Abstract
Background:
Posaconazole is superior to fluconazole/itraconazole in preventing invasive fungal diseases (IFDs) in neutropenic patients. Whether the higher cost of posaconazole is offset by decreases in IFDs in a given institute requires cost-effective analysis encompassing the spectrum of IFDs and socioeconomic factors specific to that geographic area.
Methods:
This study performed a cost-effective analysis of posaconazole prophylaxis for IFDs in an Asian teaching hospital, employing decision modeling and data of IFDs and medication costs specific to the institute, in neutropenic patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).
Results:
In the cost-effectiveness analysis, the higher cost of posaconazole was partially offset by a reduction in the cost of treating IFDs that were prevented, resulting in an incremental cost of 125,954 Hong Kong dollars/16,148 USD per IFD avoided. Over a lifetime horizon, assuming same case fatality rate of IFDs in both groups, use of posaconazole results in 0.07 discounted life years saved. This corresponds to an incremental cost of 116,023 HKD/14,875 USD per life year saved. This incremental cost per life year saved in posaconazole prophylaxis fulfilled the World Health Organization defined threshold for cost-effectiveness.
Conclusion:
Posaconazole prophylaxis was cost-effective in Hong Kong.
Introduction
Invasive fungal diseases (IFDs) are an important threat to patients with hematological malignancies, and are associated with significant morbidity and mortality. Patients who undergo induction chemotherapy for acute myeloid leukemia (AML) and hematopoietic stem cell transplantation (HSCT) are at high risk of developing IFDs. Despite the availability of potent antifungal drugs, the outcome of established IFDs remains dismal, with mortality rates often exceeding 50%Citation1.
IFDs are also associated with significant economic impacts. In patients with invasive apsergillosis, the cost of management could be 3–4-times higher than that of patients without IFD, mainly due to prolonged hospitalizationCitation2. Prophylaxis against IFDs potentially reduces the clinical and economic burden associated with IFDs in high-risk patients and is recommended by international guidelinesCitation3–5.
Posaconazole, a broad-spectrum triazole, has been shown to be effective as a prophylaxis in reducing IFDs in high-risk patients. However, its cost is comparatively higher than other commonly used azoles such as fluconazole and itraconazole. Therefore, it is important to define whether the cost of pozaconazole prophylaxis may be offset by the reduction of IFDs and, hence, the expenditure of their management.
Pharmacoeconomic studies evaluating cost-effectiveness of posaconazole prophylaxis had previously been performed in North America and EuropeCitation6–13. These studies were either performed in neutropenic patientsCitation6–8,Citation12,Citation13 or in high-risk hematologic patients undergoing chemotherapyCitation9–11. The results generally showed that posaconazole prophylaxis was both cost-effective and cost-saving. However, different countries have different incidences and spectra of IFDs, as well as diverse socioeconomic factors. Hence, data of cost effective analysis are not applicable to all geographic areas. Cost-effective analysis of posaconazole has never before been performed in Asia, which has its specific spectra of IFDsCitation14. In this study, we analyzed the cost-effectiveness of posaconazole prophylaxis against fluconazole or itraconazole in a cohort of patients with AML or myelodysplastic syndrome (MDS) treated in an Asian teaching hospital.
Materials and methods
Decision analytical model structure
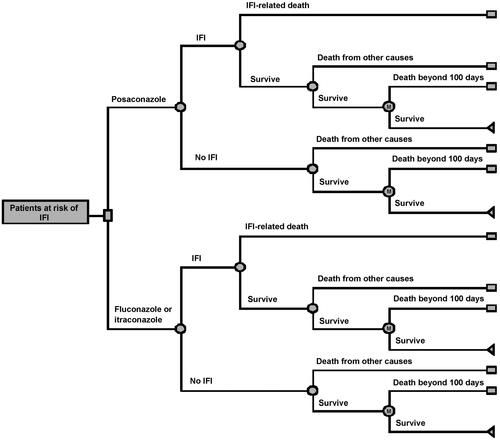
This analysis was based on and adapted from a cost-effectiveness model structure previously publishedCitation8. In this decision tree, the decision nodes denoted the decision to use posaconazole or the alternative of either fluconazole or itraconazole for prophylaxis. This model also paralleled the phase III pivotal trial showing superiority in efficacy of posaconazole over fluconazole or itraconazole in neutropenic patientsCitation15. The initial part of the decision tree represented the first 100 days of treatment, corresponding to the length of follow-up from this pivotal trialCitation15. Patients surviving this 100-day interval entered a Markov subtree, in which they progressed in 1-month increments. The risk of death in this progression was determined by the monthly risk of death from the underlying conditions (AML or MDS) and was independent of prior IFDs. The progression continued until all patients in the cohort had died. The chance nodes in the first 100 days were the probabilities that modeled the clinical events of having an IFD, surviving or dying from the IFD and dying from the underlying primary illnesses ().
Model probabilities, costs, and outcomes
The model inputs included the probability of an IFD according to treatment groups; the probability of death due to an IFD according to treatment groups; the probability of death from other causes; the 5-year relative survival for AML and MDSCitation16,Citation17; the IFD treatment costs; drug costs for posaconazole, fluconazole, and itraconazole; the mean duration of prophylaxis; and the discount rateCitation18,Citation19. The mean values for the key inputs as well as the standard deviations (SD), and the distributions used in the probabilistic sensitivity analysis (PSA), were listed in . The inputs specific to Hong Kong included the probability of dying by age (derived from the Hong Kong Census and Statistics Department)Citation20; the estimated cost (Hong Kong dollars, HKD; United States dollars, USD) of treating a patient with an IFD in Hong Kong; the daily drug costs for posaconazole (558 HKD/71.9 USD), itraconazole (132 HKD/17.0 USD), and fluconazole (7.2 HKD/0.9 USD); and the proportion of using prophylactic itraconazole (95%) in comparison with fluconazole (5%). All estimated costs were obtained from the Hospital Authority Pharmacy of Hong Kong. The proportion of use of itraconazole was based on practice data from Queen Mary HospitalCitation21,Citation22. The estimated management cost for an IFD case in Hong Kong comprised the cost of one additional week of hospital stay, and the cost of the antifungal drug (based on the use of voriconazole; intravenously 400 mg 12 hourly for 1 day and 200 mg 12 hourly for 1 week, then orally 200 mg 12 hourly for 6 weeks), which amounted to 76,469 HKD/9854 USD. The cost of diagnosis and monitoring was nominal and, therefore, not included. The outcome measures included the proportion of patients experiencing IFD, the incremental cost per IFD event avoided, and the incremental cost per life-year saved (LYS), over a lifetime period.
Table 1. Model parameters adopted.
Assumptions
Two different case scenarios were used for cost-assessment. Scenario one adopted the assumption that there was no difference in mortality due to IFD in the posaconazole vs fluconazole/itraconazole groupsCitation8. Scenario two was modeled on a lower mortality rate due to IFDs in the posaconazole group, based on the assumption that published results were repeatableCitation15. In scenario one, the mortality due to an IFD was assumed to be 45% for both the posaconazole and fluconazole/itraconazole groups during the first 100 days of prophylaxis. In scenario two, the probability of an IFD-related death within 100 days of prophylaxis was assumed to be 36% in the posaconazole group and 48% in the fluconazole/itraconazole group. These probabilities were derived from published figures, on the assumption that fungal infections were of similar clinical behavior in comparable patient populationsCitation15.
PSA
This was done for the two scenarios described. Two different thresholds were used: 50,000 USD and the gross domestic product (GDP) per capita based on the World Health Organization recommendationCitation23. Both thresholds have been used in the past for studies in Hong Kong. The GDP per capita in Hong Kong was 36,708 USD or 284,854 HKD during the study period (GDP per capita, 2012)Citation24. The parameters that were allowed to vary using the distributions described in were the probability of developing an IFD, probability of death during an IFD, the probability of death unrelated to IFD during treatment (before entering the Markov chain), and the duration of treatment. The PSA results were based on 1000 samples.
Results
Cost-effective analysis
During the 100-day clinical trial period, the drug costs were 16,126 HKD in the posaconazole group compared with 3131 HKD in the fluconazole/itraconazole group (). In scenario one, the higher cost for posaconazole was partially offset by the lower costs of treating IFDs due to the greater efficacy of prophylaxis and fewer IFDs in the posaconazole group. IFD treatment costs were 3503 HKD in the posaconazole group compared with 8412 HKD for the fluconazole/itraconazole group. In total, the costs for the posaconazole group were 8086 HKD more than the fluconazole/itraconazole group. There were 0.064 IFDs avoided during the first 100-day clinical trial period when using posaconazole as compared with fluconazole/itraconazole, corresponding to an incremental cost of 125,954 HKD/16,148 USD per IFD avoided. When the analysis was extended beyond the duration of the clinical trial (after the first 100 days) there were 0.07 discounted life years saved and the incremental cost per life year saved was 116,023 HKD/14,875 USD in scenario one. In scenario two, using the lower rate of IFD-related death for posaconazole, there were 0.09 discounted life years saved, with an incremental cost per life year saved of 89,988 HKD/11,537 USD projected over the lifetime of the patient population. These results are summarized in .
Table 2. Cost-effectiveness of posaconazole vs fluconazole/itraconazole in the prevention of invasive fungal diseases (IFD) among high-risk neutropenic patients based on two scenarios.
PSA
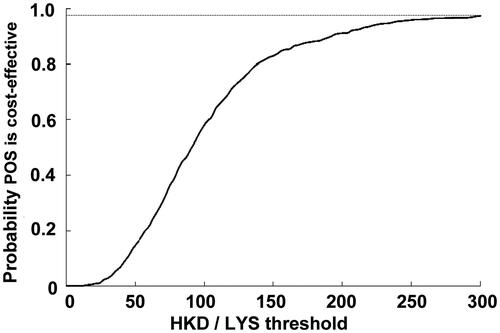
In scenario one, the PSA analysis, allowing for all the probabilities to vary around the calculated mean, showed there was a 92% probability that posaconazole would come under the 50,000 USD per life year gained threshold (i.e. cost effective), and an 87.4% probability that posaconazole would be under the 285,000 HKD based on the GDP per capital in Hong Kong. The second scenario increased this probability to 96.6% using the same thresholds for cost-effectiveness. Scatterplots with the percentage of scenarios below the 285,000 HKD per LYS are shown for both scenarios in and , respectively. All the points below the diagonal line come under the 285,000 per LY saved threshold. Similarly, PSA curves showing probabilities of different thresholds are shown in and , respectively.
Figure 2. Scatter-plot of 1000 incremental cost and incremental life-year pairs for posaconazole vs fluconazole or itraconazole according to scenario 1, which assumed that there was no difference in mortality associated with IFD in different prophylactic groups. PSA, probabilistic sensitivity analysis; ICER, incremental cost-effectiveness ratio; LYS, life-years saved.
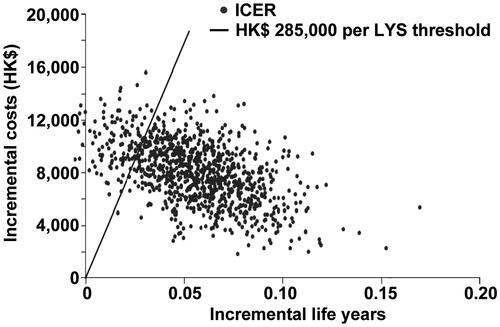
Figure 3. Scatter-plot of 1000 incremental cost and incremental life-year pairs for posaconazole vs fluconazole or itraconazole according to scenario 2, which assumed that there was a lower mortality associated with IFD in the posaconazole group as compared with the fluconazole/itraconazole group, according to published resultsCitation9. PSA, probabilistic sensitivity analysis; ICER, incremental cost-effectiveness ratio; LYS, life-years saved.
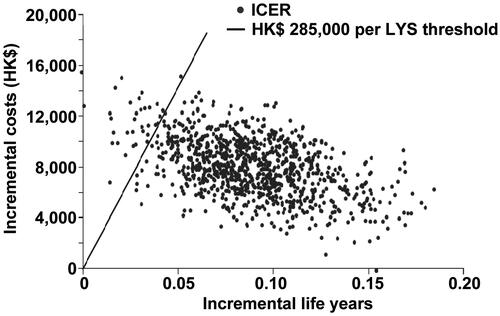
Figure 4. Probablitiy that posaconazole was cost-effective vs fluconazole or itraconazole for the prevention of IFD according to scenario 1, which assumed that there was no difference in mortality associated with IFD in different prophylactic groups. CEAC, cost-effectiveness acceptability curve; LYS, life-years saved; POS, posaconazole.
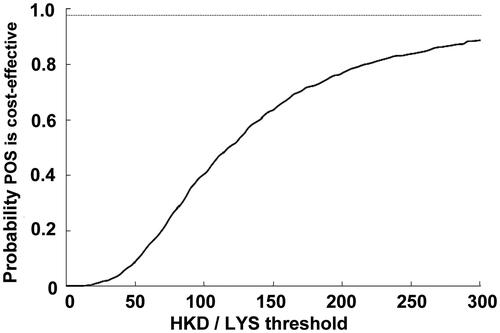
Figure 5. Probability that posaconazole was cost-effective vs fluconazole or itraconazole for the prevention of IFD according to scenario 2, which assumed that there was a lower mortality associated with IFD in the posaconazole group as compared with the fluconazole/itraconazole group, according to published resultsCitation9. CEAC, cost-effectiveness acceptability curve; LYS, life-years saved; POS, posaconazole.
Discussion
In a pivotal phase 3 studyCitation15, posaconazole demonstrated statistically significant superiority over fluconazole/itraconazole in the reduction of IFDs and IFD-related deaths in neutropenic patients following induction chemotherapy. Despite these findings, inconvenient formulation (oral solution is the only formulation available in most countries) and high drug cost had limited the use of posaconazole. It is also unknown whether the expenditures saved by reduction in breakthrough IFDs could possibly offset the high cost of posaconazole prophylaxis. Cost-effectiveness studies using decision modeling were performed in several countries, including the US, Canada, Switzerland, France and the NetherlandsCitation6–13, all favoring posaconazole as a cost-saving drug over fluconazole or itraconazole. Using similar modeling combined with local data, we were able to perform a cost-effective analysis of posaconazole prophylaxis under the healthcare settings in Hong Kong. The robustness of the analysis was further enhanced by employing two different scenarios (whether posaconazole might or might not reduce IFD-related mortality) and PSA (where the input variables were allowed to vary according to their distributions).
In this study, we were not able to confirm that the use of prophylactic posaconazole was cost-saving, as it was more expensive to prescribe posaconazole prophylaxis than to treat breakthrough IFDs when patients were on conventional triazoles. Nevertheless, monetary concerns should not be the only parameter on which to gauge the usefulness of prophylaxis. Although it might be less expensive to treat a smaller number of IFDs than to provide prophylaxis to a larger number of patients, established IFDs have profound impacts on the subsequent management options for afflicted patients. Therefore, IFDs actually have far-reaching implications on the consequent economic and human costs.
We were, however, able to show that the use of posaconazole prophylaxis was cost-effective, as the costs incurred to avoid one IFD or save one life-year were well under the GDP per capita of Hong Kong, a value used by the World Health Organizations (WHO) to define local cost-effectiveness of healthcare interventions.
There are several limitations in our study. The analysis was performed through statistical modeling simulating real life situations by fitting in data specific to Hong Kong. However, such a simulation might have deviations from the actual incidences and expenses involved. Secondly, some of the data inputs in the analysis were borrowed from other larger studies involving a substantial number of patients (the incidence of breakthrough IFD and the 5-year survivals of patients with AML or MDS). Local data derived from a smaller number of patients might not necessarily be similar. Third, we used data from early registries and studiesCitation16,Citation17 for our analysis. The treatment of AML and MDS has not undergone major changes in the last decade and survivals have been similar. To address the issue as to whether similar conclusions might be reached with more current studies, we performed another analysis employing two recent data setsCitation25,Citation26. The results showed only a slight reduction in incremental cost per life year saved, with the overall findings unchanged (Supplemental file 1). Finally, another important limitation is that our analysis might have under-estimated the costs of the management of established IFD, including treatment in the intensive care unit, hospitalization of longer than 7 days, and anti-fungal treatment of more than 6 weeks. Other hidden costs due to invasive procedures, laboratory tests and radiological investigations would also be difficult to estimate, because Hong Kong runs a national healthcare system, so that these costs are not normally calculated. These extra costs in the treatment of IFDs would put posaconazole into an even more favorable economic position and strengthen the conclusion that posaconazole prophylaxis is cost-effective compared with fluconazole/itraconazole prophylaxis.
We conclude that, with decision modeling, posaconazole was a cost-effective alternative to other conventional triazoles fluconazole and itraconazole under the healthcare system in Hong Kong. However, different from studies conducted in North America and EuropeCitation6–13, posaconazole was not found to be cost-saving. Hence, our observations highlight the fact that results from pharmacoeconomic studies may be region- or country-specific. Whether a universal switch to posaconazole prophylaxis is warranted depends on other factors, including the actual number of patients requiring such treatment and the availability of hospital funding.
Transparency
Declaration of funding
No funding was declared for this manuscript.
Declaration of financial/other relationships
SWM is an employee of Merck & Co. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary material available online Supplemental file 1
Supplemental_file.docx
Download MS Word (14.3 KB)References
- Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 2012;125(1 Suppl):S3-13
- Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health 2002;5:26-34
- Cornely OA, Böhme A, Buchheidt D, et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica 2009;94:113-22
- Segal BH, Freifeld AG, Baden LR, et al. Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 2008;6:122-74
- Walsh TJ, Anaissie EJ, Denning DW, et al; Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327-60
- Collins CD, Ellis JJ, Kaul DR. Comparative cost-effectiveness of posaconazole versus fluconazole or itraconazole prophylaxis in patients with prolonged neutropenia. Am J Health Syst Pharm 2008;65:2237-43
- Stam WB, O'Sullivan AK, Rijnders B, et al. Economic evaluation of posaconazole vs. standard azole prophylaxis in high risk neutropenic patients in the Netherlands. Eur J Haematol 2008;81:467-74
- O'Sullivan AK, Pandya A, Papadopoulos G, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health 2009;12:666-73
- Greiner RA, Meier Y, Papadopoulos G, et al. Cost-effectiveness of posaconazole compared with standard azole therapy for prevention of invasive fungal infections in patients at high risk in Switzerland. Oncology 2010;78:172-80
- Dranitsaris G, Khoury H. Posaconazole versus fluconazole or itraconazole for prevention of invasive fungal infections in patients undergoing intensive cytotoxic therapy for acute myeloid leukemia or myelodysplasia: a cost effectiveness analysis. Support Cancer Care 2011;19:1807-13
- Michallet M, Gangneux JP, Lafuma A, et al. Cost effectiveness of posaconazole in the prophylaxis of invasive fungal infections in acute leukaemia patients for the French healthcare system. J Med Econ 2011;14:28-35
- Tahami Monfared AA, O'Sullivan AK, Rotstein C, et al. Economic evaluation of posaconazole versus standard azole therapy as prophylaxis against invasive fungal infections in patients with prolonged neutropenia in Canada. Can J Infect Dis Med Microbiol 2012;23:59-64
- Lundberg J1, Höglund M, Björkholm M, et al. Economic evaluation of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infection in high-risk neutropenic patients in Sweden. Clin Drug Investig 2014;34:483-9
- Hsu LY, Lee DG, Yeh SP, et al. Epidemiology of invasive fungal diseases among patients with haematological disorders in the Asia-Pacific: a prospective observational study. Clin Microbiol Infect 2015;21:594.e7-e11
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348-59
- National Cancer Institute. SEER Cancer Statistics Review 1975–2003. Age-adjusted SEER incidence and US death rates and 5-year relative survival rates. 2006. http://seer.cancer.gov/csr/1975_2003/results_single/sect_01_table.04_2pgs.pdf. Accessed April 21, 2015
- Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer 2006;106:1099-109
- Gold MR, Siegel JE, Russell LB, et al, eds.Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
- Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health 2004;7:397-401
- HKSAR Go. Hong Kong Life Tables 1971–2012. Census and Statistics Department, 2012
- Edejer TT-T, World Health Organization. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization, 2003
- Chan TS, Hwang YY, Gill H, et al. Antifungal drug usage in haematologic patients during a 4-year period in an Asian university teaching hospital. Intern Med J 2013;43:541-6
- Edejer TT-T, World Health Organization. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization, 2003
- National income, Census and Statistics Department, Hong Kong Special Administrative Region. http://www.censtatd.gov.hk/hkstat/sub/sp250.jsp?tableID=030&ID=0&productType=8. Accessed March 27, 2015
- SEER Cancer Statistics Review (CSR) 1975–2012. National Cancer Institute, http://seer.cancer.gov/csr/1975_2012/. Accessed August 28, 2015
- Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012;120:2454-65