-related diagnosis claim), and 6.8% had both a severe and a moderate exacerbation. AbuDagga et al.Citation45 found that 42.6% of COPD patients sampled from a large US commercial payer database had at least one exacerbation during the 1-year follow-up period after initiation of maintenance therapy. The number of post-index exacerbations in the current study was considerably higher than those reported by both Pasquale et al. and AbuDagga et al., most likely due to the varying definitions applied to classify an event as an exacerbation, and heterogeneity in the types of maintenance therapy included in the analysisCitation46. Factors such as disease severity and comorbidities may also explain differences in exacerbation rates.
Exacerbations are known drivers of higher inpatient service utilization and subsequent increases in healthcare costs in COPD patientsCitation15,Citation18,Citation47. The primary differences between the two groups were significantly higher average costs associated with hospital admissions as well as re-admissions within 30 days for nebulized formoterol users compared to nebulized arformoterol users. This finding could signal substantial economic savings related to the choice of nebulized LABA. For COPD patients with ≥1 hospital admission, the average cost per inpatient stay was ∼$4483 lower per patient for nebulized arformoterol users than nebulized formoterol users. Among patients who were re-admitted to the hospital within 30 days of being discharged, the cost for nebulized arformoterol users, on average, was less than half as much as for nebulized formoterol users per inpatient stay, resulting in a potential cost saving of $10,689 per patient.
Because this study is the first to compare health and economic outcomes between nebulized arformoterol and nebulized formoterol use, it is difficult to make comparisons with other research. Past studies have reported the average total medical costs following a severe COPD-related exacerbation event to be $18,120Citation45 per patient for a single event. Such costs are comparable only to the costs associated with re-admission for nebulized formoterol users in the current study, where the median cost per inpatient stay was $18,018. The differences between treatment groups in our study may be explained by potential differences in the clinical management of nebulized arformoterol and formoterol users and patient demographics. It is interesting to note that, at index, patients in the nebulized formoterol group had more antibiotic and SABA use, and had a history of more frequent exacerbations, while nebulized arformoterol users had a higher comorbidity score. The proportion of COPD patients who had spirometry was comparable in both groups (∼36%), but the actual values were not readily available to assess whether nebulized formoterol users had greater airway obstruction and, thus, may have had more severe COPD than nebulized arformoterol users. It would be important to further investigate the role of a patient’s clinical predisposition and severity in economic outcomes in this patient population.
Study limitations
The findings of this study must be viewed in light of certain limitations inherent to retrospective claims analysis. The study results are based on medical and pharmacy claims that do not provide information on whether nebulized arformoterol or nebulized formoterol medications were used as prescribed. In addition, any use of health services that might have occurred outside of the patient’s health insurance system would typically not have been recorded in the claims and not included in our analyses. The use of the measures ‘adherent’ or ‘partially adherent’ as eligibility criteria was intended to reduce this potential bias, but serve only as surrogate measures of compliance with medication useCitation48. In addition, diagnoses can be miscoded, and chart review and verification of data were not possible. Another potential limitation is that information specific to certain clinical measures related to severity of COPD, such as lung function, was not available.
A methodological limitation included the lack of matching between the two study groups. A possible lack of comparability between the two groups was suggested on some clinical indicators at pre-index, including antibiotic use, SABA use, and history of COPD-related exacerbations. The potential confounding effects of these variables were adjusted in multivariate models since matching was not a feasible option given the relatively small sample of adherent or partially adherent nebulized LABA users. Finally, the analytic focus was on patients who met continuous enrollment criteria (180 days pre- and 1 year post-index therapy), which may have excluded patients with different treatment patterns. This type of continuous enrollment restriction was needed to ensure that full details on the patients’ treatments were captured in the pre- and post-index periods, and to allow comparison between the two nebulized LABA groups.
Conclusions
COPD is a progressive illness. Appropriate management with maintenance therapy can reduce the frequency and severity of COPD-related exacerbations and lower the cost burden associated with inpatient care and repeated re-hospitalizationsCitation18. In this study on COPD burden among adherent and partially adherent users, fewer patients receiving nebulized arformoterol experienced an exacerbation than patients receiving nebulized formoterol. COPD patients on nebulized arformoterol therapy also had lower inpatient costs as well as lower 30-day re-admission costs compared to patients receiving nebulized formoterol. Since relatively small sample sizes and potential unadjusted confounders stemming from the study design may be partially responsible for some of our results, future prospective studies with larger and more closely matched samples of nebulized arformoterol and nebulized formoterol users are needed to confirm our preliminary findings.
Transparency
Declaration of funding
Funding for this study was provided by Sunovion Pharmaceuticals, Inc.
Declaration of financial/other relationships
VB is a full-time employee of Sunovion Pharmaceuticals, Inc. and was involved with study design, data interpretation, revision of manuscript for important intellectual content, and approval of the final manuscript. At the time of this study, YJC and CM were both full-time employees of IMS Health, who were contracted by Sunovion Pharmaceuticals, Inc. to design the study and perform data analysis. Both contributed to the manuscript for important intellectual content and approved its final version. MN and BRC are affiliated with Advance Health Solutions LLC (AHS) as a full-time employee and senior consultant with expertise in pulmonary medicine serving on AHS’ Medical Advisory Board, respectively. Both performed data interpretation, revised the manuscript for important intellectual content, and approved its final version. AHS received funding for data interpretation and manuscript development from Sunovion Pharmaceuticals, Inc.
Acknowledgments
We wish to thank Soojin Cho-Reyes, PhD, and Leighla H. Sharghi, BSN, MPA from Advance Health Solutions LLC, for their assistance with literature review and synthesis as well as draft manuscript preparation.
Previous presentations
Selected content of this study was presented, in part, at the annual meetings of the International Society for Pharmacoeconomics and Outcomes Research held in Montreal, Quebec, Canada, May 31–June 4, 2014, and the American College of Chest Physicians held in Austin, TX, October 25–30, 2014.
Notes
† Brovana, Sunovion Pharmaceuticals, Inc., Marlborough, MA.
‡ Perforomist, Dey Pharma, L.P., Napa, CA.
References
- American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. Chicago, IL: American Lung Association; 2013
- Tilert T, Dillon C, Paulose-Ram R, et al. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir Res 2013;14:103
- Anto JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J 2001;17:982-94
- Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-46
- Mannino DM, Homa DM, Akinbami LJ, et al. Chronic obstructive pulmonary disease surveillance – United States, 1971–2000. MMWR Surveillance Summaries 2002;51(SS06):1-16
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2013
- Wacker ME, Hunger M, Karrasch S, et al. Health-related quality of life and chronic obstructive pulmonary disease in early stages – longitudinal results from the population-based KORA cohort in a working age population. BMC Pulm Med 2014;14:134-45
- Janson C, Marks G, Buist S, et al. The Impact of COPD on health status: findings from the BOLD study. Eur Respir J 2013;42:1472-83
- Stahl E, Lindberg A, Jansson SA, et al. Health-related quality of life is related to COPD disease severity. Health Qual Life Outcomes 2005;9:3-56
- DiBonaventura MD, Paulose-Ram R, Su J, et al. The impact of COPD on quality of life, productivity loss, and resource use among the elderly United States workforce. COPD 2012;9:46-57
- Garrido PC, de Miguel Diez J, Gutierrez JR, et al. The negative impact of chronic obstructive pulmonary disease on the health-related quality of life of patients. Results of the EPIDEPOC study. Health Qual Life Outcomes 2006;4:31
- Dalal AA, Christensen L, Liu F, et al. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis 2010;5:341-9
- Dalal AA, Liu F, Riedel AA. Cost trends among commercially insured and Medicare Advantage-insured patients with chronic obstructive pulmonary disease: 2006 through 2009. Int J Chron Obstruct Pulmon Dis 2011;6:533-42
- Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013;5:235-45
- Toy EL, Gallagher KF, Stanley EL, et al. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD 2010;7:214-28
- Yu AP, Yang H, Wu EQ, et al. Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ 2011;14:315-23
- Blanchette CM, Dalal AA, Mapel D. Changes in COPD demographics and costs over 20 years. J Med Econ 2012;15:1176-82
- Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benefits 2014;7:98-106
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. Updated 2015. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 30, 2015
- Meyer KC. COPD 2013: an update on treatment and newly approved medications for pharmacists. J Am Pharm Assoc (2003) 2013;53:e219-29
- Bollu V, Ejzykowicz F, Rajagopalan K, et al. Risk of all-cause hospitalization in COPD patients initiating long-acting or short-acting beta agonist therapy. J Med Econ 2013;16:1082-8
- Bollu V, Ernst FR, Karafilidis J, et al. Hospital readmissions following initiation of nebulized arformoterol tartrate or nebulized short-acting beta-agonists among inpatients treated for COPD. Int J Chron Obstruct Pulmon Dis 2013;8:631-9
- Rodrigo GJ, Nannini LJ, Rodriguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest 2008;133:1079-87
- Wang J, Nie B, Xiong W, et al. Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis. J Clin Pharm Ther 2012;37:204-11
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930-8
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm 2011;68:1221-32
- Dhand R, Dolovich M, Chipps B, et al. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD 2012;9:58-72
- Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care 2005;50:1313-21; discussion 1321–2
- Handley D, Senanayake C, Dutczak W, et al. Biological actions of formoterol isomers. Pulm Pharmacol Ther 2001;15:135-45
- Pahwa R, Soni V, Sharma PC, et al. Arformoterol tartrate: a review of pharmacology, analysis and clinical studies. Trop J Pharm Res 2010;9:595-603
- Hanania NA, Donohue JF, Nelson H, et al. The safety and efficacy of arformoterol and formoterol in COPD. COPD 2010;7:17-31
- Baumgartner RA, Hanania NA, Calhoun WJ, et al. Nebulized arformoterol in patients with COPD: a 12-week, multicenter, randomized, double-blind, double-dummy, placebo- and active-controlled trial. Clin Ther 2007;29:261-78
- Berger WE, Nadel JA. Efficacy and safety of formoterol for the treatment of chronic obstructive pulmonary disease. Respir Med 2008;102:173-88
- Cazzola M, Hanania NA, Matera MG. Arformoterol tartrate in the treatment of COPD. Expert Rev Respir Med 2010;4:155-62
- Gross NJ, Donohue JF. Nebulized formoterol: a review of clinical efficacy and safety in COPD. Int J Chron Obstruct Pulmon Dis 2010;5:223-32
- Hanrahan JP, Hanania NA, Calhoun WJ, et al. Effect of nebulized arformoterol on airway function in COPD: results from two randomized trials. COPD 2008;5:25-34
- Miles MC, Donohue JF, Ohar JA. Nebulized arformoterol: what is its place in the management of COPD? Ther Adv Respir Dis 2013;7:81-6
- IMS Health. IMS’ PharMetrics Plus™ Data Dictionary. http://tri.uams.edu/wp-content/uploads/2011/10/PMXPlusDataDictionaryAugust2013.dotx.pdf. Accessed March 30, 2015
- Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Low use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general population. J Gen Intern Med 2015;30:51-9
- D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993;32:382-7
- Roos LL, Stranc L, James RC, et al. Complications, comorbidities, and mortality: improving classification and prediction. Health Serv Res 1997;32:229-38; discussion 239–42
- Donaldson GC, Mullerova H, Locantore N, et al. Factors associated with change in exacerbation frequency in COPD. Respir Res 2013;14:79
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38
- Pasquale MK, Sun SX, Song F, et al. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis 2012;7:757-64
- AbuDagga A, Sun SX, Tan H, et al. Exacerbations among chronic bronchitis patients treated with maintenance medications from a US managed care population: an administrative claims data analysis. Int J Chron Obstruct Pulmon Dis 2013;8:175-85
- Seemungal TA, Hurst JR, Wedzicha JA. Exacerbation rate, health status and mortality in COPD—a review of potential interventions. Int J Chron Obstruct Pulmon Dis 2009;4:203-23
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5S-9S
- Aronson JK. Compliance, concordance, adherence. Br J Clin Pharmacol 2007;63:383-4