Abstract
Objective:
A potential complication for all new multiple myeloma (MM) patients is the clinical presentation of osteolytic lesions which increase the risk for skeletal-related events (SREs). However, the contribution of SREs to the overall economic impact of MM is unclear. The impact of SREs on healthcare resource utilization (HCRU) and costs for US patients with MM was analyzed in Truven Health Marketscan Commercial Claims and Medicare Supplemental Databases.
Methods:
Adults diagnosed with MM between January 1, 2005 and December 31, 2010 with ≥2 claims ≥30 days apart (first claim = index date) were included. SREs included: hypercalcemia, pathologic fracture, surgery for the prevention and treatment of pathologic fractures or spinal cord compression, and radiation for bone pain. Rates of HCRU (outpatient [OP], inpatient [IP], emergency room [ER], orthopedic consultation [OC], and ancillary) and healthcare costs were compared between MM patients with and without SREs. Inverse propensity weighting was applied to adjust for potential bias.
Results:
Of 1028 MM patients (mean age = 67, standard deviation = 13.2), 596 patients with ≥1 SRE and 432 without SREs were assessed. HCRU rates in IP, ER, and ancillary (p < 0.01) and mean total costs of OP, IP, and ER were significantly higher (p < 0.05) for patients with vs without SREs during follow-up. HCRU rates also increased with SRE frequency (p < 0.05 in OP, IP, ER, OC, and ancillary), as did mean total healthcare costs, except for OC (p < 0.001).
Limitations:
A broad assessment of pharmacotherapy for the treatment of MM was not an objective of the current study. Bisphosphonate use was evaluated; however, results were descriptively focused on frequency of utilization only and were not included in the broader cost and HCRU analysis.
Conclusions:
Among US patients with MM, higher SRE frequency was associated with a significant trend of higher HCRU and total healthcare costs in several settings.
Introduction
The infiltration of bone by malignant cells can occur across tumor types, including multiple myeloma (MM), and can lead to bone destructionCitation1. When tumor cells interact with the bone microenvironment they can secrete factors that disrupt the osteoclast and osteoblast balance, often in favor of increased osteoclast activityCitation1. This imbalance leads to increased bone resorption and potentially the development of skeletal-related events (SREs)Citation1. SREs can include bone fractures, bone pain, hypercalcemia, the need for bone surgery, or more serious events such as spinal cord or nerve root compression or vertebral collapseCitation2–5. SREs are associated with increased morbidity, lowered or complete loss of mobility, decreased performance status, and reduced quality-of-lifeCitation4,Citation5, all of which can impact patient eligibility for certain cancer treatmentsCitation5.
The American Cancer Society estimates that 26,850 new cases of MM will be diagnosed in the US in 2015Citation6, and 70–80% of these cases generally have detectable osteolytic lesions at diagnosis and are at risk for SREsCitation7. Evidence from several clinical trials has shown that the use of bisphosphonates (BIS), in particular Aredia (pamidronate disodium; Novartis Pharmaceuticals Corporation, East Hanover, NJ) and Zometa (zoledronic acid; Novartis Pharmaceuticals Corporation), decreases the incidence and delays the onset of SREs, as well as reduces bone pain in patients with MMCitation2,Citation5,Citation8–13. Pamidronate and zoledronic acid both received US regulatory approval in 1991 and 2002, respectively, for the treatment of bone disease associated with MMCitation14,Citation15. The International Myeloma Working Group and National Comprehensive Cancer Network recommend BIS treatment for all MM patients who are receiving anti-myeloma therapy, whether or not they have detectable osteolytic bone lesions by conventional radiographyCitation7,Citation16. In the absence of BIS use, it has been estimated that advanced cancer patients, including those with MM, could have 2–4 SREs per yearCitation2.
Economic analyses of real world data evaluating the impact of SREs in patients with MM are limited. Due to the volume of MM patients at risk for SREs, and based on published data for other cancer populationsCitation2,Citation12,Citation13,Citation17–27, the impact of SREs on overall health and cost is expected to be substantial in this population. A systematic review of published evidence reported medications, hospitalizations, and adverse events (AEs) as primary cost drivers for MM patients, in addition to progressive disease and bone or skeletal complicationsCitation28. A retrospective cost analysis revealed that skeletal complications related to MM may contribute significantly to the cost of care, up to ∼17% of the total cost of treatmentCitation29. One study reported a metastatic bone disease (MBD) rate of nearly 30% among MM patients, with a mean adjusted difference of total expenditure per MM patient between MBD cases and non-MBD controls being nearly $25,000Citation30. While a UK population-based study found that one-third of all MM patients experienced at least one SRE-associated inpatient episodeCitation31, a separate US cost study reported the mean health plan costs per inpatient event for SRE-associated hospitalizations to be for bone surgery ($31,016; standard deviation (SD) = $31,211), spinal cord compression ($43,691; SD = $53,986), and pathologic fractures ($23,347; SD = $31,605)Citation20.
Other than hospital costs for inpatients with SREs, the incremental direct costs that are attributable to SREs have not been well characterized in US patients with MM. To fill this gap, the primary objectives of this study were to evaluate the frequency of SREs in this population as well as associated incremental costs and resource use across different healthcare settings.
Methods
Study design
An analysis of Truven Health Marketscan Commercial Claims and Medicare Supplemental DatabasesCitation32 was conducted on pharmacy and medical claims data from traditional fee-for-service Medicare for individuals aged 65 and older and from non-capitated commercial health plans for individuals younger than age 65. All adult patients diagnosed with MM between January 1, 2005 and December 31, 2010 and without evidence of other cancers (with the exception of non-melanoma skin cancer [NMSC]) were included in the study. Patients with NMSC were not excluded given the condition’s prevalence in the US and availability of treatment in an outpatient setting. MM patients were required to have at least two claims for MM (ICD-9 codes 203.00, 203.01, 203.02) at least 30 days apart, and the date of the first diagnosis claim was defined as the index date (see Appendix 1/Supplemental Online Item 1 for a complete list of study codes used). Patients also had to be continuously enrolled in either fee-for-service Medicare or a non-capitated commercial health plan in the US with a medical and pharmacy benefit for at least 12 months prior to the index date and for at least 3 months following the index date. Patients were followed after the index date until disenrollment from the health plan, the end of the study period on December 31, 2011, or death. Data were analyzed beginning 12 months prior to the index date (baseline period). For the analysis, MM patients were grouped into one of the following cohorts: patients who had no SREs during the follow-up period and patients who had at least one SRE during the follow-up period. Data on the frequency of SREs, associated all-cause (defined as cancer- and non-cancer-related) and cancer-related HCRU rates, as well as all-cause and cancer-related costs in US dollars (USD) were collected during the baseline and follow-up period. All-cause (vs non-cancer) related HCRU and cost was evaluated during the baseline period given that patients with NMSC were not excluded from the analysis. Cancer-related HCRU and costs were attributed to cancer based on the presence of MM-related diagnosis codes at the associated healthcare encounter, while all-cause HCRU and costs were totaled regardless of diagnostic attribution. Rates of HCRU (outpatient, inpatient, emergency room, orthopedic consultation, and ancillary) and healthcare costs were compared between MM patients with and without SREs. Ancillary utilization and cost encompassed a range of healthcare services that included diagnostic (i.e., laboratory testing, diagnostic imaging), therapeutic (i.e., rehabilitation, physical and occupational therapy), and custodial (i.e., hospice care, long-term acute care, and urgent care) services.
Given the focus on SREs, the frequency of BIS utilization was also evaluated as a secondary objective in this study. Medications in our analysis included pamidronate, zoledronic acid, risedronate, alendronate, ibandronate, etidronate, and tiludronate. It is important to note that an evaluation of denosumab was not included as part of this study given the lack of FDA approved indication for patients with MM. Baseline Charlson/Deyo comorbidity index (CCI) scores were calculated to allow for consideration of comorbidities across the cohortsCitation33. The frequencies of comorbidities as captured by the CCI were also evaluated post-baseline in order to characterize the non-MM-related health of patients.
SREs were defined to include any of the following: hypercalcemia, pathologic fracture, surgery for the prevention or treatment of pathologic fractures, spinal cord compression, and radiation for bone pain. A treatment episode for SRE was characterized as a 90-day window of time and was informed based on recommendations for non-pharmacologic treatments for SREs, as outlined in NCCN guidelinesCitation16. Any SRE codes corresponding to the same region of the body within 90 days after the initial code for that region were considered related to the initial diagnosis of the SRE. If a region code was noted as unspecified (i.e., an SRE without a region) and was also within the 90-day window, it was incorporated into the existing SRE window. If a SRE for a different second region was detected, this created a new SRE and a subsequent 90-day window for that region. If there was a SRE classified as ‘unspecified’, and there were no other SREs within 90 days prior to this event, a new 90-day SRE window was initiated.
Statistical methods
Patient characteristics between MM patients with and without SREs were evaluated (mean, standard deviation, and median for continuous variables; frequency and percentage for categorical variables). The frequencies of SREs and BIS use in MM patients were described. The time to first SRE post-index date was assessed using Kaplan Meier analysis. Frequency of HCRU and costs per patient per month at baseline and during the follow-up period were estimated and compared between MM patients with and without SREs. The rate for each cohort was estimated by the total events (medical visits or costs contributed by all patients) divided by the cumulative length that all patients contributed. To adjust for potential bias at baseline due to non-randomness, inverse propensity score weighting (IPW) was applied to estimate ratesCitation34. Propensity score (PS) is the probability of a patient being assigned to the corresponding cohort conditional on observed baseline characteristics (age, baseline CCI, gender, insurance type, and geographic region) and estimated by logistic regression. The standard error of IPW estimators was estimated by bootstrapping methodology (replication = 1000)Citation35. Comparisons between MM patients with and without SREs were made using t-test or a Chi-square test. HCRU data by number of SREs was analyzed utilizing linear regression to observe trends.
No adjustments for multiple comparisons were made as the study objectives were exploratory in nature. Two-tailed p-values at or below a level of 0.05 were considered significant, and all statistical analyses were performed using SAS version 9.2.
Results
Characteristics of MM patients with and without SREs
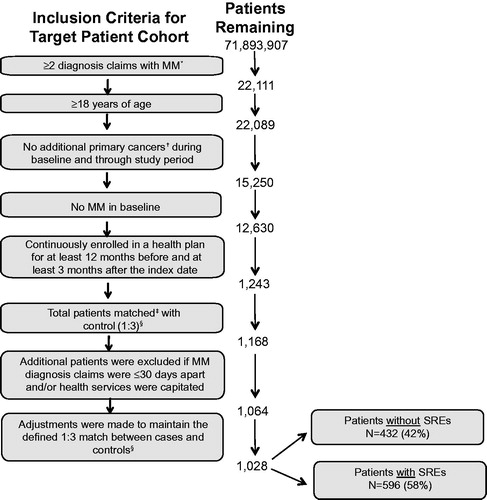
Records from 71,893,907 enrollees were screened for diagnostic claims of MM between January 1, 2005 and December 31, 2010, with 22,111 patients meeting the criteria for two or more claims of MM. The most common reasons for exclusion from the final analytic cohort were presence of other primary cancers (n = 6839), MM during the baseline period (n = 2620), and lack of continuous enrollment in the health plan (n = 11,387). The results presented here were part of a broader analysis of patients with MM that utilized a control group of patients without MM. Patient attrition based on the inclusion criteria is outlined in . For the purposes of this current analysis and report, results for MM patients relative to the control group are not included. The final MM study population consisted of 1028 patients (), of which 596 (58%) experienced at least one SRE.
Figure 1. Patient Attrition. *ICD-9-CM codes included: 203.00, 203.01, 203.02. †Excluding non-melanoma skin cancer. ‡Patients matched at index date of MM patients based on age, gender, geographic location, insurance type, baseline Charlson/Deyo comorbidity index comorbidity score, and follow-up time. See methods for additional information. §The results presented here were part of a broader analysis of patients with MM that utilized a control group of patients without MM. For the purposes of this current analysis and report, results for MM patients relative to the control group are not included. MM, multiple myeloma; N, number of patients.
Demographic characteristics of patients are shown in . MM patients who experienced SREs were slightly older, tended to have higher baseline CCI scores, had significantly greater follow-up time, and a greater proportion of patients had Medicare coverage than patients without SREs (). There were no statistically significant differences in gender, length of pre-enrollment, or geographic location between the two cohorts ().
Table 1. Patient characteristics.
The distributions of baseline comorbidities across both cohorts were evaluated in an effort to better characterize the health status of patients with MM. Despite a low frequency overall, comorbidities that were more frequent in the cohort with SREs vs those patients without SREs during the baseline period included chronic pulmonary disease (17.6% with SRE vs 10.9% without SRE, p = 0.003) and rheumatologic and connective tissue disease (5.5% with SRE vs 1.6% without SRE, p = 0.001). During the follow-up period, a significantly greater proportion of patients with SREs experienced cerebrovascular disease (24.3% vs 7.9%, p < 0.0001), chronic pulmonary disease (26.3% vs 14.4%, p < 0.001), congestive heart failure (26.2% vs 12.3%, p < 0.001), moderate or severe renal disease (31.5% vs 16.7%, p < 0.001), myocardial infarction (8.4% vs 2.5%, p < 0.001), and peripheral vascular disease (8.6% vs 4.9%, p = 0.022) than patients without SREs. No comorbidities were significantly more prevalent among patients without SREs than patients with SREs in the baseline or follow-up periods.
Frequency of SREs and bisphosphonate use in MM patients
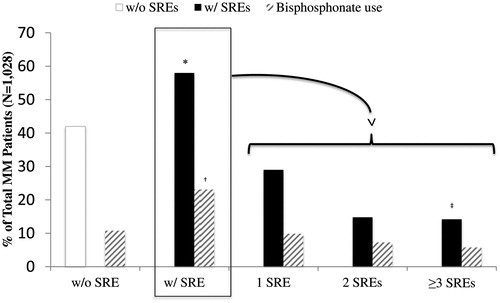
The median time to first SRE post-index date was 12.2 months (95% CI = 10–14.4). In the 58% of MM patients who experienced at least one SRE during the follow-up period, we examined the frequency of SREs during that timeframe (). Out of the 596 patients who experienced at least one SRE, 50.0% had one SRE, 26.0% had two SREs, and 24.0% had at least three SREs. Eighty-eight patients (14.8%; 88/596) had a SRE documented at their index date (MM diagnosis). For MM patients with SREs, 237 patients with SREs (39.8%; 237/596) had received BIS during the follow-up period. BIS use was reported among 34.2% (102/298) of patients with only one SRE, 49.3% (75/152) of patients experiencing two SREs, and 41.1% (60/146) among those experiencing ≥3 SREs. For MM patients without SREs, 25.7% (111/432) of patients received a BIS during the follow-up period.
Figure 2. Frequencies of SREs and bisphosphonate use in mutiple myeloma patients. *Eighty-eight patients had an SRE at the index date. †Thirty-five patients used a bisphosphonate before their first SRE. ‡Thirty-seven patients had four SREs, 19 had five SREs, and 22 had ≥6 SREs. Bisphosphonates included pamidronate, zoledronic acid, risedronate, alendronate, ibandronate, etidronate, and tiludronate. MM, multiple myeloma; SRE, skeletal-related event.
Comparison of HCRU rates for MM patients with and without SREs
The numbers of medical visits per patient month were analyzed during baseline and follow-up across several healthcare settings in an effort to characterize all-cause and cancer-related HCRU between patients with SREs vs patients without SREs. As shown in , the overall numerical trend in mean HCRU rates (PS-adjusted) across all settings was higher for MM patients with SREs during both the baseline and follow-up periods than for MM patients without SREs.
Figure 3. PS-adjusted rates of healthcare resource utilization among multiple myeloma patients. Standard error was estimated by 1000 bootstrapping replication; p-values for comparison were generated utilizing 2-sample t-tests. All-cause HCRU is comprised of cancer- and non-cancer-related HCRU. *p < 0.0001 (PS-Adjusted, All-cause: with SRE vs without SRE). †p < 0.0001 (PS-Adjusted, Cancer-related: with SRE vs without SRE). ‡p < 0.001 (PS-Adjusted, All-cause: with SRE vs without SRE). §p < 0.01 (PS-Adjusted, All-cause: with SRE vs without SRE). ¶p < 0.05 (PS-Adjusted, Cancer-related: with SRE vs without SRE). \\p ≤ 0.001 (PS-Adjusted, Cancer-related: with SRE vs without SRE). HCRU, healthcare resource use; MM, multiple myeloma; PS, Propensity score; SRE, skeletal-related events; w/, with; w/o, without.
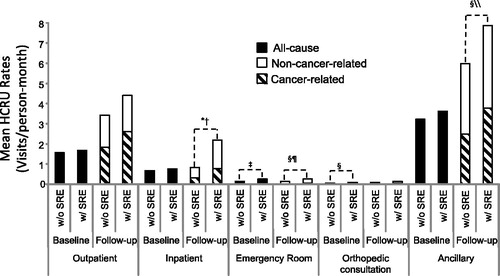
All-cause HCRU rates (medical visits/patient-month) at baseline were significantly higher for emergency room (0.26 vs 0.14; p < 0.001) and orthopedic consultation (0.10 vs 0.05; p < 0.01) for patients with SREs vs patients without SREs, respectively (). During the follow-up period, patients with SREs had significantly higher all-cause HCRU rates for inpatient (2.19 vs 0.83; p < 0.0001), emergency room (0.26 vs 0.15; p < 0.01), and ancillary (7.88 vs 5.99; p < 0.01) compared with patients without SREs, respectively ().
In addition, higher cancer-related HCRU rates during the follow-up period were observed for inpatient (mean = 0.78 vs 0.32, respectively; p < 0.0001), emergency room (mean = 0.05 vs 0.02, respectively; p < 0.05), and ancillary (mean = 3.77 vs 2.48; p = 0.001) in patients with and without SREs, respectively ().
Comparison of healthcare costs for MM patients with and without SREs
All-cause and cancer-related total healthcare costs (US dollars/patient-month) were also evaluated at baseline and during the follow-up period in MM patients with and without SREs (). Patients with SREs tended to have numerically higher total all-cause and cancer-related healthcare costs at baseline and during the follow-up period ().
Table 2. PS-adjusted costs (USD/patient-month) for multiple myeloma patients.
At baseline, all-cause total healthcare costs were similar between patients with SREs compared with patients without SREs in all settings, respectively, except for emergency room ($42 vs $22; p < 0.001) and ancillary ($346 vs $273; p < 0.05; ) costs which were higher in patients with SREs. During the follow-up period, all-cause healthcare costs specific to outpatient ($1101 vs $717; p < 0.05), inpatient ($1310 vs $620; p < 0.0001), and emergency room ($51 vs $26; p < 0.0001) were significantly greater for patients with SREs compared with patients without SREs ().
Forty-three percent and 38% of all-cause total costs were related to cancer in patients with and without SREs, respectively. Significantly higher cancer-related healthcare costs for outpatient ($644 vs $416; p < 0.05), inpatient ($761 vs $395; p < 0.001), and ancillary ($670 vs $413; p < 0.01) during the follow-up period were observed among patients with SREs compared with those without SREs, respectively ().
HCRU and costs as a function of the frequency of SREs
Since MM patients with SREs had greater HCRU and costs than patients without SREs, we also explored whether there were significant trends between the frequency of SREs and increases in HCRU and costs. As the frequency of SREs increased (no SRE, 1 SRE, 2 SREs, or ≥3 SREs), there was a significant increasing trend in HCRU rates across all healthcare settings evaluated (all p < 0.05; , top section). The same association held true for the trend in observed costs across all care settings as the frequency of SREs increased (all p ≤ 0.025), with the exception of orthopedic consultation (p = 0.250; , bottom section).
Table 3. PS-adjusted rates of HCRU and costs [USD] according to SRE frequency.
Discussion
Due to the continued positive advancements in survival for MM patients, reducing SRE incidence and burden have become increasingly important. As the literature suggests, up to 80% of newly diagnosed patients with MM have detectable bone lesions at diagnosis and are, therefore, at an increased risk for SRE developmentCitation7. More than half of the patients in the current study (58%) experienced a SRE that was reported in the medical claims during the period of observation following their diagnosis, and half of these patients experienced multiple SREs.
Clinical studies have linked BIS use to a lowered incidence of SREs in several cancersCitation2,Citation5. Of the patients with at least one SRE in the current study, ∼40% received BIS therapy. This was much lower than expected given guideline recommendations for MM patients for the time period associated with our studyCitation36–39. Despite moderate BIS use among patients in our study, SREs still occurred in more than half of MM patients (58%) within the study period, the frequency of which is consistent with other literature on this topicCitation12,Citation17,Citation18,Citation40. In a prospective, observational study (n = 238) that enrolled patients with MM, breast, prostate, and lung cancer from June 2008–December 2010, BIS use was also reportedCitation18. In this study, MM patients represented ∼20% (48/238 patients) of the total patient population and BIS use was reported among the majority of MM patients (n = 39/48; 81%)Citation18. Overall, 77% of all patients received BIS, yet 43% of all patients still experienced at least one SRE (SRE incidence per cancer type was not reported)Citation18.
In addition to describing SRE rates and BIS use in MM patients, the present study also identified the extent of resource use across different care settings. To better characterize the attribution of SREs to increasing HCRU and cost in patients with and without SREs, we PS-adjusted for baseline characteristics of patient age, CCI score, gender, insurance type, and geographic region. Overall, patients with SREs were associated with higher rates of all-cause HCRU both before (emergency room and outpatient) and after MM diagnosis (all healthcare settings except for orthopedic consultation) relative to patients without SREs.
Consistent with higher resource use, MM patients with SREs were also associated with significantly greater total costs during the baseline and follow-up periods. Forty-three percent of all-cause costs in the follow-up period were related to cancer; the remainder of which would be attributable to other co-morbidities and non-cancer diagnoses developed by these patients following their MM diagnosis. Of the total cancer-related healthcare costs, the distributions of cancer-related costs across categories evaluated were relatively similar (31–36% of total costs), except for emergency room and orthopedic consultation, which comprised a much smaller proportion of the overall total. Our study also demonstrated that, with increasing frequency of SREs, there were associated increases in the trend of HCRU for all healthcare settings and costs (except for orthopedic consultations), further emphasizing the value of preventing or reducing SREs in this patient population.
While PS analyses accounted for potential confounding factors, the accuracy of results from this analysis relies on measuring all possible confounding factors. The CCI was used to adjust for comorbidity burden across cohorts; however, there may be other unmeasured factors of health status not taken into consideration. For instance, significantly more patients with SREs had chronic obstructive pulmonary disease, rheumatoid arthritis, and connective tissue diseases at baseline than patients without SREs. Each of these diseases can be treated with corticosteroids, which in turn can induce bone loss and, thus, contribute to SRE occurrence and BIS treatment independently of MM. To address this potential bias, we adjusted for factors that could possibly affect the observed data. However, we realize that not all direct confounders could be controlled for due to the observational nature of this study. Nevertheless, it is doubtful that the above phenomenon could negate the strength of association we observed after adjusting for covariates.
This study analyzed Truven Health Marketscan Commercial Claims and Medicare Supplemental Databases, a robust claims data source that has been extensively evaluated across a number of cancer and non-cancer conditionsCitation41–46. However, while the median age of patients in our study is comparable to what has been reported elsewhereCitation47, the total sample and sub-groups of interest in the current study may not be demographically and socioeconomically representative of the overall US population with MM. Additionally, since medical insurance coding is not designed for research purposes, the accuracy of coding cannot be guaranteed and further studies utilizing medical chart review are recommended to validate the 90-day episode of care definition employed in this study. It is possible that if the region of the body affected by the SRE was not appropriately coded for by the provider, this could have led to either an over-estimation or under-estimation of the frequency of SREs, as well as an inappropriate distribution of cost and HCRU. Suggestions for future research include conducting separate analyses by SRE type and location across the multiple areas of HCRU, as only inpatient costs have been characterized previously for this patient populationCitation20. Additionally, it would be helpful to characterize costs and HCRU on the basis of whether patients had newly diagnosed or relapsed/refractory MM. This was not done in the current study given sample size constraints. Additionally, evaluation of BIS use was descriptively focused on frequency of utilization only and was not included in the broader cost and HCRU analysis. A more in-depth analysis of HCRU and costs, in particular for ancillary, would also be beneficial. Furthermore, an assessment of pharmacotherapy for the treatment of MM was not included as part of the current study given the focus on SRE objectives and previously published data on this topicCitation48.
Conclusion
This study complements the existing body of literature and offers additional insight on the association between SRE incidence, healthcare resource use, and SRE-related costs for insured individuals with MM in the US. A more thorough longitudinal investigation of pharmacotherapy use, including BIS utilization in MM patients, is warranted. This study suggests that preventing and/or reducing SREs should continue to be a priority when managing care for patients with MM.
Transparency
Declaration of funding
This study was sponsored by Eli Lilly and Company.
Declaration of financial/other relationships
All authors are employees of Eli Lilly and Company and own Eli Lilly and Company stock. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Appendix_1_Supplemental_Material.docx
Download MS Word (28 KB)Acknowledgments
The authors would like to acknowledge Jin Xie for statistical programming assistance and Karen T. Smith, PhD, and Chastity Bradley, PhD, for medical writing assistance.
References
- Theriault RL, Theriault RL. Biology of bone metastases. Cancer Control 2012;19:92-101
- Polascik TJ. Bisphosphonates in oncology: evidence for the prevention of skeletal events in patients with bone metastases. Drug Des Devel Ther 2009;3:27-40
- Coleman RE. Management of bone metastases. Oncologist 2000;5:463-70
- Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res 2013;13:483-96
- Terpos E, Dimopoulos MA, Berenson J. Established role of bisphosphonate therapy for prevention of skeletal complications from myeloma bone disease. Crit Rev Oncol Hematol 2011;77(1 Suppl):S13-S23
- Cancer Facts & Figures 2013. Atlanta: American Cancer Society, 2013. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf [Last accessed 26 August 2015]
- Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 2013;31:2347-57
- Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 1998;16:593-602
- Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med 1996;334:488-93
- Berenson JR, Rosen LS, Howell A, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 2001;91:1191-200
- Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol 2010;11:973-82
- Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 2001;7:377-87
- Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003;98:1735-44
- Zometa, Prescribing Information. East Hanover: Novartis, 2012. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021223s022lbl.pdf [Last accessed 11 March 2014].
- Aredia, Prescribing Information. East Hanover: Novartis, 2010. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020036s039lbl.pdf[Last accessed 11 March 2014].
- Anderson KC, Alsina M, Atanackovic D, et al. Multiple myeloma, version 2.2016. J Natl Compr Canc Netw 2015;13:1398-435.
- Theriault RL, Lipton A, Hortobagyi GN, et al. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J Clin Oncol 1999;17:846-54
- Mahmood A, Ghazal H, Fink MG, et al. Health-resource utilization attributable to skeletal-related events in patients with advanced cancers associated with bone metastases: results of the US cohort from a multicenter observational study. Community Oncology 2012;9:148-57
- Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care 2008;14:317-22
- Barlev A, Song X, Ivanov B, et al. Payer costs for inpatient treatment of pathologic fracture, surgery to bone, and spinal cord compression among patients with multiple myeloma or bone metastasis secondary to prostate or breast cancer. J Manag Care Pharm 2010;16:693-702
- Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol 2006;4:341-7
- Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ 2014;17:223-30
- Bahl A, Hoefeler H, Duran I, et al. Health Resource Utilization Associated with skeletal-related events in patients with advanced prostate cancer: a European subgroup analysis from an observational, multinational study. J Clin Med 2014;3:883-96
- Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol 2014;2:701-8
- Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus 2014;3:328-37.
- Duran I, Garzon C, Sanchez A, et al. Cost analysis of skeletal-related events in Spanish patients with bone metastases from solid tumours. Clin Transl Oncol 2014;16:322-9
- Hoefeler H, Duran I, Hechmati G, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: Results from a multinational retrospective - prospective observational study - a cohort from 4 European countries. J Bone Oncol 2014;3:40-8
- Rizzo M, Xu Y, Panjabi S, et al. A systematic literature review of the economic burden in multiple myeloma. ISPOR 17th Annual European Congress. November 8-12, 2014; Amsterdam, The Netherlands.
- Groot MT, Huijgens PC, Wijermans PJ, et al. Costs of multiple myeloma and associated skeletal-related events in The Netherlands. Expert Rev Pharmacoecon Outcomes Res 2004;4:565-72
- Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer 2007;109:2334-42
- Ashcroft J, Timothy B, Smith A, et al. Skeletal-related events in myeloma: A population-based study. 2013 American Society of Hematology (ASH) Annual Meeting and Exposition. December 7-10, 2013; New Orleans, LA.
- Truven Health Analytics. 2014 Truven Health Analytics MarketScan Publication and Trademark Guidelines; Ann Arbor, 2014.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statist Sci 1986;1:54-77
- Anderson KC, Alsina M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw 2009;7:908-42
- Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol 2002;20:3719-36
- Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc 2006;81:1047-53
- Durie BG. Use of bisphosphonates in multiple myeloma: IMWG response to Mayo Clinic consensus statement. Mayo Clin Proc 2007;82:516-17
- Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998;16:2038-44
- Chandran A, Bonafede MK, Nigam S, et al. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits 2015;8:148-58
- Bonafede MM, Kalra VB, Miller JD, et al. Value analysis of digital breast tomosynthesis for breast cancer screening in a commercially-insured US population. Clinicoecon Outcomes Res 2015;7:53-63
- David G, Gill M, Gunnarsson C, et al. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care 2014;20:e490-7
- Cappell KA, Shreay S, Cao Z, et al. Red blood cell (RBC) transfusion rates among US chronic dialysis patients during changes to Medicare end-stage renal disease (ESRD) reimbursement systems and erythropoiesis stimulating agent (ESA) labels. BMC Nephrol 2014;15:116
- Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest 2015;148:138-50
- Bonafede MM, Johnson BH, Watson C. Health care-resource utilization before and after natalizumab initiation in multiple sclerosis patients in the US. Clinicoecon Outcomes Res 2013;6:11-20
- SEER Stat Fact Sheets: Myeloma. Surveillance, Epidemiology, and End Results Program. Bethesda: National Cancer Institute, 2015. Available at: http://seer.cancer.gov/statfacts/html/mulmy.html [Last accessed 11 March 2014]
- Teitelbaum A, Ba-Mancini A, Huang H, et al. Health care costs and resource utilization, including patient burden, associated with novel-agent-based treatment versus other therapies for multiple myeloma: findings using real-world claims data. Oncologist 2013;18:37-45
