Abstract
Objective:
The study aimed to (1) develop a cost model for colonoscopy preparation among patients referred for colonoscopy using split-dose reduced-volume oral sulfate solution (OSS) and generic polyethylene glycol with electrolytes solution (PEG-ELS), (2) examine cost savings associated with OSS vs PEG-ELS, and (3) assess the robustness of the cost model.
Methods:
Efficacy of each agent was based on the results of a 541-patient clinical trial comparing OSS to PEG-ELS. Cleansing agent and colonoscopy procedure costs were calculated from OptumHealth Reporting & Insights claims data for 2010–Q12013. In the model, patients’ colonoscopies were tracked over a 25 or 35 year time period until the patients reached age 75. The difference per patient per year (PPPY) in total cleansing agent and colonoscopy procedure costs over the time horizon between the OSS and PEG-ELS cohort was calculated. One-way sensitivity analyses were conducted to test the robustness of the cost model.
Results:
The model showed lower cost for OSS patients over the time horizon. Total PPPY costs were $280.34 for the OSS cohort and $296.36 for the PEG-ELS cohort, resulting in a cost saving of $16.01 PPPY for the OSS cohort. This was due primarily to OSS patients having fewer colonoscopies (OSS: 0.158 vs PEG-ELS: 0.170 PPPY). Over the time horizon, cost savings of $4 763 335 were observed among 10, 000 OSS patients. Cost savings switched from OSS to PEG-ELS cohort in four cases: (1) base-case cost of a completed colonoscopy decreased by 75%, (2) base-case cost of OSS increased to over $143 per usage, (3) all non-completers were lost to follow-up, and (4) OSS bowel preparation quality dropped below PEG-ELS to 70%.
Conclusions:
From a payer’s perspective, the model showed that the use of OSS as the cleansing agent resulted in potential cost savings compared with PEG-ELS. Cost savings under OSS remained under various sensitivity analyses.
Introduction
Colorectal cancer is the third leading cause of cancer and cancer-related death in the USCitation1. Colorectal cancer is a costly disease, with costs increasing dramatically based on disease progressionCitation2. The primary goal of colorectal cancer screening is to detect and remove small pre-cancerous polyps and flat adenomas as early as possible to reduce mortality, improve patient outcomes, and decrease long-term treatment costsCitation1,Citation3. Colonoscopy screening is among the most effective means of colorectal cancer prevention through early detection of polyps or lesions, yet less than 50% of individuals, aged greater than or equal to 50 years, undergo screening colonoscopyCitation4,Citation5. An observational study was conducted and showed that increased rates of complete colonoscopy were inversely associated with death from colorectal cancerCitation6.
The quality of colonoscopy preparation plays an important role in colonoscopy; specifically, in the diagnostic accuracy of detection of adenomaCitation1. Patients who underwent colonoscopy screening reported that the bowel preparation was the worst part of the colonoscopy procedure because patients experienced adverse events such as abdominal pain, cramping, bloating, nausea, and vomiting in response to bowel preparation consumptionCitation5. Because of these reported experiences, patients who do not complete a colon-cleansing regimen may have sub-optimal bowel cleansing, which results in incomplete visualization of the colonCitation5. Rates of inadequate bowel preparation have been found to exceed 30% in some patient populationsCitation7. In a 6000-patient cohort study, the authors found that patients with inadequate bowel preparation have increased odds of immediate complications after colonoscopy by 3.5-timesCitation8.
In addition to the risk of missed colon pathology resulting in cost to treat colorectal cancer, prior research found that imperfect bowel preparation requires additional cleansing and resulted in longer proceduresCitation9, which in turn increases colonoscopy charges by 12–22%Citation10. Even with additional cleansing, the detection of polyps or lesions has been found to depend on preparation qualityCitation11. As such, endoscopists often recommend shorter follow-up time intervals for patients with poor preparation quality, resulting in additional procedural costs for colonoscopyCitation12.
Many bowel cleansing agents exist for use in colonoscopy preparation. Several studies have shown significant differences in preparation quality among themCitation13,Citation14. In a single-blind, randomized controlled trial, oral sulfate solution (OSS) was evaluated for bowel cleansing efficacyCitation15. The trial found that OSS resulted in a high rate of successful (excellent or good) preparation of 94.7%Citation15. In another randomized clinical study, Rex et al.Citation15 reported 98.4% of OSS patients experienced successful preparation. In the current cost-conscious environment, a cost analysis comparing the use of Split-Dose Reduced-Volume Oral Sulfate Solution (OSS) with Polyethylene Glycol with Electrolytes Solution (PEG-ELS) provides critical insight as payers balance the trade-off between the clinical efficacy of a cleansing agent and the total cost of colonoscopy procedures. The research study aimed to evaluate cost differences associated with using OSS (SUPREP) relative to generic PEG-ELS (GoLYTELY) in colonoscopy preparation.
Methods
A simulated cohort-based model from the payer’s perspective was developed to examine the costs of patients referred for colonoscopy using OSS and generic PEG-ELS. The model assessed the cumulative costs of the cleansing agents and colonoscopy procedures over time and computed the difference in per patient per year (PPPY) costs between the OSS and PEG-ELS cohorts.
Simulated cohort
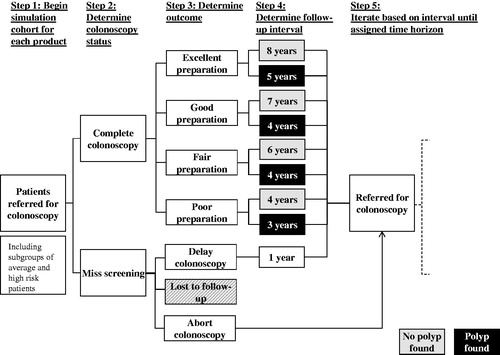
The model included a simulated cohort of 10 000 patients, including patients with a family history of colorectal cancer (i.e., considered as high risk) who were referred for screening at age 40 and patients with no family history (i.e., considered as average risk) who were referred at age 50. A time horizon of 25 or 35 years was assumed (i.e., that patients were not referred for colorectal screenings after the age of 75). In the model, patients received the cleansing agent corresponding to their cohort assignment (i.e., OSS or PEG-ELS). Patients who completed a colonoscopy procedure were assigned a preparation cleansing score (i.e., excellent, good, fair, or poor) with the probability of receiving each grade differing based on the cleansing agent. Patients were referred for follow-up colonoscopies according to the surveillance intervals observed in clinical trial data. At each surveillance time interval, patients were assigned a probability of completing the colonoscopy or missing the screening (i.e., did not start preparation and did not complete the colonoscopy). The flow of patients through screenings is illustrated in .
Data sources
Data for the simulation came from multiple sources. Clinical efficacy of the cleansing agents as well as surveillance intervals, stratified by cleansing grade and polyp detection, were obtained from the results of a 541-patient single-blind, clinical trial evaluating the safety, tolerability, and efficacy of three bowel cleansing treatments (split-dose OSS, same day-only OSS, and PEG-ELS) conducted by Braintree Laboratories, Inc (Braintree, MA).Citation16. To minimize potential bias due to differences in patient characteristics at baseline, a randomized study design scheme was employed. In this study, 95% of patients had successful cleansing with OSS-Split (i.e., 64% had ‘excellent’ preparations), compared to 77% of PEG-ELS patients (i.e., 25% had ‘excellent’ preparations)Citation16. For screening patients, the average recommended surveillance interval for OSS patients was ∼1 year longer than for PEG-ELS patientsCitation16. Cost data for completed and aborted colonoscopy procedures as well as the cost of OSS and PEG-ELS were obtained using private insurance claims data from OptumHealth Reporting & Insights for a nationwide sample of privately insured individuals for 2010–Q2013Citation17. Annual rate of death, annual colonoscopy completion rate, and proportion of patients at high risk (i.e., family history of colonoscopy) came from the published literatureCitation18–21 and were held constant over the course of the model. Additional data from the 2010 US Census was used to estimate the ratio of 40 year-old to 50 year-old individuals in the USCitation22.
Cost analysis
Costs included the cost of the cleansing agent and the screening colonoscopy procedure. Across the cleansing agents investigated, costs related to the treatment of adverse events either during the preparation or colonoscopy procedure were assumed to be the same. When patients missed colonoscopy screenings, the cost model assumed that no cost was incurred by the payer. Such patients were either referred for another colonoscopy a year later or lost to follow-up. The costs for each cohort were calculated until patients reached age 75.
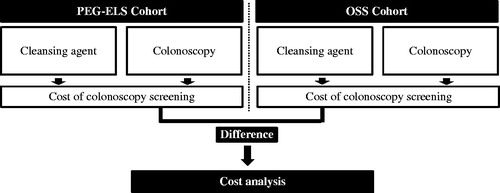
In order to assess cumulative costs, the model considered cohort demographics, time horizons for high and average risk patients, the distribution of colonoscopy completion (i.e., the percentages of patients who completed colonoscopies at initial referral, had delayed colonoscopies, had aborted colonoscopies, and were lost to follow-up), colonoscopy cleansing score (i.e., the distribution of OSS and PEG-ELS cleansing scores), polyp detection frequency (i.e., percentage of colonoscopies with a polyp detected stratified by the cleansing score), and colonoscopy cost (i.e., the cost of OSS and PEG-ELS as well as all colonoscopy procedures). Overall and per patient per year (PPPY) costs of OSS and PEG-ELS were compared. All cost values are reported in 2013 USD. illustrates the cost difference in colonoscopy screening for a single colonoscopy cycle.
Figure 2. Model flow diagram illustrating cost difference calculation for a single colonoscopy cycle. The cost difference in colonoscopy screening for a single colonoscopy cycle is illustrated in the schematic model. The cumulative cost difference in colonoscopy screenings over the time horizon accounts for varying recommended post-colonoscopy intervals, which are determined by colonoscopy cleansing score and polyp detection for each colonoscopy cycle.
Sensitivity analysis
To assess the robustness of the model assumptions, univariate sensitivity analyses were conducted to test the effect of varying key model inputs and assumptions on the costs for each cohort.
The following inputs were considered: cost of a colonoscopy, cost of each cleansing agent, assumptions about the rate of colonoscopy completion and the handling of non-completers, surveillance intervals, bowel preparation quality, age at which patients were no longer referred for screening, and proportion of high risk patients. The specific values used for these analyses as well as the initial values in the base case are displayed in .
Table 1. Summary of inputs for one-way sensitivity analyses.
Results
Base case analysis
The use of OSS resulted in fewer colonoscopies and lower overall costs over the 25 or 35 year time horizon than that of PEG-ELS. In particular, the model estimated 0.158 colonoscopies PPPY for OSS and 0.170 PPPY for PEG-ELS. While the OSS cohort had higher cleansing agent costs compared to the PEG-ELS cohort (OSS: $6.60 vs PEG-ELS: $1.22 PPPY), it had lower colonoscopy procedure costs (OSS: $273.74 vs PEG-ELS: $295.14 PPPY). Consequently, total costs were also lower for the OSS cohort. Total costs PPPY were $280.34 for OSS and $296.36 for PEG-ELS, resulting in a PPPY cost saving of $16.01 to the payer for the OSS cohort. For 10 000 patients over a 25 or 35 year time, the total procedure cost savings were $4 763 335 for the OSS cohort.
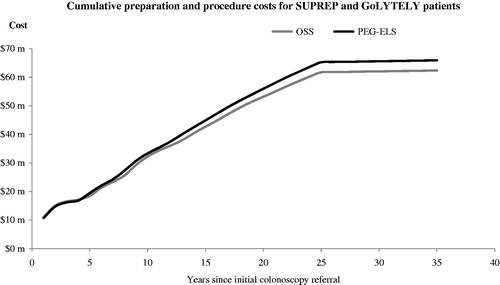
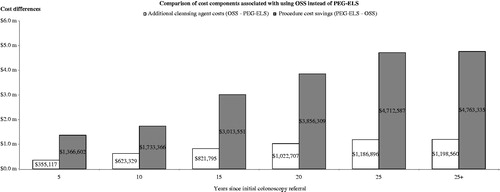
Lower PPPY costs for OSS is a consequence of lower combined preparation and procedure costs over time as illustrated in . This is primarily due to more OSS patients receiving better cleansing scores and, as a result of better cleansing, longer recommended surveillance intervals. displays the cumulative procedure cost savings of OSS relative to PEG-ELS as well as the additional cleansing agent costs. The cost model demonstrates that the relative cost savings for OSS increase more quickly than relative costs in the years after colonoscopy referral. The model shows that, in year 4, the cumulative preparation and procedure costs for OSS is lower than PEG-ELS.
Figure 4. Comparison of cost components associated with OSS use. Compares the cost differences between OSS and PEG-ELS for cleansing agent cost and colonoscopy procedure cost separately. The cost differences between OSS and PEG-ELS are observed as early as 5 years. Over the time horizon, the cost model shows that the cost savings for OSS increase more rapidly than additional costs incurred in the years after colonoscopy referral.
Sensitivity analysis
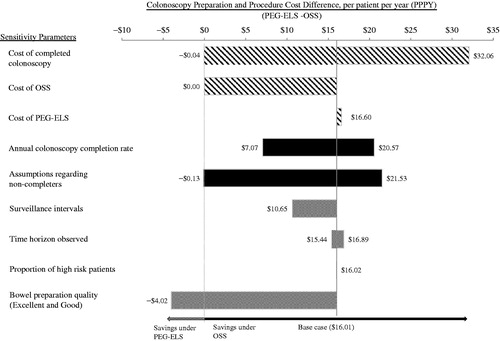
Sensitivity analyses were conducted to examine the persistence of the savings associated with OSS. The cost savings under OSS shift to PEG-ELS in each of the following four scenarios: the cost of completed colonoscopy decreases by 75% from the base-case cost; the cost of OSS increases to over $143 per usage; all non-completers are lost to follow-up; OSS bowel preparation quality dropped below PEG-ELS to 70%. Varying annual colonoscopy completion rate (i.e., using between 25–100%), cost of PEG-ELS (i.e., using up to the brand price of $10.56), surveillance intervals (i.e., using the minimum of the recommended surveillance interval), the time horizon observed (i.e., using up to age 85 years), and the proportion of high risk patients (i.e., using up to 100%) did not change the observation of cost savings under OSS. When the annual colonoscopy completion rate was varied, cost savings between $7.07–$20.57 PPPY were observed. Similarly, increasing the cost of PEG-ELS resulted in cost saving of $16.60 PPPY. illustrates the findings of the sensitivity analyses.
Figure 5. Univariate sensitivity analysis, tornado diagram. Illustrates the robustness of the cost model under different assumptions about costs (procedure and cleansing agent), rate of colonoscopy completion, and other inputs (i.e., average surveillance intervals, time horizon observed, and proportion of high risk patients). Positive cost difference means that cost savings were observed for the OSS cohort and negative cost difference means that cost savings were observed for the PEG-ELS cohort.
Discussion
This study demonstrates that the use of OSS as the cleansing agent results in potential cost savings compared with PEG-ELS from the payer perspective. Better cleansing scores and longer recommended surveillance intervals associated with OSS use resulted in fewer surveillance colonoscopies over the time horizon. In the sensitivity analysis, when bowel preparation quality of OSS was the same as PEG-ELS (i.e., at 77%), a cost saving of $2.18 PPPY for OSS was observed. The cost saving exhibited in the OSS cohort is driven by the proportion of OSS patients with an ‘excellent’ cleansing score. Therefore, in the cost model, patients using OSS incurred lower colonoscopy costs than PEG-ELS patients, which offset the increased expenditure associated with the use of OSS.
There is considerable literature comparing the efficacy of various cleansing agentsCitation23; however, there are limited studies comparing cleansing agents while considering the associated long-term healthcare costs. The results of this cost model are consistent with recent findings in the literature, mainly, on the importance of the quality of cleansing agent and cost savings incurred from a high quality cleansing agent over timeCitation1,Citation3,Citation5,Citation10,Citation24–26. Rex et al.Citation10 found that a substantial increase in cost of colonoscopy was observed with imperfect bowel preparation. More specifically, in considering cleansing agent, the cost of cleansing agents should not be the primary determinant, as reported by Gruss et al.Citation3. Gruss et al.Citation3 found that drug acquisition costs of the cleansing agent had the least impact on the overall colonoscopy cost structure and only accounts for less than 3% of the total colonoscopy procedure cost. Despite higher drug acquisition costs relative to comparator products (i.e., sodium picosulfate with magnesium citrate and sodium biphosphate/sodium phosphate), Gruss et al.Citation3 found that savings could be realized with the use of high quality cleansing agent because of the critical role that the quality of bowel preparation plays in the diagnostic accuracy of colonoscopy and the need for repeat procedures.
Cost savings from the reduction of repetitive procedures outweigh the higher drug costs for more effective colon cleansing agents. The quality of bowel cleansing determines the extent of full mucosal visualization, which is vital for two key quality indicators of colonoscopy: (1) cecal intubation rate and (2) polyp detection rateCitation1,Citation3,Citation5,Citation27. If full mucosal visualization is not achieved either as a result of poor patient compliance or a lack of efficacy of the cleansing agent, repeat procedures with additional costs may be administered to ensure that pathological changes are not missedCitation1,Citation3,Citation5,Citation27. Additionally, when faced with intermediate quality preparation that prevents full mucosal visualization, many gastroenterologists recommend a significantly shorter follow-up interval resulting in additional cost of colonoscopy proceduresCitation1,Citation3. Thus, cleansing agent choice should be determined by not only the acquisition cost of the cleaning agent, but also the cost of the initial colonoscopy procedure as well as any repeat procedures due to incomplete visualization of all areas of the colon mucosaCitation1,Citation3. Gruss et al.’sCitation3 cost model considered these factors and found that the higher acquisition costs for OSS were completely offset by savings from fewer colonoscopies over the time horizon. As depicted in our cost model in , this result of savings associated with the use of OSS was robust to a number of sensitivities. In order for PEG-ELS to show cost savings compared to OSS, extreme assumptions about costs of completed colonoscopy and OSS, bowel preparation quality, and outcomes of non-completers must be made.
The cost model findings are consistent with the reported literature on the importance of high quality cleansing agents in overall cost savingCitation3. However, the cost model has some limitations. For the clinical efficacy input parameters, the regimen for administration of the bowel cleansing agents was limited to their approved indications. This study only focused on results from the US, where OSS has been approved for split administration and was compared to PEG-ELS which has been approved as an evening regimen only. Pricing and approved administration regimens of OSS and PEG-ELS vary by country, which may limit the generalizability of cost savings globally. Rex et al.Citation15 and Di Palma et al.Citation28 published OSS split-dose and evening that were consistent with the McGowan et al.Citation16 published conference abstract. Despite the limitation in publication, additional sensitivity analyses demonstrated robustness of the analysis that cost savings remained under OSS. For example, under the assumption that the success rate of OSS and PEG-ELS was 95%, a marginal cost savings of $6.81 PPPY was observed in the OSS cohort because of the proportion of OSS patients with an ‘excellent’ cleansing score. The cost model focused on the direct cost of the cleansing agent and colonoscopy procedures over the time horizon of up to age 75 years. The model assessed a time horizon of over 25 years and did not account for changes in medication over time (e.g., new or generic cleansing agents entering the market). Sensitivity analysis was performed for patients up to age 85 years and a cost saving under OSS of $16.89 PPPY was observed. Finally, the cost model does not consider indirect costs associated with sub-optimal bowel preparation where additional resources are required for intra-procedural cleansing to correct for inadequate colonic preparation, as recently shown by MacPhail et al.Citation29, where inadequate preparation markedly increases washing volume and suctioning time. Other indirect costs may include lost time from work due to repeated colonoscopy procedures or the cost of not detecting adenocarcinomas at an earlier stage where the cost of colonoscopy and the cost of treating adenocarcinomas may be over 100-fold higher than the cost of colonoscopy screeningCitation24,Citation30. These limitations may be expected to increase the cost differences between preparations. Future studies are warranted to examine the association between better colon cleansing agents and earlier detection of cancer with lower costs.
From a payer’s perspective, the cost model demonstrated that potential near-term and long-term cost savings associated with more efficacious cleansing agents were observed even if these agents have higher acquisition costs than comparators. The quality of bowel preparation is one critical component to the diagnostic accuracy of colonoscopy, resulting in a lower detection rate of adenomas and increased cost of colorectal cancer prevention in addition to patient compliance and co-morbid conditionsCitation25. The US Multi-Society Task Force (MSTF) on colorectal cancer recommends that clinicians meet or exceed a target rate of 85% for adequate bowel preparationCitation25. In order to achieve a MSTF target of 85% for adequate bowel preparation, a high-quality bowel cleansing agent (such as OSS) is required.
Transparency
Declaration of funding
This work was supported by Braintree Laboratories, Braintree, MA.
Declaration of financial/other relationships
RC, LH, and MY are employees of Analysis Group, Inc., a consulting company that has received research grant from Braintree Laboratories. During the cost model’s development, SY and MD were employees of Analysis Group, Inc. MC is an employee of Braintree Laboratories. FAF is a consultant for Braintree Laboratories. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
We would like to thank Audrey Tiew for helping with the preparation of the manuscript.
References
- Hillyer GC, Basch CH, Lebwohl B, et al. Shortened surveillance intervals following suboptimal bowel preparation for colonoscopy: results of a national survey. Int J Colorectal Dis 2013;28:73-81
- Cost of Colorectal Cancer. Cost of illness handbook. US Environmental Protection Agency, 2007
- Gruss HJ, Crockett A, Leicester RJ. Budget-impact model for colonoscopy cost calculation and comparison between 2 litre PEG + ASC and sodium picosulphate with magnesium citrate or sodium phosphate oral bowel cleaning agents. J Med Econ 2012;15:758-65
- Luo Z, Bradley CJ, Dahman BA, et al. Colon cancer treatment costs for medicare and dually eligible beneficiaries. Health Care Financ Rev 2009;31:35-50
- Cohen LB, Kastenberg DM, Mount DB, et al. Current issues in optimal bowel preparation: excerpts from a roundtable discussion among colon-cleansing experts. Gastroenterol Hepatol 2009;5(11 Suppl 19):3-11
- Rabeneck L, Paszat LF, Saskin R, et al. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol 2010;105:1627-32
- Kazarian ES, Carreira FS, Toribara NW, et al. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol 2008;6:438-42
- Chan AO, Lee LN, Chan AC, et al. Predictive factors for colonoscopy complications. Hong Kong Med J = Xianggang yi xue za zhi/Hong Kong Acad Med 2015;21:23-9
- Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc 2005;61:378-84
- Rex DK, Imperiale TF, Latinovich DR, et al. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002;97:1696-700
- Harewood GC, Sharma VK, Garmo Pd. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003;58:75-9
- Ben-Horin S, Bar-Meir S, Avidan B. The impact of colon cleanliness assessment on endoscopists' recommendations for follow-up colonoscopy. Am J Gastroenterol 2007;102:2680-5
- Rex DK, Palma JAD, Rodriguez R, et al. A randomized clinical study comparing reduced-volume oral sulfate solution with standard 4-liter sulfate-free electrolyte lavage solution as preparation for colonoscopy. Gastrointest Endosc 2010;72:328-36
- Tepes B, Mlakar DN, Metlicar T. Bowel preparation for colonoscopy with magnesium sulphate and low-volume polyethylene glycol. Eur J Gastroenterol Hepatol 2014;26:616-20
- Rex DK, DiPalma JA, McGowan J, et al. A comparison of oral sulfate solution with sodium picosulfate: magnesium citrate in split doses as bowel preparation for colonoscopy. Gastrointest Endosc 2014;80:1113-23
- McGowan J, Cleveland M, Rex D. Comparison of oral sulfate solution regimens to 4l PEG-ELS in adult subjects undergoing colonoscopy. Am J Gastro 2013;108:S603
- Optum Health Reporting & Insights. www.optum.com. Accessed 2014
- Mai PL, Wideroff L, Greene MH, et al. Prevalence of family history of breast, colorectal, prostate, and lung cancer in a population-based study. Public Health Genomics 2010;13:495-503
- Turner BJ, Weiner M, Yang C, et al. Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment–keeping behavior. Ann Intern Med 2004;140:528-32
- Denberg TD, Melhado TV, Coombes JM, et al. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med 2005;20:989-95
- Actuarial Life Table. Social Security Administration, 2010
- United States Census. Bureau USC, 2010
- Hookey LC, Vanner S. A review of current issues underlying colon cleansing before colonoscopy. Can J Gastroenterol 2007;21:105-11
- Bini EJ, Rajapaksa RC, Weinshel EH. The findings and impact of nonrehydrated guaiac examination of the rectum (FINGER) study: a comparison of 2 methods of screening for colorectal cancer in asymptomatic average-risk patients. Arch Intern Med 1999;159:2022-6
- Cohen LB. Advances in bowel preparation for colonoscopy. Gastrointest Endosc Clin N Am 2015;25:183-97
- Cohen SL, Einarsson JI. The role of mechanical bowel preparation in gynecologic laparoscopy. Rev Obstet Gynecol 2011;4:28-31
- Hassan C, Bretthauer M, Kaminski MF, et al. Bowel preparation for colonoscopy: guideline. European Society of Gastrointestinal Endoscopy (ESGE), 2013. p 142-50
- Di Palma JA, Rodriguez R, McGowan J, et al. A randomized clinical study evaluating the safety and efficacy of a new, reduced-volume, oral sulfate colon-cleansing preparation for colonoscopy. Am J Gastroenterol 2009;104:2275-84
- MacPhail ME, Hardacker KA, Tiwari A, et al. Intraprocedural cleansing work during colonoscopy and achievable rates of adequate preparation in an open-access endoscopy unit. Gastrointest Endosc 2015;81:525-30
- Henry SG, Ness RM, Stiles RA, et al. A cost analysis of colonoscopy using microcosting and time-and-motion techniques. J Gen Intern Med 2007;22:1415-21