Abstract
Background:
Acromegaly is a rare disorder characterized by the over-production of growth hormone (GH). Patients often experience a range of chronic comorbidities including hypertension, cardiac dysfunction, diabetes, osteoarthropathy, and obstructive sleep apnea. Untreated or inadequately controlled patients incur substantial healthcare costs, while normalization of GH levels may reduce morbidity and mortality rates to be comparable to the general population.
Objective:
To assess the 3-year budget impact of pasireotide LAR on a US managed care health plan following pasireotide LAR availability.
Methods:
Two separate economic models were developed: one from the perspective of an entire health plan and another from the perspective of a pharmacy budget. The total budget impact model includes costs of drug therapies and other costs for treatment, monitoring, management of adverse events, and comorbidities. The pharmacy cost calculator only considers drug costs.
Results:
The total estimated budget impact associated with the introduction of pasireotide LAR is 0.31 cents ($0.0031) per member per month (PMPM) in the first year, 0.78 cents ($0.0078) in the second year, and 1.42 cents ($0.0142) in the third year following FDA approval. Costs were similar or lower from a pharmacy budget impact perspective. For each patient achieving disease control, cost savings from reduced comorbidities amounted to $10,240 per year.
Limitations:
Published data on comorbidities for acromegaly are limited. In the absence of data on acromegaly-related costs for some comorbidities, comorbidity costs for the general population were used (may be under-estimates).
Conclusions:
The budget impact of pasireotide LAR is expected to be modest, with an expected increase of 1.42 cents PMPM on the total health plan budget in the third year after FDA approval. The efficacy of pasireotide LAR in acromegaly, as demonstrated in head-to-head trials compared with currently available treatment options, is expected to be associated with a reduction of the prevalence of comorbidities.
Keywords::
Introduction
Acromegaly is a rare disorder characterized by the over-production of growth hormone (GH), often as a result of a pituitary adenomaCitation1. The prevalence of acromegaly has been estimated at 58 per million population from a review of epidemiological studiesCitation2. A recent administrative claims analysis of a large dataset of US patients estimated an overall annual prevalence of acromegaly across the 5 years from 2008–2012 of 69–77 cases per million person-years (PY), which is similar to the estimate from the reviewCitation3.
Patients often experience a range of chronic comorbidities including hypertension, cardiac dysfunction, diabetes, osteoarthropathy, and obstructive sleep apneaCitation4. Mortality rates are elevated ∼2-fold in patients with uncontrolled acromegalyCitation5,Citation6. Untreated or inadequately controlled patients incur substantial healthcare costsCitation7,Citation8, while normalization of GH levels may reduce morbidity and mortality to levels similar to the general populationCitation5,Citation9,Citation10. All these factors contribute to the potential for increased healthcare resource utilization and an economic burden to patients and payers. Data available from health economic studies of acromegaly are limited. One recent study published in 2013 analyzed acromegaly or acromegaly-related administrative claims data from US health plans and found total all-cause medical costs averaged $2255.34 per month and total costs of managing acromegaly averaged $1005/month. Another study performed a cost-of-illness assessment health resource consumption due to acromegaly and associated comorbidities in northern ItalyCitation8. This study found total annual costs were ∼1.6-times higher in patients with uncontrolled compared to controlled disease. While cost savings from reduction in comorbidities are expected from improved disease control, such savings have not been reported in the literature.
The goals of treatment of acromegaly are to normalize GH and IGF-1, control tumor mass, treat comorbidities (hypertension, cardiac dysfunction, diabetes, osteoarthropathy, and sleep apnea), and minimize mortality. Treatment options for patients with acromegaly include surgery, pharmacologic therapy, and radiotherapyCitation11–13. Surgery remains the primary treatment option for acromegaly, while somatostatin analogs (SSA) are the primary first-line therapy after surgery. Treatment with SSA is also the primary option if surgery is not appropriate (for example, in patients with medical contraindications such as recent myocardial infarction, if surgery is delayed, or when the patient refuses the surgical option). In the US and in Europe, an SSA is most often used as primary drug treatment for patients with acromegalyCitation14.
Signifor LAR (pasireotide LAR) is a SSA approved by the FDA in 2014 for the treatment of patients with acromegaly who have had an inadequate response to surgery and/or for whom surgery is not an optionCitation15. The objective of this study was to assess the 3-year budget impact of pasireotide LAR on a US managed care health plan following pasireotide LAR availability.
Methods
Model design
This study assesses the budget impact of pasireotide LAR availability from two perspectives. The first perspective evaluates the total budget impact to a US managed care health plan, including costs of drug therapies and other costs for treatment, monitoring, management of adverse events (AEs), and comorbidities. The second perspective, from that of the pharmacy budget, includes only drug therapy costs. The budget impact of pasireotide LAR is calculated as the incremental budget impact of adding pasireotide LAR to the existing set of options treatments for acromegaly patients. Model outcomes include annual estimates of total treatment costs for the first 3 years of pasireotide LAR availability, as well as the total annual cost over the period. All costs were inflated to $2014 using the Consumer Price Index for Medical Care ServicesCitation16. Due to the short time horizon, post-2014 inflation is not included. There is no discounting in this model per recommendations from the ISPOR Task Force on Good Research Practices–Budget Impact Analysis, which suggest that, because the budget impact analysis presents financial streams over time, it is not necessary to discount these costsCitation17. The models were built in Microsoft Excel 2010.
Patient population
Both models calculate the economic impact of pasireotide LAR availability on a health plan with 1 million covered lives. An estimate of the prevalence of acromegaly was used to determine the size of the expected population with acromegaly. The target population considered in the population model is patients with acromegaly who have had an inadequate response to surgery and/or for whom surgery is not an option. The model excludes patients appropriate for treatment with surgery or radiotherapy and includes pasireotide LAR and alternative drug therapy options. The model calculates the total number of eligible patients receiving either initial drug therapy (1L+ population) or subsequent drug therapy (2L+ population) for a hypothetical health plan with 1 million covered lives.
The total number of plan members with acromegaly is based on published estimates of the prevalence of the disease. The prevalence estimate of 58 per million population from the widely-cited Holdaway 1999 review is based on an average of four studies published in the years 1980 (UK), 1988 (Sweden), 1990 (Northern Ireland), and 1993 (Spain)Citation2. A recent claims analysis of a large dataset of US patients is consistent with the estimates in Holdaway 1999, with an estimated prevalence of 69–77 per million for the years 2008–2012Citation3. Some studies outside of the US with smaller patient populations suggest the prevalence may be over 100 per millionCitation18–21. A patient flow model illustrates progression of acromegaly patients from early to advanced stages of disease (). Default inputs and assumptions are shown in .
Figure 1. Patient flow for acromegaly treatment. Patient counts are based on a hypothetical health plan with 1,000,000 members. Total acromegaly patients is estimated as the prevalent population (58 patients per million) multiplied by the proportion diagnosed (75%). See discussion for assumptions and default input values.
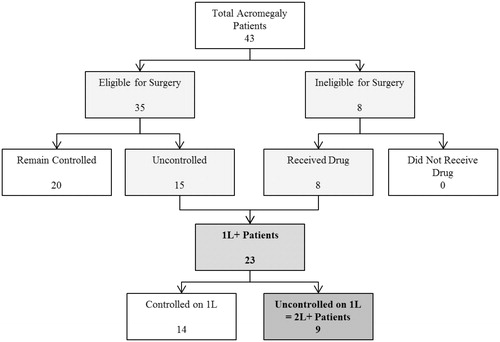
Table 1. Treatment-eligible patient population estimates.
The size of the covered population and other model inputs may be modified by the user based on varying plan population or other assumptions. The proportion of the prevalent population diagnosed with acromegaly is assumed to be 75%. Diagnosed acromegaly patients are then divided into patient groups who either are or are not appropriate for surgery. Medical therapy is appropriate for patients who have either progressed following surgery or are not appropriate for surgery. Of those who receive surgery, 45% are expected to progress following surgery, of whom 96% are expected to be appropriate for subsequent medical treatmentCitation12,Citation22. Of those not appropriate for surgery, 89% are expected to be appropriate for medical treatment. On a patient level, of 23 patients eligible for initial drug therapy (1L+ population), 40% of patients remain uncontrolled, and thus nine patients are eligible for subsequent drug therapy (2L+ population).
Treated shares
Market shares were obtained from unpublished primary market research with physicians and applied to the eligible patient populationCitation23. Treatment options include medical therapies and projected shares are presented along with pre-launch shares. In the scenario without pasireotide LAR entry, shares are static across the 3 year horizon. In the scenario with pasireotide LAR entry, market share changes reflect the uptake of pasireotide LAR with peak share in the third year after commercial availability. Shares for combination therapies were calculated as weighted from total drug shares across monotherapies and combinations. The use of pasireotide LAR in this model is limited to the labeled indication. No combination treatments have been approved by the FDA. Information about combination therapies used to treat acromegaly is limited and estimates are based on expert opinion and market researchCitation23.
Treatment costs
Drug cost is calculated as the product of the total expected amount of drug consumed each year based on prescribing information recommendations and the cost per unit. Patients are assumed to begin treatment at the beginning of the year, remain on treatment until the end of the year and be 100% compliant to drug regimens. The base case cost of pasireotide LAR is assumed to be $140,000 per yearCitation24. Combination drug costs are assumed to be the sum of monotherapy costs reduced in proportion to the dose reduction. Based on a chart review, the dose reduction for the octreotide LAR/lanreotide + pegvisomant combinations are 12% (SSA) and 60% (pegvisomant), respectivelyCitation25. There is no evidence in the published literature of specific dose reduction amounts for other drug combinations (0% dose reduction in the base case).
Octreotide LAR and pegvisomant require different initiation drugs or doses. Octreotide LAR requires an initial 2 weeks of treatment with octreotide injection at 3 doses a day before beginning the full regimenCitation26. Pegvisomant requires a higher initial dose of 40 mg, administered under a physician’s supervision before treatment can begin normallyCitation27.
Adverse events
No grade 3 or higher AEs were reported in the prescribing information for octreotide LAR, lanreotide, pegvisomant, bromocriptine or cabergoline monotherapies. Hyperglycemia was the only grade 3+ AE reported in >5% of pasireotide LAR subjects in both the C2305 and C2402 studies. Adverse event cost reflects the 33% of patients on pasireotide LAR 40 mg experiencing any grade of hyperglycemia in the C2402 study.
Comorbidities
The cost savings from preventing cases of acromegaly comorbidities are estimated as a function of disease control. The prevalence of comorbidities decreases with improved acromegaly disease control (see ). While acromegaly is associated with a number of comorbidities; those included in the model are reversible to a degree with disease controlCitation28. The prevalence rate for lipid abnormalities represent the prevalence of risk factors, measured by total cholesterol levelsCitation29 and left ventricular hypertrophy (mass index)Citation30, respectively. Arthropathy is defined as the thickening of the shoulder and right knee jointsCitation31. Spinal involvement is reported as the incidence of patients who experience vertebral fracturesCitation32. Glucose abnormalities including hyperglycemia and metabolic syndrome are frequently observed at diagnosisCitation33 and following transsphenoidial surgeryCitation34.
Table 2. Acromegaly budget impact model: comorbidity prevalence.
Comorbidity cost offsets are gained with more effective treatments. Disease control is defined as a GH level of <2.5 ng/mL and normalized IGF-1Citation35,Citation36. Base rates of control for octreotide LAR (42%) and lanreotide ATG (38%) and alternatives (10%) were assumed from pivotal clinical trial experience. Using the primary endpoint of disease control from the C2402 study (15.4% for pasireotide LAR and 0% for octreotide LAR and lanreotide ATG), pasireotide LAR rate of control (55%) is +15.4% above the alternatives. Disease control was not measured as a composite end-point in the pivotal clinical trials of pegvisomant, bromocriptine and cabergoline (see )Citation36.
Table 3. Acromegaly budget impact model: treatment effect.
The cost of comorbidities is obtained from a variety of sources, of which only oneCitation37 presents annual comorbidity cost data specific to an acromegalic patient population. Comorbidity reduction from combination treatments is modeled as the sum from monotherapy (may be over-estimates, especially given dose reduction in combination).
Monitoring
Medical resources include costs related to disease monitoring and maintenance, and treatment administration. Treatment options that are administered intramuscularly are assumed to be physician administered only. Subcutaneous drugs are assumed to require an initial consultation in which patients are trained to self-administer the treatment (some patients may self-administer subcutaneous drugs without training, and a conservative estimate of this cost was included in the model). All treatment options require regular outpatient monitoring at a rate assumed to be every 4 weeks, based on the octreotide LAR recommendationsCitation38, in which at every drug administration every 4 weeks, patients are also tested for disease progress.
Regularly performed laboratory procedures for disease monitoring include tests for growth hormones and IGF-1. Plasma prolactin testing is required for cabergoline treatmentCitation39. Side-effects monitoring is also necessary in the case of octreotide LAR, which requires glucose and thyroid function monitoringCitation26, and pasireotide LAR, which also requires glucose monitoring. According to AACE 2011 guidelines, tumor enlargement has been infrequently associated with the use of pegvisomantCitation11. Therefore, serial monitoring with pituitary MRI scans is suggested (frequency of MRI is assumed to be once every 9 months).
Costs for medical resources are obtained from the 2011 Medicare physician fee schedule and adjusted to 2014 dollarsCitation40. Where available, the Medicare listed price is used. Because the Medicare listed price is unavailable for laboratory procedures, the lower limit of the national fee range is used instead.
Results
Total cost of treatment
Total costs for each treatment option comprise costs related to treating acromegaly as well as cost savings from disease control from treatment. Combination therapy costs are assumed to be the sum of monotherapy costs, except for reduced combination drug costs as the result of cost reductions proportional to dose reductions. Annual costs are listed in .
Table 4. Acromegaly budget impact model: total costs.
Budget impact
The budget impact associated with the introduction of pasireotide LAR is calculated as the difference between the total cost to the health plan of treating acromegaly with and without pasireotide LAR included in the health plan formulary. With an eligible population of 23 acromegaly patients for initial drug therapy (1L+ population), and nine acromegaly patients for subsequent drug therapy (2L+ population), the health plan budget is expected to increase with the introduction of pasireotide LAR (see , and ).
Figure 2. Acromegaly budget impact model: budget impact with and without the introduction of Pasireotide LAR (1L+ Population).
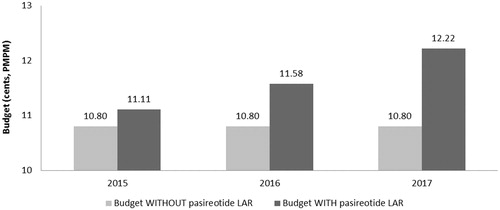
Figure 3. Acromegaly budget impact model: budget impact with and without the introduction of Pasireotide LAR (2L+ Population).
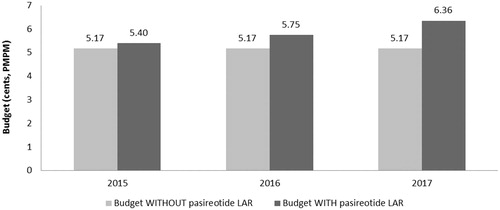
Table 5. Acromegaly budget impact model: summary of results.
Based on a covered population of 1 million members, the expected total difference in the budget for use in the 1L+ population for the entire US managed care health plan is $36,864 in the first year, $93,079 in the second year, and $169,986 in the third year. On a per member per month (PMPM) basis, the estimated budget impact for use in the 1L+ population on a health plan with one million covered lives is 0.31 cents ($0.0031) in the first year, increasing to 0.78 cents ($0.0078) in the second year, and 1.42 cents ($0.0142) in the third year. The expected total difference in the budget for use in the 2L+ population for the entire US managed care health plan is $27,792 in the first year, $69,432 in the second year, and $142,250 in the third year. On a PMPM basis, the estimated budget impact for use in the 2L+ population on a health plan with 1 million covered lives is 0.23 cents ($0.0023) in the first year, increasing to 0.58 cents ($0.0058) in the second year, and 1.19 cents ($0.0119) in the third year.
Pharmacy budget impact results
The estimated pharmacy budget impact of pasireotide LAR in the 1L+ population is 0.31 cents ($0.0031) in the first year, 0.77 cents ($0.0077) in the second year, and 1.41 cents ($0.0141) in the third year after launch (see and ). The estimated pharmacy budget impact of pasireotide LAR in the 2L+ population is 0.23 cents ($0.0023) in the first year, 0.57 cents ($0.0057) in the second year, and 1.17 cents ($0.0117) in the third year after launch (see and ).
Figure 4. Acromegaly pharmacy cost calculator: pharmacy budget impact with and without the introduction of Pasireotide LAR (1L+ Population).
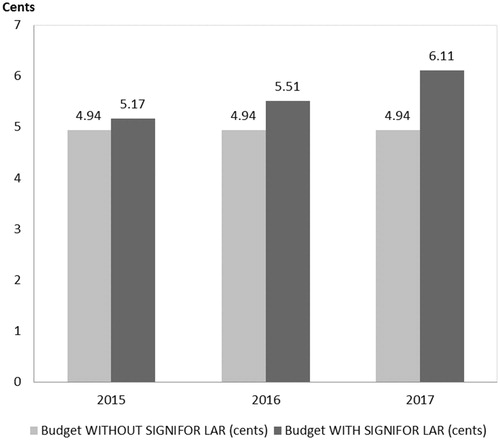
Figure 5. Acromegaly pharmacy cost calculator: pharmacy budget impact with and without the introduction of Pasireotide LAR (2L+ Population).
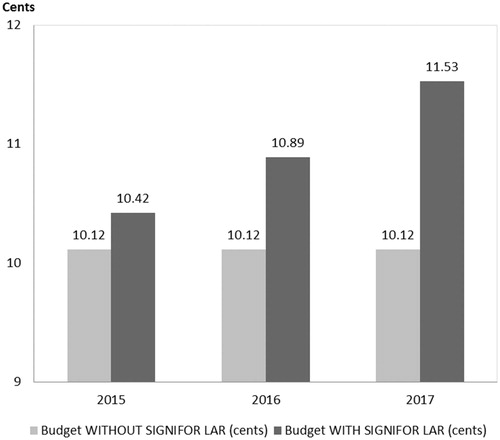
Table A7. Acromegaly budget impact model: medical resource cost per unit.
Table 6. Acromegaly pharmacy cost calculator: results.
Sensitivity analysis
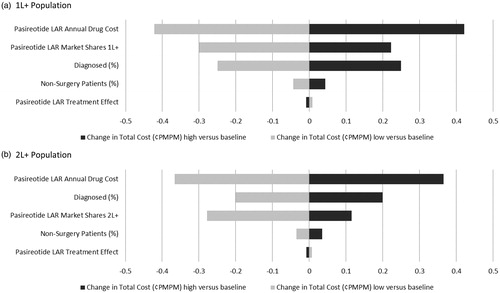
Five model parameters are closely examined in a one-way sensitivity analysis: (1) pasireotide LAR annual drug cost, (2) pasireotide LAR market shares, (3) portion of patients who do not receive surgery, (4) pasireotide LAR treatment effect, and (5) portion of acromegaly patients who are diagnosed. By varying parameters by ±10%, the most significant impact is caused by pasireotide’s drug price, and is expected to alter the budget impact by a magnitude of less than 0.50 cents PMPM over the course of 3 years. The range of the 3 year budget impact is presented in a tornado diagram in .
Discussion
This analysis is impacted by the limited source data specific for patients with acromegaly. The areas most impacted by this deficit of information are the estimation of the eligible patient population model and market shares. Because acromegaly is a rare disorder, the pharmacy budget impact associated with introduction of pasireotide LAR is small, less than 2 cents PMPM for the acromegaly population eligible for medical therapy (1L+) in the third year after introduction to the health plan formulary.
Published clinical and economic data for acromegaly is limited. In absence of data on acromegaly-related costs for some comorbidities, comorbidity costs for the general population had to be used (may be under-estimates). Differences in these annual comorbidity costs between an acromegaly patient population and an average patient may be significantly different, as sleep apnea and CVD annual costs are $2402 and $6877 for the general population, respectivelyCitation28, but are $10,675 and $14,261 for an acromegalic patient populationCitation37. Because the difference in the cost of comorbidities between an acromegalic and normal patient population is substantial, this could under-estimate the impact of the cost offsets due to controlled disease, and over-estimate the overall budget impact of pasireotide LAR.
Conclusion
Introduction of pasireotide LAR to a health plan formulary is expected to result in a very small increase in costs to the health plan. Budget impact should be considered in the context of reduced use of other costly treatments, including off-label use of combinations of multiple branded agents such as SSAs with pegvisomant. The efficacy of pasireotide LAR in acromegaly, as demonstrated in head-to-head trials compared with currently available treatment options, is expected to be associated with a reduction of the prevalence of comorbidities.
Transparency
Declaration of funding
This research was sponsored by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
JJZ and DN are employees of Analysis Group and have disclosed receiving consulting fees from Novartis Pharmaceuticals Corporation. MPN and WHL have disclosed that they are employees of Novartis Pharmaceuticals Corporation. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Notes
*Signifor LAR is a registered trademark of Novartis Pharmaceuticals Corporation, East Hanover, NJ.
References
- Del Porto LA, Liubinas SV, Kaye AH. Treatment of persistent and recurrent acromegaly. J Clin Neurosci 2011;18:181-90
- Holdaway IM, Rajasoorya C. Epidemiology of acromegaly. Pituitary 1999;2:29-41
- Burton T, Le Nestour E, Neary M, et al. Prevalence of acromegaly in a large US managed care population. ENDO. 2015;FRI-489.
- Ribeiro-Oliveira An, Jr, Barkan A. The changing face of acromegaly–advances in diagnosis and treatment. Nat Rev Endocrinol 2012;8:605-11
- Holdaway IM. Excess mortality in acromegaly. Horm Res 2007;68(5 Suppl):166-72
- Dekkers OM, Biermasz NR, Pereira AM, et al. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab 2008;93:61-7
- Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab Clin N Am 2008;37:101-22, viii
- Didoni G, Grottol S, Gasco V, et al. Cost-of-illness study in acromegalic patients in Italy. J Endocrinol Invest 2004;27:1034-9
- Orme SM, McNally RJQ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. J Clin Endocrinol Metab 1998;83:2730-4
- Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol 2008;159:89-95
- Katznelson L, Laws ER, Melmed S, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:3933-51
- Melmed S, Colao A, Barkan A, et al. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 2009;94:1509-17
- Katznelson L, Laws ER, Melmed S, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:3933-51
- Giustina A, Bronstein MD, Casanueva FF, et al. Current management practices for acromegaly: an international survey. Pituitary 2011;14:125-33
- Signifor LAR (pasireotide) for injectable suspension, for intramuscular use [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp, 2014
- Bureau of Labor Statistics. US Department of Labor. Consumer Price Index (CPI) for Medical Care Services (CUSR0000SAM2). 2014. http://www.bls.gov/cpi/. Accessed December 11, 2015
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health 2007;10:336-47
- Rosario PW. Frequency of acromegaly in adults with diabetes or glucose intolerance and estimated prevalence in the general population. Pituitary 2011;14:217-21
- Daly AF, Rixhon M, Adam C, et al. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 2006;91:4769-75
- Cannavo S, Ferrau F, Ragonese M, et al. Increased prevalence of acromegaly in a highly poluuted area. Eur J Endocrinol 2010;163:509-13
- Schneider HJ, Sievers C, Saller B, et al. High prevalence of biochemical acromegaly in primary care patients with elevated IGF-1 levels. Clin Endocrinol 2008;69:432-5
- Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000;85:526-9
- RedBook ReadyPrice Online. New York, NY: Thomson Reuters, 2010. http:// Q24 www.thomsonhc.com/micromedex2/librarian. Accessed 11 December, 2015
- Data on file. Internal Communication. Novartis Pharmaceuticals Corp, 2014
- Novartis Data on File. A Medical Chart Review to Study the Association between Biochemical Control and Comorbidity in Treated Acromegaly Patients. 2014
- Novartis. Budget impact analysis of Signifor in Cushing's disease. 2011:Data on File
- Somavert (pegvisomant for injection) [prescribing information]. New York, NY: Pfizer Inc, 2010
- Ben-Shlomo A, Sheppard MC, Stephens JM, et al. Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 2011;14:284-94
- Vilar L, Naves LA, Costa SS, et al. Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 2007;13:363-72
- Colao A, Marzullo P, Ferone D, et al. Cardiovascular effects of depot long-acting somatostatin analog Sandostatin LAR in acromegaly. J Clin Endocrinol Metab 2000;85:3132-40
- Colao A, Cannava S, Marzullo P, et al. Twelve months of treatment with octreotide-LAR reduces joint thickness in acromegaly. Eur J Endocrinol 2003;148:31-8
- Bonadonna S, Mazziotti G, Nuzzo M, et al. Increased prevalence of radiological spinal deformities in active acromegaly: a cross-sectional study in postmenopausal women. J Bone Miner Res 2005;20:1837-44
- Ciresi A, Amato MC, Pivonello R, et al. The metabolic profile in active acromegaly is gender-specific. J Clin Endocrinol Metab 2013;98:E51-9
- Tzanela M, Vassiliadi DA, Gavalas N, et al. Glucose homeostasis in patients with acromegaly treated with surgery or somatostatin analogues. Clin Endocrinol (Oxf) 2011;75:96-102
- Fleck J, Pedroncelli A, Kodama L, et al. A phase III, multicenter, randomized, parallel-group study to assess the efficacy and safety of double-blind pasireotide LAR 40 mg and pasireotide LAR 60 mg versus open-label octreotide LAR or lanreotide ATG in patients with inadequately controlled acromegaly (CSR Study No. CSOM230C2402). Novartis, 2013
- Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2014;2(1);875-884.
- Broder MS, Neary MP, Chang E, et al. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary 2014;17:333-41
- Truong HL, Nellesen D, Ludlam WH, et al. Budget impact of pasireotide for the treatment of Cushing's disease, a rare endocrine disorder associated with considerable comorbidities. J Med Econ 2014;17:288-95
- Greenstone LLC. Cabergoline (tablet) [prescribing information]. Peapack, NJ: Greenstone LLC, December, 2017.
- Centers for Medicare and Medicaid Services (CMS). Medicare Physician Fee Schedule. 2011.
- Acromegaly. National Endocrine and Metabolic Diseases Information Service. NIH, 2008. Publication No. 08-3924. http://www.endocrine.niddk.nih.gov/pubs/acro/acro.aspx. Accessed December 11, 2015
- Novartis Data on File. Acromegaly Treatment Flow Analysis. 2014
- Davi MV, Dalle Carbonare L, Giustina A, et al. Sleep apnoea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur J Endocrinol 2008;159:533-40
- RedBook ReadyPrice Online. New York, NY: Thomson Reuters, 2010. http://www.thomsonhc.com/micromedex2/librarian. Accessed December 11, 2015
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics 2011. Circulation 2011;123:e18â-e18â
- Ray GT, Collin F, Lieu T, et al. The cost of health conditions in a health maintenance organization. Med Care Res Rev 2000;57:92-109
- Tosteson AN, Burge RT, Marshall DA, et al. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care 2008;14:605-605
Appendix
The full market shares for patients in the 1L+ and 2L+ populations are presented in Tables A1 and A2.
Table A1. Acromegaly budget impact model: projected market shares for initial drug therapy (1L+ Population) (%).
Additional clinical inputs for estimates of drug use at treatment initiation and prevalence of comorbidities are included in Table A4 and Table A5. Total costs listed in Table A3 include initial costs for octreotide LAR and pegvisomant. Medical resource utilization rates and cost per unit are provided in Table A6 and Table A7.