Abstract
Background
For many years, the standard of care for patients diagnosed with deep vein thrombosis (DVT) has been low-molecular-weight heparin (LMWH) bridging to an oral Vitamin-K antagonist (VKA). The availability of new non-VKA oral anticoagulants (NOAC) agents as monotherapy may reduce the likelihood of hospitalization for DVT patients.
Objective
To compare hospital visit costs of DVT patients treated with rivaroxaban and LMWH/warfarin.
Methods
A retrospective claim analysis was conducted using the MarketScan Hospital Drug Database for care provided between January 2011 and December 2013. Adult patients using rivaroxaban or LMWH/warfarin with a primary diagnosis of DVT during the first day of a hospital visit were identified (i.e., index hospital visit). Based on propensity-score methods, historical LMWH/warfarin patients (i.e., patients who received LMWH/warfarin before the approval of rivaroxaban) were matched 4:1 to rivaroxaban patients. The hospital-visit cost difference between these groups was evaluated for the index hospital visit, as well as for total hospital-visit costs (i.e., including index and subsequent hospital visit costs).
Results
All rivaroxaban users (n = 134) in the database were well-matched with four LMWH/warfarin users (n = 536). The mean hospital-visit costs were $5257 for the rivaroxaban cohort and $6764 in the matched-cohort of patients using LMWH/warfarin. The $1508 cost difference was statistically significant between cohorts (95% CI = [−$2296; −$580]; p-value = 0.002). Total hospital-visit costs were lower for rivaroxaban compared to LMWH/warfarin users within 1, 2, 3, and 6 months after index visit (significantly lower within 1 and 3 months, p-values <0.05)
Limitations
Limitations were inherent to administrative-claims data, completeness of baseline characteristics, adjustments restricted to observational factors, and lastly the sample size of the rivaroxaban cohort.
Conclusion
The availability of rivaroxaban significantly reduced the costs of hospital visits in patients with DVT treated with rivaroxaban compared to LMWH/warfarin.
Introduction
For the last two decades, therapy with low-molecular-weight heparin (LMWH) bridging to an oral vitamin-K antagonist (VKA), typically warfarin, has been the standard of care for patients diagnosed with venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE)Citation1. This treatment was often initiated in the hospital, and requires coagulation monitoring with serial assays of the international normalized ratio (INR) and directed dose adjustments, all adding to the cost and inconvenience of this approach.
In the last few years, large international phase-III clinical trials comparing that standard of care to the non-VKA oral anticoagulants (NOAC) agents rivaroxaban, dabigatran, apixaban, and edoxaban have shown similar or superior efficacy and safety of these new agentsCitation2–6. More recently, the US Food and Drug Administration (FDA) approved rivaroxaban (in November 2012), dabigatran (in April 2014), apixaban (in August 2014), and edoxaban (in January 2015) for the prevention and treatment of VTECitation7–10. As the NOAC agents rivaroxaban and apixaban do not require neither bridging nor lead-in therapy with parenteral agent, they may offer the advantage of convenient initiation of therapy and discharge home from the site of diagnosis (frequently an emergency department [ED]), thereby potentially decreasing costs.
With an estimated prevalence of 950,000 VTE patients (of which two thirds were diagnosed with DVT) in the US in 2006 that is expected to increaseCitation11–13, the impact of these new agents is of importance for the healthcare system. The purpose of this analysis is to compare hospital visit costs among a sample of patients with DVT treated with rivaroxaban, with a matched sample of patients treated with LMWH/warfarin, in a real world setting.
Methods
Data source
Between January 2011 and December 2013, health-insurance claims from the Truven MarketScan Hospital Drug Database (HDD) for care were used to conduct the study. MarketScan HDD is a large US hospital-based, service-level database that represents more than 550 teaching and non-teaching hospitals. In comparison to traditional claims data in which the information was collected from the insurer’s point of view, the MarketScan HDD database contains information collected from the hospital’s point of view. The MarketScan HDD includes patient information such as demographics (e.g., age, gender, primary-payer type, geography), primary and secondary diagnoses, primary and secondary procedures, length of hospital stay, drug-administration on a daily basis, department charge and cost details, days-of-stay, and primary physician’s specialty. The MarketScan HDD data are de-identified and fully compliant with all Health Insurance Portability and Accountability Act (HIPAA) privacy and security requirements to protect the anonymity and confidentiality of the patients.
Study design
A retrospective matched-cohort design was used to compare hospital visit costs among a population of patients with DVT who presented at a hospital and were treated with rivaroxaban and patients treated with LMWH/warfarin before November 2012 (historical cohort). Although different research questions and, therefore, different endpoints were evaluated, the study employed the same sample of patients with DVT treated with either rivaroxaban or LMWH/warfarin as the one reported in a study previously published in Hospital Practice by the present group of authorsCitation14. As in the previous study, rivaroxaban users were not matched to current warfarin users because the availability of rivaroxaban would have already impacted this comparison. Rivaroxaban users were matched to LMWH/warfarin users based on patient characteristics.
Patients included in the study had a primary diagnosis of DVT during the first day of a hospital-based evaluation (i.e., index hospital visit), were administered rivaroxaban or LMWH/warfarin (before November 2, 2012) during that first day, and were aged 18 years or older. Patients were excluded if they were diagnosed with a PE during the associated inpatient stay. Patients were observed until discharge (i.e., only the first day if they were discharged within 24 hours of their hospital visit or until the date of discharge from the hospital if they had been admitted to hospital). Records up to 12 months before and on the index hospital visit were used to evaluate the baseline characteristics.
Study endpoints
The main endpoint of this study was the total cost associated with the index hospital visit of patients with DVT, including costs of patients who were admitted and discharged the same day as well as the costs of patients who were hospitalized. Costs of the index hospital visit were also stratified between patients hospitalized and patients admitted and discharged the same day. In addition, the total hospital visit costs including the index and subsequent hospital visits (i.e., hospital visits after the index hospital visit) within 1, 2, 3, and 6 months after the index hospital visit were determined.
Statistical analysis
Each patient in the rivaroxaban cohort was matched with four patients from the LMWH/warfarin cohort using propensity-score calipers of 5%. Propensity scores were calculated using a multivariate logistic-regression model that incorporated the following baseline characteristics: age, gender, primary-payer type, geographic region of the hospital, hospital characteristics (i.e., urban, large [≥500 beds], teaching), admission source (i.e., physician referral, clinical referral, and transfer from hospital), and VTE risk factors (e.g., age at least 60 years, active malignancy, congestive heart failure).
Cost differences between rivaroxaban treated patients and their matched LMWH/warfarin patients were assessed with a gamma distribution and a log link. In the HDD database, data on the hospital charges were available for all patients. However, hospital costs were available for only 89% of rivaroxaban users and 81% of LMWH/warfarin users. For the subset of patients with only charges information, the costs were imputed by applying the mean charge to cost ratio of each cohort to obtain costs for all patients. All costs were inflation-adjusted to 2013 $US based on the medical-care component of the Consumer Price Index.
The total hospital visit cost including the index and subsequent hospital visits was also reported for patients treated with rivaroxaban and their matched patients treated with LMWH/warfarin among subgroups of patients whose follow-up (time to end of data) was at least 1 month, 2 months, 3 months, and 6 months. Patients in the rivaroxaban cohort were matched exactly with four patients of the LMWH/warfarin cohort using propensity-score calipers of 5% to form the rivaroxaban and LMWH/warfarin matched-cohorts among patients with sufficient follow-up for each of the time period evaluated (i.e., 1, 2, 3, and 6 months). Comparisons between cohorts were assessed based on the incremental total hospital visit costs, with a gamma distribution and a log link.
Descriptive characteristics were generated to summarize the baseline characteristics of both the unmatched- and matched-cohorts. Means, medians, and standard deviations (SDs) were used to describe continuous variables, while frequencies and percentages were used to describe categorical variables. Standardized differences were used to compare the baseline characteristics between cohorts and all baseline characteristics with standardized differences below 10% were considered well balancedCitation15–17. All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
summarizes the study sample selection. The rivaroxaban and LMWH/warfarin cohorts included 134 and 6347 patients, respectively. The 134 rivaroxaban patients were matched with 536 LMWH/warfarin patients. After matching, the mean age of both cohorts was 62 years and ∼50% of the patients were female (). The most prevalent risk factors for VTE were hypertension, age ≥60 years and tobacco use in both cohorts.
Figure 1. Patients’ disposition. DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PE, pulmonary embolism. This figure has been adapted from: Merli GJ, Hollander JE, Lefebvre P, et al. Rates of hospitalization among patients with deep vein thrombosis before and after the introduction of rivaroxaban. Hospital practice (1995). 2015;43(2):85–93, with permission from Taylor & Francis Ltd (http://www.tandfonline.com/).
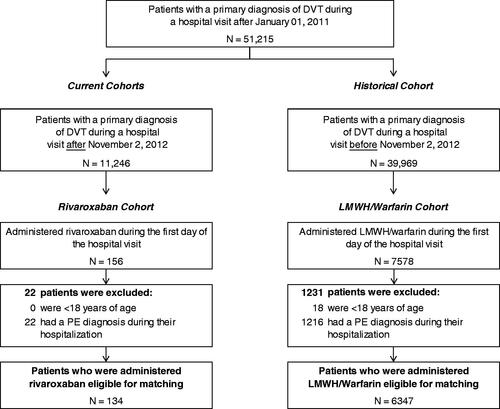
Table 1. Patients demographics and clinical characteristics—unmatched and matched rivaroxaban and LMWH/warfarin cohorts.*
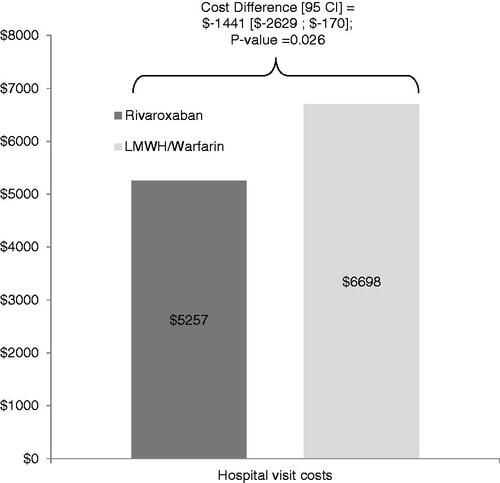
presents the index hospital visit cost of the rivaroxaban and matched LMWH/warfarin cohort, the cost difference and the corresponding 95% confidence interval and the p-value. The mean index hospital visit cost was $5257 and $6764 for patients treated with rivaroxaban and LMWH/warfarin, respectively. The corresponding cost difference was significantly lower by $1508 (22%), for the rivaroxaban cohort (95% CI = [−$2296; −$580]; p-value = 0.002).
Figure 2. Index hospital visit costs of rivaroxaban and LMWH/warfarin users. CI, confidence interval; LMWH, low molecular weight heparin.
presents the index hospital visit costs stratified by the type of hospital visit. The mean hospital visit costs for patients hospitalized were $7520 for patients treated with rivaroxaban and $7758 for patients treated with LMWH/warfarin (cost difference [95% CI] = −$238 [−$1535; $1328]; p = 0.747). Patients admitted and discharged the same day and treated with rivaroxaban also had similar hospital visit costs, at $1904, compared to $2150 for patients treated with LMWH/warfarin (cost difference [95% CI] = −$247 [−$542; $103]; p = 0.157). Those results show that the main driver of the cost difference of $1508 for index hospital visit costs (i.e., including both patients hospitalized and patients admitted and discharged the same day) between cohorts is the lower proportion of patients hospitalized in the rivaroxaban cohort (60%) compared to the LMWH/warfarin cohort (82%).
Table 2. Index hospital visit costs of rivaroxaban and LMWH/warfarin users stratified by type of visits.
presents the total hospital visit costs including subsequent hospital visits after the index hospital visit. The total hospital visit costs were lower for rivaroxaban compared to LMWH/warfarin users within 1, 2, 3, and 6 months (significantly lower within 1 and 3 months, p-values <0.05).
Table 3. Comparison of total hospital visit costs (i.e., index and subsequent hospital visits) among rivaroxaban and LMWH/warfarin users.
Discussion
This retrospective matched-cohort study compared the hospital visit costs following an initial hospital evaluation between DVT patients treated with rivaroxaban and a matched sample of DVT patients treated with the standard of care LMWH/warfarin. There was a 22% reduction in hospital costs among patients treated with rivaroxaban compared to LMWH/warfarin treatment.
A previous study by our group of authors estimated the hospitalization rate of patients with DVT who presented at a hospital after the availability of rivaroxabanCitation14. The study found a significant 27% reduction in the rate of admission to hospital compared to what the rate was prior to the availability of rivaroxaban. After adjusting for the possible trends in hospital admissions over time for patients treated with rivaroxaban, the results remained significant, with reduction rates varying from 23–25%. These trends in hospital admissions found in the previous study, thus, estimated a 3–6% reduction in the rate of hospital admission before vs after November 2012, which could also affect the hospital costs reduction found between patients treated with rivaroxaban and those treated with LMWH/warfarin in the current study. Of note, in the current study all costs were inflation-adjusted to 2013 $US based on the medical-care component of the Consumer Price Index to compare price on the same year of reference.
Recent studies, more targeted towards a broader definition of VTE patients than the present study that is restricted to DVT patients, have found that rivaroxaban is a cost-effective alternative to warfarin and enoxaparin/warfarin in treating patients with acute VTECitation18,Citation19. Both studies used data from the EINSTEIN trials and Markov models and found that rivaroxaban was a cost-effective option for the treatment of acute VTE and in the prevention of recurrent VTECitation19. Furthermore, although they were applied to different conditions than DVT, rivaroxaban and other NOACs were compared to warfarin in several other studiesCitation20–23, which analyzed the impact on costs and LOS among patients with non-valvular atrial fibrillation. Consistent with our study, they found that healthcare costs were significantly lower than the costs incurred using warfarinCitation23, and they also found that NOAC agents were associated with a shorter LOS than warfarin treatments.
This study has several limitations. First, the sample size of the rivaroxaban cohort was relatively small. Second, like all studies using administrative-claims data, it is subject to inherent limitations, such as the possible inaccuracies in billing and missing data. Third, the matching of patients was performed based on the baseline characteristics that were collected during hospital visits and hospitalizations occurring at the same hospital up to 12 months before and on the index hospital visit. This particular limitation could affect the comprehensiveness of the baseline characteristics. Fourth, like all observational studies, all adjustments that can be accounted for in the model can only be made for observable factors. In other words, unmeasured confounders cannot be modeled. Finally, the recorded data may have been subject to potential biases such as those of information or classification (e.g., identification of false-positive or false-negative DVT events). Despite these limitations, the current study has several strengths, including reliance on the real-world utilization of rivaroxaban and LMWH/warfarin anticoagulant agents among DVT patients.
Conclusion
In this real-world retrospective study, the mean hospital visit costs were found to be significantly lower for patients with DVT presenting at a hospital and treated with rivaroxaban compared to LMWH/warfarin. Furthermore, the total costs including the index and subsequent hospital visits were also found to be lower for patients treated with rivaroxaban.
Transparency
Declaration of funding
This research was funded by Janssen Scientific Affairs, LLC, Raritan, NJ.
Declaration of financial/other relationship
GJM and JEH have received consulting fees from Janssen Scientific Affairs. MKR and BB are employees of Janssen Scientific Affairs. PL, FL, and GG are employees of Analysis Group, Inc., a consulting company that has received research grants from Janssen Scientific Affairs. CVP reports receiving consulting fees for scientific activities from Janssen, Boehringer-Ingelheim, Pfizer, and Daiichi-Sankyo. CVP and JEH have engaged in no promotional activities.
Acknowledgments
The authors would like to acknowledge Jeff R. Schein, Janssen Scientific Affairs, LLC, Raritan, NJ, who provided assistance during the study.
References
- Tsiara S, Pappas K, Boutsis D, et al. New oral anticoagulants: should they replace heparins and warfarin? Hellenic J Cardiol 2011;52:52-67
- Landman GW, Gans ROB. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2011;364:1178; author reply 1178
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808
- Büller HR, Décousus H, Grosso Ma, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15
- Agnelli G, Becattini C, Franco L. New oral anticoagulants for the treatment of venous thromboembolism. Best practice & research. Clin Haematol 2013;26:151-61
- U.S. Food and Drug Administration. FDA expands use of Xarelto to treat, reduce recurrence of blood clots. Maryland, 2012. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm326654.htm. Accessed February 2015
- Boehringer Ingelheim. FDA Approves Pradaxa® (dabigatran etexilate mesylate) for treatment and reduction in the risk of recurrence of deep venous thrombosis and pulmonary embolism. Maryland, 2014. http://us.boehringer-ingelheim.com/news_events/press_releases/press_release_archive/2014/04-07-14-fda-approves-pradaxa-dabigatran-etexilate-mesylate-treatment-reduction-risk-of-recurrence-deep-venous-thrombosis-pulmonary-embolism.html. Accessed February 2015
- Bristol-Myers Squibb. U.S. FDA Approves Eliquis (apixaban) for the Treatment of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), and for the reduction in the risk of recurrent DVT and PE following initial therapy BMS Newsroom. Assessed
- U.S. Food and Drug Administration. FDA approves anti-clotting drug Savaysa. Maryland, 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm429523.htm. Accessed February 2015
- Deitelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol 2011;86:217-20
- Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med 2010;38(4 Suppl):S495-501
- Dobesh PP. Economic implications of inadequate treatment of venous thromboembolism and potential solutions. J Pharm Pract 2014;27:178-86
- Merli GJ, Hollander JE, Lefebvre P, et al. Rates of hospitalization among patients with deep vein thrombosis before and after the introduction of rivaroxaban. Hosp Pract (1995) 2015;43:85-93
- Cohen J. Statistical power analysis for the behavioral sciences. Toronto: Academic Press, Inc, 1977. p 19-24
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387-98
- Austin PC. Using the Standardized Difference To Compare The Prevalence Of A Binary Variable Between Two Groups In Observational Research. Commun Stat Simul Comput 2009;38:1228-34
- Lefebvre P, Coleman CI, Bookhart BK, et al. Cost-effectiveness of rivaroxaban compared with enoxaparin plus a vitamin K antagonist for the treatment of venous thromboembolism. J Med Econ 2014;17:52-64
- Seaman CD, Smith KJ, Ragni MV. Cost-effectiveness of rivaroxaban versus warfarin anticoagulation for the prevention of recurrent venous thromboembolism: a U.S. perspective. Thromb Res 2013;132:647-51
- Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay: is rivaroxaban associated with shorter inpatient stay compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:645-53
- Fonseca E, Walker DR, Hill J, et al. Abstract 282: Dabigatran Etexilate Is Associated With Shorter Hospital Length Of Stay Compared To Warfarin In Patients With Nonvalvular Atrial Fibrillation. Circul Cardiovasc Qual Outcomes 2012;5:A282
- Cowper PA, Pan W, Anstrom K, Stafford J, et al. Apixaban reduces hospitalization in patients with atrial fibrillation: an analysis of the effect of Apixaban therapy on resource use in the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation trial. J Am Coll Cardiol 2013;61:E1576
- Laliberté F, Pilon D, Raut MK, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:1521-8
