Abstract
Objective:
Falls are associated with neurogenic orthostatic hypotension (nOH) and are an economic burden on the US healthcare system. Droxidopa is approved by the US FDA to treat symptomatic nOH. This study estimates the cost-effectiveness of droxidopa vs standard of care from a US payer perspective.
Methods:
A Markov model was used to predict numbers of falls and treatment responses using data from a randomized, double-blind trial of patients with Parkinson’s disease and nOH who received optimized droxidopa therapy or placebo for 8 weeks. The severity of falls, utility values, and injury-related costs were derived from published studies. Model outcomes included number of falls, number of quality-adjusted life-years (QALYs), and direct costs. Incremental cost-effectiveness ratios (ICERs) were calculated. Outcomes were extrapolated over 12 months.
Results:
Patients receiving droxidopa had fewer falls compared with those receiving standard of care and gained 0.33 QALYs/patient. Estimated droxidopa costs were $30,112, with estimated cost savings resulting from fall avoidance of $14,574 over 12 months. Droxidopa was cost-effective vs standard of care, with ICERs of $47,001/QALY gained, $24,866 per avoided fall with moderate/major injury, and $1559 per avoided fall with no/minor injury. The main drivers were fall probabilities and fear of fall-related inputs.
Limitations:
A limitation of the current study is the reliance on falls data from a randomized controlled trial where the placebo group served as the proxy for standard of care. Data from a larger patient population, reflecting ‘real-life’ patient use and/or comparison with other agents used to treat nOH, would have been a useful complement, but these data were not available.
Conclusion:
Using Markov modeling, droxidopa appears to be a cost-effective option compared with standard of care in US clinical practice for the treatment of nOH.
Introduction
Patients with neurogenic orthostatic hypotension (nOH) experience a decrease in blood pressure upon standing that is the result of an inadequate norepinephrine response from vasomotor neuronsCitation1. Patients with Parkinson’s disease (PD) or other neurodegenerative diseases in which there is autonomic nervous system dysfunction may develop nOH. The most frequently reported symptoms of nOH include lightheadedness, dizziness, feeling ‘like you might black out’, and syncopeCitation1,Citation2.
In 2014, droxidopa (Northera, Lundbeck LLC, Deerfield, IL), a norepinephrine pro-drug, was approved by the US Food and Drug Administration for the treatment of orthostatic dizziness, lightheadedness, or the ‘feeling that you are about to black out’ in adult patients with symptomatic nOH caused by primary autonomic failure (PD, multiple system atrophy, and pure autonomic failure [PAF]), dopamine β-hydroxylase deficiency, or non-diabetic autonomic neuropathyCitation3. During a 10-week trial in which falls were assessed as a secondary efficacy end-point, patients receiving droxidopa experienced fewer falls and fall-related injuries compared with placeboCitation4,Citation5. A significant difference in the rate of falls per patient-week between droxidopa and placebo (0.40 vs 1.70; p = 0.018) was found in post-hoc analysis using a Poisson-inverse Gaussian (PIG) distribution model because of non-normal distribution of the falls data in this studyCitation6.
As nOH is a relatively rare disorder with few treatment options, limited information identifying burden of illness or cost-effectiveness of treatment is available. However, falls and fall-related injuries associated with nOH may represent a substantial burden of disease for individual patients as well as the healthcare system in general. Patients with nOH, or sub-optimal nOH management, may limit their daily activities and experience a decreased quality-of-life because of the increased potential for falls and fall-related injuries. The resultant healthcare costs from fall events are likely to represent a significant economic burden on the US healthcare system. Using data from the 10-week droxidopa clinical trial, a previous analysis estimated the costs of falls and drug therapy for patients with nOH associated with PD but did not examine other treatment benefits or costs not related to fallsCitation7.
To further understand the impact of droxidopa compared with standard of care in nOH treatment, a cost-effectiveness analysis was performed from a US payer perspective.
Methods
For the cost-effectiveness analysis, the population of interest included patients with symptomatic nOH associated with PD. The interventions of interest were droxidopa treatment for 6 months, followed by standard of care for 6 months, compared with standard of care for 12 months. A Markov model with two modules was developed; the first module predicted the number of falls of different severity and the second module predicted the impact of improvement in nOH symptoms (treatment response) with droxidopa treatment.
Model structure
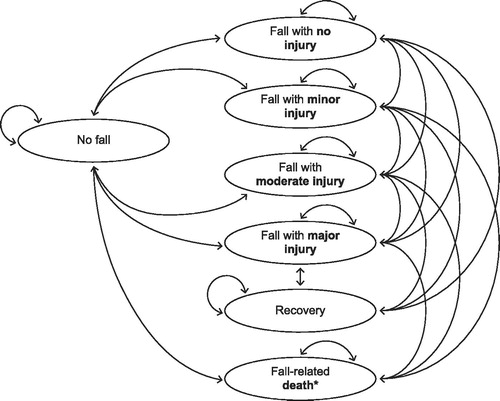
The Markov model included a total of eight health states, seven of which were fall-related (including fall-related death). Death from any cause was included as the eighth health state. The health states and possible transitions between them are depicted in . A second component of the model accounted for treatment-induced improvement in nOH symptoms (i.e., treatment response). Treatment response was defined as a ≥50% improvement at week 8 from baseline in the Orthostatic Hypotension Symptom Assessment for dizziness or lightheadedness (item 1)Citation2. The time horizon was 12 months; the cycle length was 1 week. The assumptions used in the development of the scenario are outlined in .
Table 1. Summary of model assumptions.
Patients and treatments
Data from a phase 3, double-blind, placebo-controlled randomized trial of droxidopa (NOH306; ClinicalTrials.gov identifier: NCT01176240) provided the basis of the population cohort, fall probability data, and treatment response rates used in the simulation. Details and primary results from this study have been previously describedCitation4,Citation5. Briefly, patients with PD and symptomatic nOH received either placebo or an optimized dose of droxidopa (100–600 mg 3-times daily) for 8 weeks, after an initial titration period of up to 2 weeks. The number of patient-reported falls from baseline to the end of the study, as recorded daily in electronic diaries, was assessed as a secondary efficacy variable.
Because all patients participating in the randomized trial were allowed to use non-pharmacologic interventions for nOH management, as well as fludrocortisone (a frequently used treatment for nOH), the placebo group was used as a proxy for standard of care. Of note, however, the use of midodrine (the only other FDA-approved treatment for nOH) was not permitted during the study. A comparison to midodrine was not possible because no information on the effect of midodrine on falls could be identified. Furthermore, comparisons of other efficacy outcomes were hampered by the different assessments and scales used in the midodrine and droxidopa studies.
After 6 months of treatment, patients in the droxidopa treatment group were assumed to switch to standard of care and its associated probability of fall estimates.
Model inputs
A hypothetical cohort of 10,000 patients with a mean age of 72.4 years (the average participant age in study NOH306) was used in the model. The key model inputs derived from the NOH306 trial and published literature are summarized in .
Table 2. Key model inputs for base analysis and DSA analysis.
Falls
Weekly fall probabilities were calculated based on fall rate data from NOH306 as determined from the PIG modelCitation6. The distribution of falls by severity was derived from a published study of fall probability for elderly people in a residential setting in AustraliaCitation8; reported probabilities were re-calculated to re-integrate the probability of fall-related death.
Based on published data from a study that showed that 6940 of 14,665 fall-related injuries among elderly adults involved fractures likely requiring lengthy bed rest, the probability of recovery after a major injury was determined to be 47.3%Citation9.
Cost estimation
Costs were estimated from the perspective of payers in the US healthcare system (i.e., only direct US costs). Direct costs were the costs of droxidopa treatment, nOH management, and falls. To calculate the cost of droxidopa treatment, the average dose from study NOH306 was used; a contract dispensing rate of 86% was assumed, and the patient co-payment of $7 per week was deducted from the treatment cost. Costs associated with nOH management were general practitioner and neurologist visits at treatment initiation and follow-up. Throughout the model timeframe, it was assumed that patients treated with standard of care and non-responders in the droxidopa group had two general practitioner visits and one neurologist visit every 3 months. For the period between the treatment assessment (8 weeks) and the end of droxidopa treatment, it was assumed that treatment responders had two general practitioner visits and one neurologist visit every 4 months. The unit costs of physician visits were estimated from the MarketScan (Truven Health Analytics, Ann Arbor, MI) Medicaid database for 2012.
Costs associated with falls varied according to fall severity and were derived from published reports. For an injurious fall (considered a moderate injury) and a fall requiring hospitalization (considered a major injury), costs were derived from a study published by Davis et al.Citation10. The cost for a fall-related death was derived from a report published by Stevens et al.Citation11. All costs were adjusted to 2014 values using the Consumer Price Index (restricted to medical care) from 2006–2014. Cost for a fall with minor injury was taken from a study published by Haines et al.Citation8, updated to 2014 using the Australian healthcare Consumer Price Index and converted to US dollars. No cost discounts were applied, because the time horizon was only 1 year.
Mortality
The model included fall-related death and all-cause death. The probability of death resulting from a fall was calculated as 0.37% based on published literatureCitation8. The weekly probability of all-cause death for patients between 72–73 years of age was calculated to be 0.046%, based on US life tablesCitation12.
Utilities
The health state utility corresponding to stage 2 of PD in the Hoehn and Yahr scaleCitation13 (the stage reported by 39.3% of all patients [45.5% of symptomatic patients] in NOH306) was 0.7, which was derived from data reported by Siderowf et al.Citation14. The utilities of fall states were calculated by applying utility decrements associated with fall, fracture, and fear of falling (FoF) to the base utility assigned to the ‘no fall’ state. The utility of the ‘no fall’ state was selected to reflect the average utility of patients with PD, regardless of whether they had nOHCitation14. Utility decrements for fall, fracture, and FoF were determined from a study published by Iglesias et al.Citation15. Utility decrements for different degrees of FoF identified by Iglesias et al.Citation15 were applied using the approach reported by Latimer et al.Citation16 and include the following: fall with no injury assumed to cause FoF ‘a little bit of the time’, fall with minor injury assumed to cause FoF ‘a little bit of the time’, fall with moderate injury assumed to cause FoF ‘a good bit of the time’, and fall with major injury assumed to cause FoF ‘all the time’. The duration of FoF was set to 26 weeks for the purpose of the model, as previous studies have indicated that falls in the previous 6–12 months had an impact on FoF and avoidance of activitiesCitation15,Citation17,Citation18.
To determine the utility increment of treatment response, it was assumed that treatment responders would have less activity avoidance than non-responders and that this would translate into a similar improvement in health-related quality-of-life reported by patients with PD when they have better control over their PD symptoms. As such, Palmer et al.Citation19 reported that patients with PD in Hoehn and Yahr stages 1.5 and 2.5 had mean health state values of 0.85 and 0.78, respectively, for no recurrence of PD symptoms at any time. This increase of 0.07 (for improvement in nOH symptoms) was applied as the utility increment to treatment response in the current analysis.
Estimation of outcomes
The outcomes evaluated in the model were the number of falls (total and by severity, including the number of fall-related deaths) and the number of quality-adjusted life-years (QALYs). The number of QALYs was determined from the utility decrements associated with falls, fractures, FoF, and time (patient-weeks) spent in each health state. A utility decrement associated with potential fall-related injury was subtracted for each fall according to the severity of the injury. An average utility decrement for all falls except those with major injury was considered. For falls with major injury, a utility decrement corresponding to the impact of a fracture in terms of utility was considered.
The model did not include a specific health state to account for FoF; therefore, the losses in utility associated with FoF were calculated a posteriori based on the calculated number of falls by severity. A QALY decrement was subtracted a posteriori for each fall (i.e., several QALY decrements were subtracted for patients experiencing multiple falls over the 26 weeks).
The calculation of QALYs also took into account treatment response. The utility increase for treatment responders was applied for the time between the response assessment (8 weeks) and the end of treatment.
Cost-effectiveness analysis
The cost-effectiveness of treatment was evaluated using incremental cost-effectiveness ratios (ICERs), which were calculated from the difference in costs between the droxidopa treatment and standard-of-care groups (incremental costs), divided by the difference in the number of falls, or the difference in the number of QALYs between treatment groups. ICERs were calculated per QALY gained, per avoided fall with no/minor injury, and per avoided fall with moderate/major injury.
Sensitivity analyses
Two types of sensitivity analyses, a deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA), were used to determine the robustness of the cost-effectiveness results. The varied parameters and corresponding values used in the DSA are shown in . In the PSA, the model was run 5000 times, with the inputs being drawn at random from their statistical distributions. The parameters and sources used for the PSA are shown in . The results were presented in the form of ‘tornado’ charts, cost-effectiveness planes, and cost-effectiveness acceptability curves.
Table 3. PSA parameters and sources.
Results
Effectiveness outcomes
Patients treated with droxidopa had fewer falls vs those treated with standard of care in every fall severity category (). During the time horizon of 12 months, droxidopa treatment was associated with a total of 106,643 avoided falls per 10,000 patients. Treatment with droxidopa provided a gain of 0.33 QALYs per patient vs standard of care. The QALY gain with droxidopa treatment was associated with a lower number of falls and a higher number of responders (patients with improved nOH symptoms).
Table 4. Number of falls per 10,000 patients by treatment group.
Cost outcomes
The cost of droxidopa treatment for 6 months was estimated to be $30,112 (). However, avoidance of falls as a result of treatment with droxidopa provided an estimated cost savings of $14,574 compared with standard of care. Total costs were $66,414 and $50,827 for droxidopa and standard of care, respectively, for a difference of $15,587.
Table 5. Total and incremental costs per patient by treatment group in US dollars.
Cost-effectiveness outcomes
Treatment with droxidopa was found to be cost-effective based on the provision of greater health benefits compared with standard of care. The ICERs from a payer perspective were $47,001 per QALY gained, $24,866 per avoided fall with moderate/major injury, and $1559 per avoided fall with no/minor injury.
Sensitivity analyses
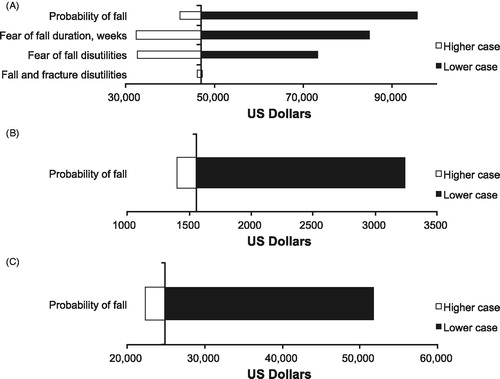
In the DSA, fall probability was the only parameter with an impact on costs, with a higher probability of falls in both the droxidopa and standard-of-care groups associated with a smaller cost difference. QALYs were impacted by fall probabilities, FoF duration, FoF disutilities, and fall/fracture disutilities. The parameters with the greatest impact on QALYs were the FoF parameters, with a longer FoF duration and greater FoF disutilities generating a greater difference in QALYs between droxidopa and standard of care. ICER per QALY results were most sensitive to the variation in fall probabilities and the FoF parameters (). Higher fall probabilities were associated with a smaller ICER per QALY gained, whereas shorter FoF duration and lower FoF disutilities were associated with a higher ICER per QALY gained. ICER per avoided fall was the most sensitive to variations in probabilities of falling, irrespective of the fall type ().
Figure 2. Deterministic sensitivity analyses for ICER per QALY (A), avoided fall with no/minor injury (B), and avoided fall with moderate/major injury (C). ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
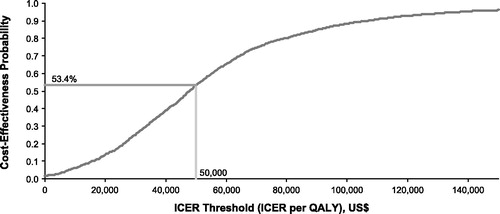
The PSA suggested that droxidopa had an ∼53% probability of being cost-effective vs standard of care for a threshold of $50,000 per QALY gained (). The ICER threshold corresponding to the 50% probability for droxidopa to be cost-effective was $1550 per avoided fall with no/minor injury and $24,600 per avoided fall with moderate/major injury.
Discussion
The cost-effectiveness of droxidopa was evaluated using falls data from a randomized controlled trialCitation4,Citation5 of droxidopa for input into a Markov model. From a US payer perspective, the ICER was $24,866 per year per avoided fall with moderate/major injury and $47,001 per year per QALY gained compared with standard of care. Although $50,000 per QALY gained has been arbitrarily considered the cost-effectiveness threshold, a recent report suggests that use of this single value may be a conservative estimate (especially for resource-abundant countries, such as the US), which does not account for inflation, increases in healthcare spending, and shifts in societal perception of value associated with health gains. Therefore, it has been suggested that cost-effectiveness analyses using multiple thresholds (i.e., $50,000, $100,000, and $200,000 per QALY) may be more appropriateCitation20. Additionally, the World Health Organization (WHO) considers interventions to be cost-effective if they are 1–3-times the gross domestic product (GDP) per capitaCitation21. Using the WHO benchmark, our economic analysis of droxidopa suggests a treatment benefit, based on the estimated US GDP per capita for 2014 (slightly more than $54,000)Citation22,Citation23.
To our knowledge, no economic analyses of nOH treatment have been reported other than our previous study of the financial impact of care for falls and drug acquisition during the 10-week droxidopa clinical trial linearly expanded to 6 monthsCitation7. Compared with our earlier analysis, the current Markov model cost-effectiveness study may provide more conservative estimates for 6-month drug supply costs ($26,281 vs $30,112) and cost savings due to an avoidance of falls ($18,823 vs $14,574). The differences may be attributed to the enhanced ability of the Markov model to estimate the impact of treatment beyond the clinical trial duration and a more conservative assumption on falls.
The key drivers of the model were the probability of a fall and treatment response (i.e., improvement of NOH symptoms); sensitivity analyses have indicated that the results are sensitive to changes in the distribution of falls by severity, the costs of falls, and the inputs associated with FoF. Because no data for the distribution of fall severity in orthostatic hypotension or nOH populations could be identified, data from a fall severity study of Australian residential seniors was selected as a surrogateCitation8. In sensitivity analyses using fall severity data from other patient populationsCitation16,Citation24, droxidopa was considered to be a dominant option compared with standard of care for both QALY and avoided fall outcomes.
A study in a residential care population by Haines et al.Citation8 was selected as the source of falls requiring medical attention because it provided a systematic capture of all falls and it used the WHO definition of a fallCitation25, which was comparable to the definition used in the droxidopa clinical trial. Additionally, the Haines et al. study may provide more conservative estimates of the proportion of falls leading to medical attention (and, thus, subsequent costs) than other identified studies. For example, Haines et al. report higher rates for falls resulting in no harm (81.1% vs 64.7%) and lower rates of falls requiring hospitalization (2.8% vs 3.6%) than another study of falls in hospitalized patientsCitation26. In other studies, reported rates of falls resulting in a fracture varied from 6.9% (11/160 institutionalized patients)Citation27 to at least 10% (in a modeling study of nursing home falls)Citation28. Of note, the fall-related hospitalization rate in Haines et al. (2.8%) is similar to the rates observed in other studies of analogous fall outcomes in cohorts of community-dwelling elderly (e.g., 1.9% of falls leading to hospitalization [13 patients hospitalized following 677 falls]Citation29 and a 1.7% hip fracture rate in patients with ≥1 fall [5085 fractures/294,184 fall events])Citation30. The transferability of the Haines et al. findings (patients in an aged-care facility without PD) to patients with PD is supported by a study that suggests a similar proportion of falls resulting in injury in patients with PD and in a community-dwelling healthy elderly population without PDCitation31.
The values used for a fall with moderate injury and a fall with major injury in our study were based on the costs of an injurious fall and costs per fall requiring hospitalization reported in a systematic literature reviewCitation10. However, because some values appear to represent charges rather than costs, their use may over-estimate actual costs. For the sensitivity analyses, cost data represented immediate-care costs only (e.g., doctor visits and emergency department/hospitalization costs related to falls). Because any other associated costs of falling, such as rehabilitation or follow-up care, were not included, the cost estimates may not account for the full economic impact associated with falls in patients with nOH. Also, because falls are associated with increased admission to long-term care facilitiesCitation32,Citation33, falls may represent a greater burden (in both financial and human terms) to patients, their families, and society in general than could be estimated by our analysis.
Fear of fall-related inputs (i.e., duration and utility decrement values) also had a large impact on the cost-effectiveness results as measured by QALY outcomes. Because studies determining actual duration of FoF were not available, a 26-week duration for FoF was assumed, based on previous reportsCitation15,Citation17,Citation18,Citation34. In our model, patients who fell multiple times were attributed an FoF utility decrement with each fall. Our model did not account for the possibility that FoF effects do not accumulate after a certain number of falls, although increased falls are associated with increased FoF and activity avoidanceCitation17.
Other assumptions used to limit the complexity of our models may have affected the results. In our model, the number of falls per week was limited to one; however, this simplification may have under-estimated fall-related costs and treatment-associated cost savings. Patients with nOH may fall multiple times during a week; in the droxidopa clinical trial, the average fall rate in the placebo group was 2.1-times per week. Also, it was assumed that non-responders continued treatment, despite those non-responders in standard of care not benefiting from droxidopa treatment.
For our evaluation, the placebo group in the droxidopa clinical trial was used as a proxy for standard of care. This limits the interpretation of our findings because in the US midodrine is also approved for the treatment of nOHCitation35; however, no direct information on falls in the midodrine trials nor direct comparisons between droxidopa and midodrine for the treatment of nOH are available, and indirect comparisons between droxidopa and midodrine were not feasible in our study. Although the inability to compare with midodrine may be a limitation of our study, in the droxidopa clinical trial all patients were instructed to use non-pharmacological interventions and pharmacotherapy with fludrocortisone was allowed. Thus, employment of these nOH management strategies may be considered a realistic assumption of standard of care for the placebo group and support the interpretation of our findings. In a study of alcohol dependency, the use of placebo as a proxy in an analysis was accepted by the National Institute for Health and Care Excellence for a Single Technology AppraisalCitation36,Citation37. Additionally, the exact mechanisms by which droxidopa reduced falls in this trial, or whether midodrine might reduce falls in a similar fashion and to a similar extent as droxidopa, are not known. Although reduced falls may be expected because of symptomatic improvements in nOH, other mechanisms may contribute to the reduction in falls observed with droxidopa treatment, including benefits for freezing of gait or attentionCitation38,Citation39.
A limitation of the current study is the reliance on falls data from patients participating in a randomized controlled trial. Data from a larger and more diverse patient population with nOH that reflected ‘real-life’ use of droxidopa would have been a useful complement, but these data are not available yet. The assumptions and methods employed in our analysis (e.g., FoF utility decrements, number of falls per week limits, and continuation of treatment in non-responders, as previously discussed) may also have affected the results. Furthermore, falls in patients with PD may have many causes (e.g., freezing/festination, neurologic factors, and postural instability)Citation40, and it has been suggested that droxidopa could have a positive effect on freezing of gaitCitation39. However, any effects of droxidopa beyond improvements related to nOH were not evaluated during the clinical trial.
Another limitation of this study was the use of short-term clinical trial results to extrapolate effects for a 6-month period. However, use of modeling to estimate economic outcomes is considered appropriate, especially if limited data are available or the collection of additional data would be challengingCitation41. Subsequent evaluations of the long-term effectiveness of droxidopa will be bolstered by data from a 6-month observational study of the drug that is currently underway.
Conclusions
Further research on the probability of injurious fall, the costs related to the negative consequences of FoF, and the impact on quality-of-life and functioning is needed for more precise estimates of the cost-effectiveness of droxidopa treatment. With the approval of droxidopa, treatment data from an expanded patient population will serve to help refine the model. Using 10-week clinical trial data, droxidopa appears to be a cost-effective option compared with standard of care for the treatment of patients with nOH from a US payer perspective, based on the savings associated with avoiding falls and fall-related injuries.
Transparency
Declaration of funding
The data reported in this work were derived from clinical trials and a post-hoc analysis funded by Lundbeck LLC.
Declaration of financial/other relationships
CF and LAH are employees of Lundbeck. RAH has received honoraria or payments for consulting, advisory services, or speaking services in the past 12 months from Acadia Pharmaceuticals, Auspex Pharmaceuticals, Acorda Therapeutics, AstraZeneca, Cowan Therapeutics, Gerson Lehrman Group (GLG), Allergan, AbbVie, Biotie Therapies, Chelsea Therapeutics (now Lundbeck NA Ltd), Cynapsus Therapeutics, Impax Laboratories, Lundbeck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson’s Research, Neurocrine Biosciences, Novartis, Pfizer, Inc., Teva Pharmaceuticals, GuidePoint Global, and UCB BioSciences, Inc. RAH is employed by the University of South Florida (FL) and is supported in part by a Center of Excellence grant from the National Parkinson Foundation. RAH’s institution has received research support in the past 12 months from Abbott Laboratories, Addex Therapeutics, Allergan, AstraZeneca, Chelsea Therapeutics, Inc., GE Healthcare, Impax Laboratories, Ipsen Biopharmaceuticals, Merck/MSD, Merz, the Michael J. Fox Foundation for Parkinson’s Research, Schering-Plough, Teva Neuroscience, UCB, and Vita-Pharm. JD, EK, and SA have provided consultancy services to Lundbeck. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors received medical writing and editorial assistance from the CHC Group (North Wales, PA), which was supported by Lundbeck.
References
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69-72
- Kaufmann H, Malamut R, Norcliffe-Kaufmann L, et al. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 2012;22:79-90
- Northera (droxidopa). Full Prescribing Information. Deerfield, IL: Lundbeck NA Ltd, 2014
- Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson's disease (NOH306A). J Parkinsons Dis 2014;4:57-65
- Hauser RA, Isaacson S, Lisk JP, et al. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (nOH306B). Mov Disord 2015;30:646-54
- Rowse G, Heritier S, Hewitt L. Falls during a 10-week placebo-controlled study of droxidopa for neurogenic orthostatic hypotension in Parkinson’s disease. Clin Auton Res 2014;24:230
- François C, Hauser RA, Kaufmann H, et al. Impact of reduction in falls for patients with PD and NOH: post hoc economic analyses of phase 3 clinical trial data on droxidopa. Mov Disord 2015;30:86-7
- Haines TP, Nitz J, Grieve J, et al. Cost per fall: a potentially misleading indicator of burden of disease in health and residential care settings. J Eval Clin Pract 2013;19:153-61
- Roudsari BS, Ebel BE, Corso PS, et al. The acute medical care costs of fall-related injuries among the U.S. older adults. Injury 2005;36:1316-22
- Davis JC, Robertson MC, Ashe MC, et al. International comparison of cost of falls in older adults living in the community: a systematic review. Osteoporos Int 2010;21:1295-306
- Stevens JA, Corso PS, Finkelstein EA, et al. The costs of fatal and non-fatal falls among older adults. Inj Prev 2006;12:290-5
- Bell FC, Miller ML. Life tables for the United States Social Security Area 1900–2100. United States of America Social Security Administration. http://www.ssa.gov/OACT/NOTES/as120/LifeTables_Body.html. Accessed June 24, 2015 Baltimore, MD; August 2005
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42
- Siderowf A, Ravina B, Glick HA. Preference-based quality-of-life in patients with Parkinson's disease. Neurology 2002;59:103-8
- Iglesias CP, Manca A, Torgerson DJ. The health-related quality of life and cost implications of falls in elderly women. Osteoporos Int 2009;20:869-78
- Latimer N, Dixon S, Drahota AK, et al. Cost-utility analysis of a shock-absorbing floor intervention to prevent injuries from falls in hospital wards for older people. Age Ageing 2013;42:641-5
- Bertera EM, Bertera RL. Fear of falling and activity avoidance in a national sample of older adults in the United States. Health Soc Work 2008;33:54-62
- Delbaere K, Crombez G, Vanderstraeten G, et al. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing 2004;33:368-73
- Palmer CS, Schmier JK, Snyder E, et al. Patient preferences and utilities for ‘off-time’ outcomes in the treatment of Parkinson's disease. Qual Life Res 2000;9:819-27
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- World Health Organization. Cost-effectiveness Thresholds. http://www.who.int/choice/costs/CER_levels/en/. Accessed July 10, 2015 Washington, DC;
- International Monetary Fund. World Economic Outlook Database, April 2015. 2015. http://www.imf.org/external/pubs/ft/weo/2015/01/weodata/index.aspx. Accessed July 14, 2015 Washington, DC
- World Bank. GDP per capita, PPP (current international $), World Development Indicators database. 2015. http://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD?order=wbapi_data_value_2013+wbapi_data_value+wbapi_data_value-last&sort=desc. Accessed July 14, 2015 Washington, DC
- Wielinski CL, Erickson-Davis C, Wichmann R, et al. Falls and injuries resulting from falls among patients with Parkinson's disease and other parkinsonian syndromes. Mov Disord 2005;20:410-15
- Carroll NV, Delafuente JC, Cox FM, et al. Fall-related hospitalization and facility costs among residents of institutions providing long-term care. Gerontologist 2008;48:213-22
- Heinrich S, Rapp K, Rissmann U, et al. Cost of falls in old age: a systematic review. Osteoporos Int 2010;21:891-902
- Sorensen SV, de Lissovoy G, Kunaprayoon D, et al. A taxonomy and economic consequences of nursing home falls. Drugs Aging 2006;23:251-62
- Nurmi I, Sihvonen M, Kataja M, et al. Falls among institutionalized elderly–a prospective study in four institutions in Finland. Scand J Caring Sci 1996;10:212-20
- Irvine L, Conroy SP, Sach T, et al. Cost-effectiveness of a day hospital falls prevention programme for screened community-dwelling older people at high risk of falls. Age Ageing 2010;39:710-16
- Craig J, Murray A, Mitchell S, et al. The high cost to health and social care of managing falls in older adults living in the community in Scotland. Scott Med J 2013;58:198-203
- Allcock LM, Rowan EN, Steen IN, et al. Impaired attention predicts falling in Parkinson's disease. Parkinsonism Relat Disord 2009;15:110-15
- Basic D, Hartwell TJ. Falls in hospital and new placement in a nursing home among older people hospitalized with acute illness. Clin Interv Aging 2015;10:1637-43
- Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med 1997;337:1279-84
- Bowling A, Seetai S, Morris R, et al. Quality of life among older people with poor functioning. The influence of perceived control over life. Age Ageing 2007;36:310-15
- Parsaik AK, Singh B, Altayar O, et al. Midodrine for orthostatic hypotension: a systematic review and meta-analysis of clinical trials. J Gen Intern Med 2013;28:1496-503
- Laramee P, Brodtkorb TH, Rahhali N, et al. The cost-effectiveness and public health benefit of nalmefene added to psychosocial support for the reduction of alcohol consumption in alcohol-dependent patients with high/very high drinking risk levels: a Markov model. BMJ Open 2014;4:e005376
- Stevenson M, Pandor A, Stevens JW, et al. Nalmefene for reducing alcohol consumption in people with alcohol dependence: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 2015;33:833-47
- Study to assess the clinical benefit and safety of droxidopa in Parkinson’s Disease 2015. https://clinicaltrials.gov/ct2/show/study/NCT02066571. Accessed December 16, 2015
- Devos D, Moreau C, Dujardin K, et al. New pharmacological options for treating advanced Parkinson's disease. Clin Ther 2013;35:1640-52
- Rudzinska M, Bukowczan S, Stozek J, et al. Causes and consequences of falls in Parkinson disease patients in a prospective study. Neurol Neurochir Pol 2013;47:423-30
- Buxton MJ, Drummond MF, Van Hout BA, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ 1997;6:217-27