Abstract
Objective:
To assess the cost-effectiveness of delayed-release dimethyl fumarate (DMF, also known as gastro-resistant DMF), an effective therapy for relapsing forms of multiple sclerosis (MS), compared with glatiramer acetate and fingolimod, commonly used treatments in the US.
Methods:
A Markov model was developed comparing delayed-release DMF to glatiramer acetate and fingolimod using a US payer perspective and 20-year time horizon. A cohort of patients, mean age 38 years, with relapsing-remitting MS and Kurtzke Expanded Disability Status Scale (EDSS) scores between 0–6 entered the model. Efficacy and safety were estimated by mixed-treatment comparison of data from the DEFINE and CONFIRM trials and clinical trials of other disease-modifying therapies. Data from published studies were used to derive resource use, cost, and utility inputs. Key outcomes included costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios. Alternative scenarios tested in a sensitivity analysis included drug efficacy, EDSS-related or relapse-related costs, alternative perspectives, drug acquisition costs, and utility.
Results:
Base-case results with a 20-year time horizon indicated that delayed-release DMF increased QALYs +0.450 or +0.359 compared with glatiramer acetate or fingolimod, respectively. Reductions in 20-year costs with delayed-release DMF were −$70,644 compared with once-daily glatiramer acetate and −$32,958 compared with fingolimod. In an analysis comparing delayed-release DMF to three-times-weekly glatiramer acetate and assuming similar efficacy and safety to the once-daily formulation, 20-year costs with delayed-release DMF were increased by $15,806 and cost per QALY gained was $35,142. The differences in costs were most sensitive to acquisition cost and inclusion of informal care costs and productivity losses. The differences in QALYs were most sensitive to the impact of delayed-release DMF on disease progression and the EDSS utility weights.
Conclusion:
Delayed-release DMF is likely to increase QALYs for patients with relapsing forms of MS and be cost-effective compared with fingolimod and glatiramer acetate.
Introduction
Multiple sclerosis (MS) is a chronic, recurrent inflammatory disorder of the central nervous system characterized by periodic exacerbations accompanied or followed by progressive neurologic disabilityCitation1. MS generally is first diagnosed in young adults between 20–50 years of age, with the peak incidence at 30 yearsCitation1. Life expectancy for those with MS may be reduced by 5–10 years compared to those without MSCitation2. The annual prevalence in the non-institutionalized US adult population has been estimated to be 0.21% based on the nationally representative Medical Expenditure Panel Survey (MEPS) data from 1998–2009Citation3. MS has a significant detrimental and highly debilitating effect on the lives of most patients over many years, with the disease lasting an average of 30 years. The average direct annual medical care costs per patient for those with a diagnosis of MS in the US were estimated to be $23,434 in 2009, varying with disease symptoms and other co-morbid conditions and accounting for the fact that many people with MS are not taking disease-modifying therapies (DMTs)Citation4. DMTs that reduce the rate of relapse and slow disease progression in relapsing forms of MS account for the majority of the annual medical care costsCitation4.
Several DMTs are currently indicated for relapsing forms of MS, including the beta interferons, glatiramer acetate, natalizumab, delayed-release dimethyl fumarate, teriflunomide, and fingolimod. In randomized clinical trials, all these drugs have been shown to reduce the annualized risk for a relapse, and some have also been shown to slow disease progression, measured using the Kurtzke Expanded Disability Status Scale (EDSS)Citation5–14. These drugs vary both in their efficacy and safety profiles and in their dosing formulations and convenience to patients.
Delayed-release dimethyl fumarate, the most recently approved oral drug, has been shown in a pooled analysis of two clinical trials to reduce the annualized relapse rate, slow disease progression, and reduce the rate of appearance of new brain lesions on a magnetic resonance image. In one of the clinical trialsCitation5, glatiramer acetate was also included as a reference comparator and, in a post-hoc analysis, twice-a-day delayed-release dimethyl fumarate was shown to have greater efficacy for T2 lesions than glatiramer acetate and similar clinical efficacy. The most common adverse events associated with delayed-release dimethyl fumarate treatment were flushing and gastrointestinal events including diarrhoea, nausea, and abdominal pain. Other safety signals of note included a decrease in mean white blood cell and lymphocyte counts and a transient increase in hepatic transaminasesCitation15.
Because of the high costs associated with DMTs for MS, an economic evaluation for a new DMT, in addition to the efficacy and safety data, is an important piece of information for healthcare decision-makers. Many health plans in the US use estimates of the cost-effectiveness of a new drug as an input into their decision on whether to add the new drug to their formulary and what co-pays or co-insurance rates or other restrictions to apply. In this article we present the results of a cost-effectiveness analysis for delayed-release dimethyl fumarate compared with glatiramer acetate, a commonly used injectable DMT, and fingolimod, an oral DMT, for the treatment of relapsing-remitting multiple sclerosis (RRMS).
Methods
Overview
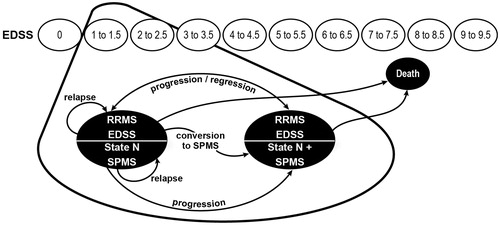
A Markov model was developed in which a cohort of patients with RRMS progressed through a series of disability states based upon the EDSSCitation16. The model structure was adapted from a previously developed UK model for delayed-release dimethyl fumarateCitation17, which was similar in structure to previous published modelsCitation18–20.
A cohort of patients with RRMS distributed among the different EDSS states as observed in the delayed-release dimethyl fumarate DEFINE and CONFIRM clinical trials entered the model. In this cohort, ∼60% of patients were treatment-naïve, and 40% had previously received at least one DMTCitation5,Citation6. Because delayed-release dimethyl fumarate is indicated for patients with relapsing forms of MS in the US without mention of previous treatments, this cohort is likely to be representative of those who might be prescribed the drug.
During the model time horizon patients could either progress to higher or lower EDSS states or remain in the same state at rates that depended on their EDSS state and the DMT treatment. Although no patients with secondary progressive MS (SPMS) were included in the DEFINE and CONFIRM clinical trials, in the model, patients could also progress to SPMS, where patients typically experienced fewer relapses but progressed to higher EDSS states at a faster rate than patients with RRMS. Patients who progressed to SPMS were assumed to enter the next stage of disease severity, as defined by the EDSS at the moment of transition.
Costs and utility values were assigned to each EDSS health state, as well as to adverse events (AEs) using published data or treatment algorithms. The model used efficacy data from all published trials and assumed discontinuation rates were the same for all DMTsCitation20 and all patients who were still receiving a DMT discontinued it when their EDSS score reached 7. Patient mortality rate was assumed dependent on age, sex, and EDSS state (i.e., disease severity).
The effect of treatment was modeled by changes to the EDSS progression/regression risks and relapse rates and the associated changes in health state—related costs and utilities. The model used 1-year cycles and was programmed to provide estimates for any time horizon between 1–50 years, with a base case of 20 years. The model outcomes, costs, life-years, and quality-adjusted life-years (QALYs) per patient during the model time horizon were dependent on the time spent in each EDSS state, the incidence of MS relapses, AEs from treatment, and the DMT received, as well as the time spent on treatment. A diagram of the model is shown in .
Setting and perspective
The model was developed for the US and presents the results from a payer perspective including only direct medical and formal care costs.
Population
The RRMS population initiating treatment was assumed to match that included in the delayed-release dimethyl fumarate clinical trials DEFINE and CONFIRMCitation5,Citation6. Patients with SPMS were excluded from these trials.
Treatment comparisons
The primary intervention in the model was a twice-daily regimen of delayed-release dimethyl fumarate. The two comparators used in the model were glatiramer acetate 20 mg once daily and fingolimod. Glatiramer acetate 40 mg three times weekly was used in an alternative scenario analysis.
Input parameter values
The model input parameters can be broadly classified into the following categories: population demographics; natural history parameters; treatment efficacy and safety and duration on treatment; costs; and utilities.
Population demographics
The population characteristics for the entering patient cohort included mean age, female-to-male ratio, and the distribution of EDSS scores at model entry. These characteristics were taken from the delayed-release dimethyl fumarate clinical trial data (DEFINE and CONFIRM) or from a US national survey (see ). In these trials, ∼60% of the patients were treatment naive and 40% had previously taken one or more DMTs.
Table 1. Cohort characteristics used in the delayed-release dimethyl fumarate cost-effectiveness model (all patients).
Natural history
To fully evaluate progression of disease without DMT, three independent annual transition probability matrices were used in the model: (1) transition matrix representing the probabilities of movement between EDSS states for the patient with RRMS (EDSS 0–9), (2) transition matrix representing the probabilities of the patient moving from RRMS to SPMS (EDSS 1–9), and (3) transition matrix representing the probabilities of movement between EDSS states for the patient with SPMS (EDSS 1–9). The natural history transition probability rates for patients with RRMS are shown in Supplementary Appendix A, Table A1. These transition probabilities were taken from the observed placebo data from the delayed-release dimethyl fumarate clinical trials (DEFINE and CONFIRM), supplemented with data from 806 patients with RRMS followed for an average of 34.7 years from 1972–2000 in the London Ontario Multiple Sclerosis registryCitation21. Alternative natural history disease progression rates were tested in the sensitivity analysis. The delayed-release dimethyl fumarate placebo transition rates for those with RRMS were used for EDSS states up through 7, and transition probabilities derived from the London Ontario registry data were used for EDSS states 8 and 9 because of the limited number of observations beyond EDSS 7 in the delayed-release dimethyl fumarate trials. The delayed-release dimethyl fumarate clinical trial placebo data indicated regression as well as progression in EDSS states in the population not receiving DMTs. The impact of excluding regression of the EDSS was tested in the sensitivity analysis. The probability of converting from RRMS to SPMS in each 1-year cycle was estimated using the time-to-SPMS data from the London Ontario database (see Supplementary Appendix A, Table A2). The transition probability matrix for patients with SPMS was also estimated using the data from the London Ontario database (Supplementary Appendix A, Table A3).
Annualized natural history relapse rates per person per year were taken from pooled baseline data from the delayed-release dimethyl fumarate clinical trials, which documented the annual relapse rate in the 12 months before enrollment in the studies. Data with adequate sample size were available only up to EDSS 5. Therefore, the relapse rate was computed using the relative risk of relapse in EDSS states 6–9 compared with the previous EDSS state from Patzold and PocklingtonCitation22. The Patzold and PocklingtonCitation22 study followed 102 patients with MS for 2 years and documented relapse rates. The relapse rates for those with SPMS were also derived from Patzold and PocklingtonCitation22, by adjusting them to reflect the higher relapse rates for those with RRMS in the DEFINE and CONFIRM clinical studies. The resulting annual relapse rates used in the model are shown in Supplementary Appendix A, Table A4. These represent the relapse rates that patients would experience if they were untreated with any DMT. Alternative natural history relapse rates were tested in the sensitivity analysis.
Finally, mortality rates in patients with MS have been shown to be significantly higher than those of the general population, including suicide as a significant cause of deathCitation23–26. Age- and sex-specific all-cause mortality rates for the general population were taken from US life tablesCitation27. These mortality rates were then adjusted upwards using the relative risk of death in an MS population compared with the general population taken from a study by PokorskiCitation24 performed for an insurance company in the US (see Supplementary Appendix A, Table A5). The relative risk of death for those with MS compared with the general population in this study varied by EDSS state, ranging from 1.3 for those with EDSS 1 to 5.31 for those with EDSS 9. It was assumed that the increased probability of mortality by age and EDSS state was the same across the RRMS and SPMS populations.
Treatment efficacy and duration on treatment
The effect of treatment with the DMTs was included in the model in three ways: (1) treatment effect on disability progression (hazard ratio for disability progression rates relative to placebo), (2) treatment effect on annualized relapse rates (relative rate in comparison to placebo), and (3) treatment effect multiplier on conversion to SPMS (multiplier set to 0% in the base case, i.e., no treatment effect on conversion to SPMS). The first two types of efficacy inputs were derived for all the DMTs included in the model using a mixed-treatment comparison (MTC) analysisCitation17,Citation28 based on a systematic literature search to identify all randomized clinical trials of DMTs for MS. The disability progression hazard ratios and the rate of relapses relative to placebo for the DMTs included in the model are shown in . The trials included in these estimates are shown in Supplementary Appendix A, Table A6.
Table 2. Treatment effect parameters for disability progression and relapse rate.
In the model, patients who discontinued from their initial therapy were assumed to take no further DMT. This assumption was made to be consistent with other published models of the cost-effectiveness of DMTs for RRMS, including the cost-effectiveness analysis sponsored by the US Agency for Healthcare Research and Quality (AHRQ) by Tappenden et al.Citation20. In addition, we assumed that the DMT annual discontinuation rates are the same for all DMTs and equal to 10% for the first 2 years after starting treatment and then fall to 3% per year for the remaining 10 years. These discontinuation rates were used in the Tappenden et al.Citation20 US cost-effectiveness analysis sponsored by the AHRQ. In the model, all patients still taking DMTs were assumed to stop treatment when their EDSS score reached 7 or above, although stopping treatment after conversion to SPMS if before EDSS reached 7 was tested in the sensitivity analysis.
Disease-modifying therapy and multiple sclerosis health state costs
DMT costs are reported in 2015 US dollars using published wholesale acquisition costs for April 8, 2015. Other MS-related medical care costs are reported in 2015 US dollars and were inflated to 2015 values where necessary using inflation indices for May 2015 from the Bureau of Labor StatisticsCitation29.
For each DMT, acquisition, administration, and monitoring costs were estimated in May 2015 US dollars. The annual acquisition cost was calculated using wholesale acquisition cost per pack for the anticipated dose multiplied by the number of expected packs used per year (see Supplementary Appendix A, Table A7). Annual acquisition costs were $66,612 for delayed-release dimethyl fumarate, $74,547 for daily glatiramer acetate, $65,103 for three times weekly glatiramer acetate, and $70,752 for fingolimod. Administration costs were assumed to be zero for the oral drugs and require some nurse education time for the injectable drug glatiramer acetate ($109.92 in year 1 and $36.64 in year 2) (see Supplementary Appendix A, Table A8). The annual cost of monitoring while on treatment was considered separately for the first year on treatment ($435.42 for delayed-release dimethyl fumarate, $342.38 for glatiramer acetate, $657.13 for fingolimod) and the subsequent years ($435.42 for delayed-release dimethyl fumarate, $342.38 for glatiramer acetate, $365.64 for fingolimod). The cost of monitoring was estimated from the expected resource use per patient per year on treatment, multiplied by the appropriate unit costs (see Supplementary Appendix A, Table A9).
The cost-effectiveness model takes into account two different types of MS disease-related costs: costs associated with disability progression through EDSS states, excluding the costs for DMTs, and an average incremental cost associated with an MS relapse. The base-case values for the direct costs (medical care + formal care costs) for each EDSS health state were calculated using linear interpolation from the values estimated from a survey of 1909 US patients by Kobelt et al.Citation30 using their values for EDSS <4, EDSS 4–6, and EDSS >6 and inflated to May 2015 US dollars using the Consumer Price Index for Medical Care from the US Bureau of Labor StatisticsCitation29 (see ). In the model, the EDSS-related costs for those with RRMS or SPMS are assumed to be the same for the same EDSS state. The cost for a relapse was estimated to be $2217, based on an estimate of the difference in annual costs for those with and without a relapse in the Kobelt et al.Citation30 US study, inflated to May 2015 US dollars.
Table 3. EDSS state costs used in the cost-effectiveness analysis: excluding costs of disease-modifying therapies.
Utilities
Utility weights for EDSS states without a relapse and during a relapse were derived from the delayed-release dimethyl fumarate clinical trial data by pooling observations for each EDSS state (0–9) and calculating the mean EuroQol EQ-5D index score for each state. The resulting base-case values are shown in . SPMS utilities were derived from the delayed-release dimethyl fumarate clinical trial data adjusted for the relationship between RRMS and SPMS utilities estimated in the UK MS surveyCitation31.
Table 4. Base-case utility scores per EDSS state.
Adverse events
The annual costs and disutility associated with AEs for each DMT were estimated based on the percentage of people experiencing each type of AE, the percentage of those AEs that were serious, the costs for serious and non-serious AEs, and the disutility and duration of each AE. AEs were included in the model if they met the following criteria: the most common AEs listed on the delayed-release dimethyl fumarate labelCitation15 (≥5% incidence in any treatment group of delayed-release dimethyl fumarate studies) or the common delayed-release dimethyl fumarate AEs on the label that have been reported in the published literature. AEs that had an incidence at least 3% higher in the total delayed-release dimethyl fumarate group than in the placebo group were also included, even if overall incidence in the delayed-release dimethyl fumarate arm was less than 5%. The AEs included for comparators were only those reported in delayed-release dimethyl fumarate studies, a conservative assumption. The proportion of each AE that was serious (grade 3 or 4) was also estimated using trial data. The incidence (and hence cost and disutility) of AEs was assumed to remain constant for all years that the patient continues to take the DMT.
The cost of treating each non-serious or serious AE that was included in the model was estimated from the expected resource use of treating a patient with a specific AE, taken from publicly available literature, validated by clinical expert opinion, and multiplied by appropriate unit costs and presented in May 2015 US dollars. In some situations—for example, where publicly available data were not available—a clinical expert who treats patients with MS provided the resource use estimates. The average annual cost for the treatment of all AEs for a patient on each DMT was estimated based on the estimates of annualized incidence of each AE, the proportion of AEs that were serious for that DMT, the cost for each serious AE, and the cost for each non-serious AE.
The disutility from AEs was obtained from a variety of sources or assumed where no data were available. The duration for each AE was based on clinical expert opinion. The average annual QALY loss for all AEs for a patient on each DMT was estimated based on the estimated annualized incidence of each AE, the proportion of AEs that were serious for that treatment, the utility loss for each serious AE, and the utility loss for each non-serious AE.
The average annual cost and QALYs lost attributable to AEs for each DMT are shown in . A detailed description of the methods and the input data used to estimate these values is provided in Supplementary Appendix B.
Table 5. Estimates of annual adverse event costs and quality-adjusted life-years lost.
Model assumptions
To construct a valid and robust model, a series of assumptions were made where there were no readily accessible data or where data limitations restricted the scope and flexibility of the disease pathway. Many of these have been mentioned in the sections above. They include: the assumption that those discontinuing treatment do not switch to another DMT, but follow natural history disease progression; no direct impact of treatment on conversion to SPMS or mortality but an indirect effect through the estimated delay in disease progression; and relapse treatment cost and utility loss not variable by EDSS state. A comprehensive list of the model assumptions is shown in Supplementary Appendix A, Table A10.
Base-case and sensitivity analyses
The following outcomes were estimated by the model: total costs, QALYs, and life-years; incremental costs, QALYs, and life-years gained; and incremental cost per QALY gained for the base-case input parameter values. In line with common US practice, a base-case discounting rate of 3.0% was applied to both costs and outcomes in the cost-effectiveness model.
To evaluate the effect of parameter uncertainty on the cost-effectiveness estimates, one-way and probabilistic sensitivity analyses were performed. The one-way sensitivity analysis tested the sensitivity of the results to changes in specific variables as follows:
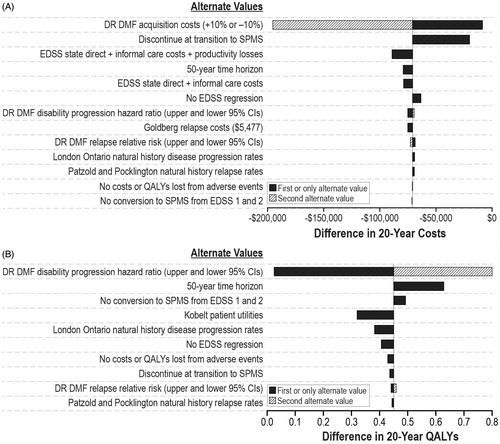
alternative estimates of delayed-release dimethyl fumarate treatment efficacy for disease progression and relapse using the 95% confidence interval estimates from the MTC analysis (see );
inclusion of informal care costs or informal care costs plus productivity losses in addition to direct medical and formal care costs for each EDSS state (see );
alternative values of the direct costs associated with each EDSS state, increasing and decreasing these values by 10%;
alternative estimates of the cost of a relapse, $5704 (in May 2015 US dollars), using the estimate presented in Goldberg et al.Citation32, based on treatment algorithms for a mild, moderate, and severe relapse developed by O’Brien et al.Citation33;
alternative annual costs of delayed-release dimethyl fumarate, increasing and decreasing these costs by 10%;
alternative assumptions about natural history rates of disease progression assuming either no regression of EDSS for the trial-based estimates or using only data from the London Ontario survey;
alternative assumptions about natural history relapse rates using the Patzold and PocklingtonCitation22 rates (see Supplementary Appendix A, Table A4);
alternative utility values for EDSS states using the estimates from the Kobelt et al.Citation30 study (the Kobelt US survey estimated a decrease of 0.094 in utility during a relapse, and utility values by EDSS were not presented separately for those with RRMS and SPMS [see Supplementary Appendix A, Table A11]);
assuming no costs or QALY losses associated with AEs;
assuming a 50 year time horizon instead of 20 years; and
assuming no transition from RRMS to SPMS until EDSS 3.
The probabilistic sensitivity analyses tested the sensitivity of the results in 1000 iterations allowing simultaneous changes in all the input parameter values using the following distributions: log-normal distributions for the efficacy parameters; gamma distributions for the cost inputs; log-normal distributions for treatment and AE disutility estimates and natural history relapse rates; and beta distributions for AE rates, discontinuation rates, and rates of transferring from RRMS to SPMS. For the efficacy parameters, the 95% confidence limits from the MTC were used to estimate the variability. For the other input parameters, a standard error of 10% was assumed.
Results
Results of the base-case analysis are shown in , with delayed-release dimethyl fumarate compared with glatiramer acetate, once daily and fingolimod. Base-case results with a 20-year time horizon indicated that delayed-release dimethyl fumarate was less costly and produced more QALYs than both fingolimod (−$33,059, +0.359 QALY) and glatiramer acetate, once a day (−$70,810, +0.450 QALY).
Table 6. Discounted (3.0%) cost-effectiveness model outcomes per patient: 20-year costs and QALYs.
Results of the sensitivity analysis of delayed-release dimethyl fumarate compared with glatiramer acetate once a day and fingolimod are shown in and , respectively. The impact of delayed-release dimethyl fumarate on the disability progression rate, the use of the Kobelt utility values and the model time horizon had the greatest impact on the incremental QALYs for both comparisons. The acquisition cost for delayed-release dimethyl fumarate and including informal care and productivity losses in the EDSS state costs had the greatest impact on the incremental costs for both comparisons. The results of the probabilistic sensitivity analysis showed that, compared with fingolimod or glatiramer acetate once a day, there was at least a 98% probability that delayed-release dimethyl fumarate was the cost-effective treatment at all threshold levels.
Table 7. Cost-effectiveness results from sensitivity analyses of a comparison with Fingolimod.
Discussion
The results of the base-case analysis comparing treatment of RRMS with delayed-release dimethyl fumarate with treatment with glatiramer acetate or fingolimod indicated that use of delayed-release dimethyl fumarate would result in an increase in quality-adjusted life years with a decrease in total treatment costs, including the cost of the DMT, over a 20-year time horizon. In an extensive one-way sensitivity analysis, the results were most sensitive to the acquisition cost of delayed-release dimethyl fumarate, the cost categories (direct costs, informal care costs, productivity costs) included in the disease-related costs, the efficacy of delayed-release dimethyl fumarate, and EDSS utility weights. Our results for the comparison with fingolimod are similar to those estimated by Zhang et al.Citation34. This economic evaluation use a Markov model with a shorter (5-year) time horizon to estimate the cost-effectiveness of delayed-release dimethyl fumarate compared with fingolimod and teriflunomide, and showed that delayed-release dimethyl fumarate had lower costs (−$39,802 compared with fingolomod and −$25,940 vs teriflunomide) and higher quality-adjusted life years (0.03 compared with fingolimod and 0.04 vs teriflunomide).
Several studies have shown that the economic burden of patients with MS increases with increasing levels of disabilityCitation30,Citation33,Citation35. The economic burden of MS includes both direct medical care and formal care (e.g., nursing home and home help) costs, as well as informal care costs by family members or friends and productivity losses for the patient and, possibly, also for family membersCitation4,Citation30,Citation36,Citation37. In the Kobelt et al.Citation30 US survey, direct costs, other than those for DMTs, made up 29% of the total annual costs of MS, and informal care cost and productivity losses made up 37% of the total annual costs. Treatment with DMTs that can slow the rate of disease progression will prolong the time during which the patient is able to be functionally independent and is likely to reduce both the direct costs of care for MS symptoms and the costs associated with informal care and productivity losses.
There are several strengths in the modeling approach used in this article. First, we have presented the results of a detailed economic evaluation of delayed-release dimethyl fumarate compared with two other commonly prescribed DMTs for RRMS that was designed to meet the recently published ISPOR Task Force guidelines for cost-effectiveness analysisCitation38. The cost-effectiveness analysis used a model structure and assumptions that have been used extensively in RRMS. Second, since the clinical trials for DMTs for MS included both placebo-controlled studies and active-comparator studies, a formal MTC analysis was performed based on a systematic literature search to identify all published clinical data. The results of this analysis were used to estimate reduction in annualized risk of relapse and the hazard ratio for disease progression for the DMTs compared in this analysis.
Limitations
Limitations in the modeling approach include the lack of long-term data for the more recently approved DMTs. Thus, assumptions were needed about long-term efficacy and safety and discontinuation rates for delayed-release dimethyl fumarate, fingolimod and 3-times weekly glatiramer acetate. Our sensitivity analyses indicated that the results would be sensitive to these assumptions.
Our focus on adverse events that were associated with delayed release dimethyl fumarate might have resulted in the omission of some adverse events associated only with fingolimod or glatiramer acetate. Our sensitivity analysis indicated that our results changed very little when adverse events were excluded from the analysis.
Finally, after discontinuation, the model did not include the possibility of switching to a second DMT, but assumed return to symptomatic treatment only. This allowed us to isolate the impact of the initial treatments being compared but did not provide any information on the optimal sequencing of treatments for those with RRMS. Currently data are not available indicating how response to treatment differs depending on the sequence in which the treatment is used.
Conclusions
Delayed-release dimethyl fumarate is likely to increase QALYs for patients with relapsing forms of MS and be cost-effective compared with other commonly used DMTs.
Transparency
Declaration of funding
The study was sponsored by Biogen, Inc. and funding provided to RTI Health Solutions to develop the model. Employees from Biogen worked collaboratively with RTI Health Solutions to design the model, collect the input data, interpret the results and prepare the manuscript.
Declaration of financial/other relationships
JM is an employee of RTI Health Solutions, a consulting company that received funding from Biogen, the manufacturer of delayed-release dimethyl fumarate, to develop the cost-effectiveness model. RI, MF, SS, and TL are employees of Biogen Idec. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary material is available online.
IJME_A_1135805_Supplementary_material.pdf
Download PDF (197.3 KB)Acknowledgments
The US model was an adaptation of a UK cost-effectiveness model which was developed for the UK by Maria Malmenas and Anna Walker from HERON Commercialization.
References
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 2010;9:A387-94
- Runia TF, van Pelt-Gravesteijn ED, Hintzen RQ. Recent gains in clinical multiple sclerosis research. CNS Neurol Disord Drug Targets 2012;11:497-505
- Campbell JD, Ghushchyan V, McQueen B, et al. Burden of multiple sclerosis on direct, indirect costs and quality of life: National US estimates. MS Relat Disord 2014;3:227-36
- Owens GM, Olvey EL, Skrepnek GH, et al. Perspectives for managed care organizations on the burden of multiple sclerosis and the cost-benefits of disease-modifying therapies. J Manag Care Pharm 2013;19(1 Suppl A):S41-S53. http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=16103. Accessed July 9, 2015
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97
- Gold R, Kappos L, Arnold DL, et al.; DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107
- IFNB Multiple Sclerosis Study Group [No authors listed]. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1995;45:1277-85
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268-76
- Kappos L, Radue EW, O'Connor P, et al.; FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387-401
- O'Connor P, Wolinsky JS, Confavreux C, et al.; TEMSO Trial Group. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293-303
- Polman CH, O'Connor PW, Havrdova E, et al.; AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899-910
- PRISMS Study Group [No authors listed]. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352:1498-504; erratum in Lancet 1999:353:678
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group [No authors listed]. PRISMS-4: long term efficacy of interferon-b-1a in relapsing MS. Neurology 2001;56:1628-36; erratum in Neurology 2001;57:1146
- Biogen Idec Inc. Tecfidera (dimethyl fumarate) delayed-release capsules, for oral use. 2013. http://www.tecfidera.com/pdfs/full-prescribing-information.pdf. Accessed April 16, 2013
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52
- Biogen Idec. Cost-effectiveness analysis of Tecfidera® in patients with relapsing remitting multiple sclerosis. Version 18.0 technical report. Data on file. Cambridge MA: Biogen, Inc.; 2013
- Chilcott J, McCabe C, Tappenden P, et al.; Cost Effectiveness of Multiple Sclerosis Therapies Study Group. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ 2003;326:522
- Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics 2008;26:617-27
- Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease-modifying therapies in the management of multiple sclerosis for the Medicare population. Value Health 2009;12:657-65
- Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain 2010;133:1914-29
- Patzold U, Pocklington PR. Course of multiple sclerosis. First results of a prospective study carried out of 102 MS patients from 1976–1980. Acta Neurol Scand 1982;65:248-66
- Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004;127:844-50
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med 1997;29:101-6
- Sadovnick AD, Ebers GC, Wilson RW, et al. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992;42:991-4
- Sumelahti ML, Tienari PJ, Wikström J, et al. Survival of multiple sclerosis in Finland between 1964 and 1993. Mult Scler 2002;8:350-5
- Kochanek KD, Xu J, Murphy SL, et al. Deaths: final data for 2009. Natl Vital Stat Rep 2011;60:601-117
- Hutchinson M, Fox RJ, Havrdova E, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin 2014;30:613-27
- Bureau of Labor Statistics (BLS). Consumer price index (CPI) for medical care. 2015. Washington DC. http://www.bls.gov/cpi/cpid1505.pdf. Accessed June 26, 2015
- Kobelt G, Berg J, Atherly D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006;66:1696-702
- Orme M, Lerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54-60
- Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm 2009;15:543-55
- O'Brien JA, Ward AJ, Patrick AR, et al. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res 2003;3:17
- Zhang X, Hay JW, Niu X. Cost effectiveness of fingolimod, teriflunomide, dimethyl fumarate, and intramuscular interferon-β1a in relapsing-remitting multiple sclerosis. CNS Drugs 2015;29:71-81
- Karampampa K, Gustavsson A, Miltenberger C, et al. Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler 2012;18(2 Suppl):7-15
- Morrow TJ. The costs and consequences of multiple sclerosis relapses: a managed care perspective. J Neurol Sci 2007;15(1 Suppl):S39-44
- Whetten-Goldstein K, Sloan FA, Goldstein LB, et al. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler 1998;4:419-25
- Caro JJ, Briggs AH, Siebert U, et al.; ISPOR-SMDM Modeling Good Research Practices Task Force. Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—1. Value Health 2012;15:796-803