Abstract
Objective:
Brain metastases among lung cancer patients can impair cognitive and functional ability, complicate care, and reduce survival. This study focuses on the economic burden of brain metastasis in lung cancer—direct healthcare costs to payers and indirect costs to patients, payers, and employers—in the US.
Methods:
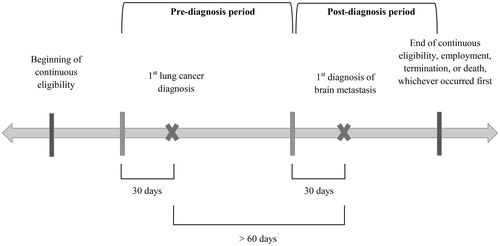
Retrospective study using claims data from over 60 self-insured Fortune 500 companies across all US census regions (January 1999–March 2013). Adult, non-elderly lung cancer patients with brain metastasis were evaluated over two study periods: (1) pre-diagnosis (≤30 days prior to first observed lung cancer diagnosis to ≤30 days prior to first-observed brain metastasis diagnosis) and (2) post-diagnosis (≤30 days prior to first observed brain metastasis diagnosis to end of continuous eligibility or observation).
Outcome measures:
Healthcare costs to payers and resource utilization, salary loss to patients, disability payouts for payers, and productivity loss to employers.
Results:
A total of 132 patients were followed for a median of 8.4 and 6.6 months in the pre- and post-diagnosis periods, respectively. At diagnosis of brain metastasis, 21.2% of patients were on leave of absence and 6.1% on long-term disability leave. Substantial differences were observed in the pre- vs post-diagnosis periods. Specifically, patients incurred much greater healthcare utilization in the post-diagnosis period, resulting in $25,579 higher medical costs per-patient-per-6-months (PPP6M). During this period, patients missed significantly more work days, generating an incremental burden of $2853 PPP6M in salary loss for patients, $2557 PPP6M in disability payments for payers, and $4570 PPP6M in productivity loss for employers.
Limitations:
Type of primary lung cancer and extent of brain metastasis could not be assessed in the data. The analysis was also limited to patients with comprehensive disability coverage.
Conclusions:
Development of brain metastasis among lung cancer patients is associated with a substantial economic burden to payers, patients, and employers.
Introduction
Brain metastases are a prevalent and devastating neurologic complication of cancer. Although their precise incidence is unclear, due to under-detection and under-reporting, Gavrilovec and PosnerCitation1 estimate that 37,000 new cases emerged in the US in 2003 among patients with primary lung, breast, melanoma, renal, or colorectal cancers. Due to increasing incidence of primary cancers and extension of survival from new therapy options, the incidence of brain metastasis is rising, putting a greater population at riskCitation2,Citation3. Although all primary tumors can progress in the brain, lung cancer accounts for at least half of the cases with brain metastasisCitation4–6. In fact, brain metastases are estimated to afflict as many as 17–88% of patients with small cell and non-small cell lung cancers during their disease courseCitation7–10.
Development of brain metastasis can disrupt brain function and cause a range of symptoms including neurologic dysfunctions, behavioral abnormalities, cognitive failures, and even deathCitation2. Common presentations include headaches, gait disturbances and nausea, hemiparesis, memory problems, and mood/personality changesCitation11–14. Supportive treatment for brain metastasis is often focused on preserving patients’ neurocognitive and functional ability. However, these efforts can sometimes add to patients’ comorbidity burden via side-effects from medications like steroids and anti-epileptic drugsCitation15. In addition, survival following the development of brain metastasis is generally short, even among patients with initially effective treatment responsesCitation4. Median survival ranges from 2–15 months, depending on primary tumor location, number and type of metastases, systemic disease control, and the patient’s overall healthCitation3,Citation16. Patients with newly diagnosed brain metastases are typically managed with surgery and/or radiation therapyCitation17, and studies have suggested that certain chemotherapy (pemetrexedCitation18, or platinum-based therapies with etoposideCitation19 or topotecanCitation20) and targeted therapies (epidermal growth factor receptor [EGFR]-targeted therapiesCitation21,Citation22, bevacizumabCitation23, or anaplastic lymphoma kinase [ALK] inhibitorsCitation24–26) may improve survival.
The economic burden of brain metastasis has been demonstrated to be substantial. A claims analysis of data records from 2004–2010 estimated that the mean monthly, all-cause, healthcare costs for lung, melanoma, and breast cancer patients with brain metastasis were $17,007, $23,426, and $19,708, respectively (2010 US dollars (USD))Citation3. More strikingly, a study examining breast cancer during 2002–2004 found that the mean 6-month healthcare costs for patients with brain metastasis were $60,045 compared with $28,193 for patients without brain metastasis (2006 USD)Citation27. Drivers of this difference included more physician office visits, more pharmacy claims, and longer and more frequent hospital stays among patients with brain metastasisCitation27. Another claims study over 2011–2013 examined healthcare costs of crizotinib-treated patients with ALK positive (ALK+) non-small cell lung cancer (NSCLC) following the development of brain metastasis. This study found that monthly healthcare costs increased from an average of $5983–$22,645 per patient after the development of brain metastasis (2013 USD)Citation28. The main contributors to this near 4-fold increase included costs for pharmacy prescriptions and hospital-administered therapies, inpatient care, and outpatient services.
A more comprehensive assessment of the economic burden of brain metastasis among lung cancer patients in terms of direct and indirect costs has not yet been done. The objective of this study was to investigate not only healthcare utilization and direct costs of care, but also indirect costs in the US, which have not been previously examined in the literature for brain metastasis. Further, the study examines the drivers of both direct and indirect costs, outlining a detailed review of the economic burden of brain metastasis.
Methods
Data source and patient sampling
The Optum Health Reporting and Insights Database is a large administrative-claims database, representing 82 self-insured Fortune 500 companies from across all US census regions. Claims data from this database, containing enrolment records, as well as all medical and pharmacy claims, for over 18 million privately-insured individuals covered by these companies were included, spanning the period from January 1, 1999 to March 31, 2013. For 41 of the 82 companies, productivity loss data were available for ∼4.2 million lives, including short- and long-term disability and medically-related absenteeism. For these individuals, salary information was also available. Consistent with the requirements of the Health Insurance Portability and Accountability Act, all patient data were de-identified prior to collection and use for this study and were exempt from Institutional Review Board (IRB) review.
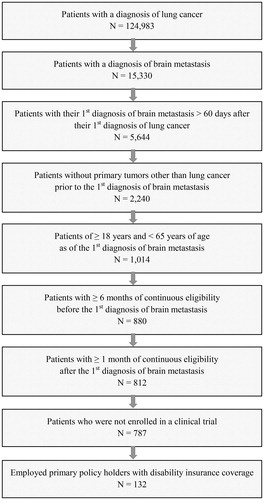
Patients were selected for the study if they met the following requirements: (1) ≥1 diagnosis of lung cancer (ICD-9-CM: 162.xx); (2) ≥1 diagnosis of brain metastasis (ICD-9-CM: 198.3x and 198.4x); (3) first diagnosis for brain metastasis >60 days after first diagnosis for lung cancer; (4) no primary tumors other than lung cancer prior to the first diagnosis of brain metastasis; (5) ≥18 and <65 years of age as of the first diagnosis of brain metastasis; (6) ≥6 months of continuous health plan enrollment before the first diagnosis of brain metastasis; (7) ≥1 month of continuous health plan enrollment following the first diagnosis of brain metastasis; (8) no claims indicative of participation in a clinical trial (ICD-9-CM: V70.7x); and (9) employment as a primary policy-holder with disability insurance coverage. Patient counts following the application of each of the requirements are shown in .
For each patient meeting the sample selection criteria, the observation period was divided into a pre-diagnosis period and a post-diagnosis period (). The pre-diagnosis period started 30 days prior to the first observed lung cancer diagnosis and ended at the start of the post-diagnosis period 30 days prior to the first observed brain metastasis diagnosis. The start of the pre-diagnosis period was chosen in order to better capture the costs related to the detection and diagnosis of lung cancer, and the end of the pre-diagnosis period was similarly chosen, to better capture the costs related to the detection and diagnosis of brain metastasis. The post-diagnosis period extended through the end of continuous eligibility or the end of data availability, whichever occurred first.
Outcomes
Study outcomes included healthcare resource utilization, direct, and indirect healthcare costs. For healthcare resource utilization, five outcomes were assessed—(1) inpatient admissions, (2) inpatient days, (3) outpatient visits, (4) emergency room (ER) visits, and (5) other medical services. For the last category, the following care sites were examined separately: patient home care, hospice, and extended care facility (e.g. skilled nursing facilities, nursing homes).
Direct healthcare costs were evaluated from a payer perspective and encompassed all treatments and care from inpatient, outpatient, ER, pharmacy, and other care settings. Furthermore, aggregate costs were stratified by procedures, broad disease and co-morbid condition categories (defined by the ICD-9 classification), drugs, and sites of care. For all analyses, healthcare costs were adjusted for inflation and expressed in 2013 USD, using the Bureau of Labor Statistics (BLS) Consumer Price Index (CPI) for medical care.
Indirect costs were considered from patient, payer, and employer perspectives. From the patient perspective, disability- and absenteeism-related salary losses were considered. Disability-related salary loss was calculated as the difference between the wages that patients would have received if they had not been on disability leave and the disability benefits they received. Absenteeism-related salary loss was calculated as a patient’s number of unpaid sick days multiplied by their daily wage. From the payer perspective, the outcome was the total disability benefit paid out to patients. Both short- and long-term disability wage replacement indemnities were considered. Finally, from the employer perspective, the outcome was the total cost that they incurred due to productivity loss. This was measured as the number of working days lost due to employees’ disability leave and medically-related absenteeism multiplied by the employees’ respective daily wage rates. To clarify, medically-related absenteeism and disability leave are mutually exclusive categories of absenteeism, where medically-related absenteeism is defined as sick days/sick leave taken off from work due to ill health that is not related to a specific disability, while disability leave is defined as paid time off work for a reason related to someone’s disability. The following assumptions were made for the indirect cost: (1) six sick leave days were assumed to be fully covered by employers per calendar yearCitation29; (2) 261 days of paid work (assuming a 5-day working week) were assumed to take place per year; and (3) inpatient days and ER admissions were assumed to account for a full day of absenteeism while outpatient and other medical service visits were assumed to account for half a day of absenteeism. All indirect costs were adjusted for inflation and expressed in 2013 USD, using the BLS’s CPI.
Statistical analysis
All outcomes were compared between the pre- and post-diagnosis periods and reported on a per-patient-per-six-months (PPP6M) basis. Statistical comparisons between patients’ pre- and post-diagnosis periods were made using non-parametric tests, specifically McNemar’s test for dichotomous variables and Wilcoxon’s signed-rank tests for continuous variables.
Results
Patient characteristics
A total of 15,330 patients with brain metastasis were identified from an initial pool of 124,983 lung cancer patients. Of these, 787 met all sample selection criteria except for the disability insurance criterion, and 132 met all criteria. The mean age of the selected patients at the first diagnosis of brain metastasis was 55.4 years, with 17% of patients between 41–50 years of age and 63% between 51–60 years of age, inclusive (). Approximately 42% were female, and 73% had health plans with preferred provider organization or point of service arrangements. With respect to region of residence, the South, Midwest, Northeast, and West each comprised 37%, 27%, 26%, and 10% of the sample, respectively.
At the time of the first diagnosis of brain metastasis, ∼21% were already on a leave of absence (non-disability), and 6% were on long-term disability leave. Patients came from a variety of industries, with almost 75% from the following four industries: technology, transport, manufacturing and packaging, and telecommunications. Patients had a mean Charlson-Quan comorbidity index (CCI)Citation30 of 5.7, with a median of 6.0. Brain metastasis was not included in the CCI score calculation. The three most common comorbidities were chronic obstructive pulmonary disease, hypertension, and diabetes, experienced by 46%, 30%, and 14% of patients, respectively.
The mean observation length for the pre-diagnosis period was 12.8 months, with a median of 8.4 months. The observation length for the post-diagnosis period was slightly shorter, with a mean of 12.5 months and median of 6.6 months.
Healthcare utilization
Following the development of brain metastasis, patients’ utilization of hospital-based care increased both in their number of inpatient admissions as well as their number of inpatient days. Inpatient admissions more than doubled from a mean value of 0.8 PPP6M in the pre-diagnosis period to 1.9 PPP6M in the post-diagnosis period (p-value <0.001) (). Consistently, the number of inpatient days increased from a mean of 6.0 PPP6M to 16.7 PPP6M (p-value <0.001). ER visits also rose for patients following the development of brain metastasis, but appeared only marginally significant (p = 0.054).
Outpatient visits showed no significant change from the pre-diagnosis to the post-diagnosis period (p = 0.261), remaining fairly constant at mean values of 41.5 PPP6M and 37.7 PPP6M. Use of medical services for day-to-day patient care, however, increased following the development of brain metastasis. Specifically, patients had 3.3 PPP6M (p-value <0.001) more home care visits (e.g., related to infusion/injection procedures and equipment, nursing services, oxygen and respiratory equipment), and 0.8 PPP6M (p-value = 0.007) more extended care facility visits during the post-diagnosis period. Additionally, the number of days in a hospice increased from 0 PPP6M to 3.6 PPP6M in the post-diagnosis period (p-value <0.001).
Direct healthcare costs
Direct healthcare costs of patients increased markedly following development of brain metastasis, as shown in . Specifically, the mean all-cause cost increased from $70,157 PPP6M to $86,027 PPP6M (p-value =0.021). This increase was driven by the mean medical service cost increasing from $44,608 PPP6M to $70,187 PPP6M, with the increase in medical costs being particularly driven by the costs of inpatient care. The average inpatient care cost increased from $17,586 PPP6M to $40,481 PPP6M (p-value <0.001). For ER services, the mean cost rose from $337 PPP6M to $846 PPP6M, a 150% increase (p-value = 0.037). For the other medical services, including patient home care service, hospice, and extended care facility, the mean costs rose from $872 PPP6M to $2097 PPP6M, a 140% increase. Outpatient care costs, in contrast, showed no significant change, with mean values of $25,813 PPP6M and $26,763 PPP6M in the pre-diagnosis and post-diagnosis periods, respectively (p-value = 0.558). These trends in costs, by type of service, were consistent with expectations of brain-metastasis patients’ greater severity, more intensive care needs, and reduced autonomy; accordingly, these patients displayed increased costs for more intensive treatment as found in hospital settings and day-to-day support services from sources like home health, extended care facilities, and hospice.
Table 1. Patient characteristics at first observed diagnosis of brain metastasis.
Table 2. Comparison of healthcare resource utilization during the pre- and post-diagnosis periods.
The average cost of the four procedures frequently used to treat and monitor brain tumors—radiotherapy, SRS, imaging, and x-ray tests—increased significantly: cost for radiotherapy increased by $731 PPP6M, SRS increased by $686 PPP6M, and imaging tests $412 PPP6M (all p-values <0.05). X-ray tests, in contrast, showed no significant change (p-value = 0.128).
Medical costs by disease category were also summarized. shows the results for a sub-set of categories and conditions (as defined by the ICD-9 classification) related to the development of brain metastasis: (1) neoplasms, (2) diseases of the nervous system and sense organs, (3) diseases of the circulatory system, and (4) symptoms, signs, and ill-defined conditions. Costs for conditions within the categories with significant pre-/post-diagnosis differences and absolute values greater than $100 PPP6M are presented in . Costs associated with neoplasm-related care were the largest among the four broad disease categories, and experienced the greatest increase from the pre-diagnosis to the post-diagnosis period. For the pre-diagnosis period, these costs averaged $35,341 PPP6M, while for the post-diagnosis period they averaged $58,646 PPP6M, a 66% increase of $23,305 PPP6M (p-value <0.001). Within this disease category, malignant neoplasm care accounted for a vast majority of the costs. Major contributors to this expense category included care for brain metastasis and lung cancer, which moved in opposite directions from the pre-diagnosis to the post-diagnosis period. More specifically, brain metastasis care costs totaled $0 in the pre-diagnosis period (by study construction), increasing to a mean value of $26,263 PPP6M in the post-diagnosis period. Lung cancer care costs, in contrast, averaged $32,499 PPP6M in the pre-diagnosis period, falling to an average of $23,532 PPP6M in the post-diagnosis period. It is unclear, however, if this drop reflects a true decrease in lung cancer care costs or if it is an artifact of coding. The latter could occur if care services were received for a combination of conditions, but physicians and/or coders only noted a sub-set of these on patients’ claims. For instance, an oncologist visit in the post-diagnosis period may have addressed both brain metastasis and lung cancer concerns, but have only received a claim code for brain metastasis, the more recent and pressing condition.
Table 3. Comparison of healthcare costs between pre- and post-diagnosis periods.
Increasing medical costs were also observed for the other three broad disease categories from the pre-diagnosis to the post-diagnosis period. Significance, however, was only present for one of them—diseases of the nervous system and sense organs. For this disease group, mean costs rose from $451 PPP6M to $2367 PPP6M from the pre-diagnosis to the post-diagnosis period, a 425% increase (p-value <0.001). Notable contributors to this included complications related to brain metastasis, such as cerebral edemas ($1297 PPP6M cost increase; p-value <0.001), epilepsy and recurrent seizures ($152 PPP6M cost increase; p-value = 0.012), and degenerative or hereditary central nervous system diseases ($201 PPP6M cost increase; p-value = 0.003).
Although the total cost increases of the other two broad disease categories—circulatory system diseases and symptoms, signs, and ill-defined conditions—were not statistically significant overall, some statistically significant increases were found when these costs were stratified into components relevant to brain metastasis. For diseases of the circulatory system, these included cerebrovascular diseases and pulmonary circulation diseases. For the former, medical costs rose from a mean value of $197 PPP6M to $2019 PPP6M from the pre-diagnosis to the post-diagnosis period (p-value <0.001). Among these cerebrovascular diseases, intracerebral hemorrhage accounted for a considerable portion of the cost increase, climbing from a mean value of $0 PPP6M to $1119 PPP6M from the pre-diagnosis to the post-diagnosis period (p-value = 0.031). For pulmonary circulation diseases, medical costs experienced a smaller increase of $581 PPP6M, rising from a mean value of $671 PPP6M to $1252 PPP6M (p-value = 0.043).
Among symptoms, signs, and ill-defined conditions, a number of common symptoms of brain metastasis exhibited significant cost increases. These included alteration of consciousness ($442 PPP6M cost increase; p-value <0.001), convulsions ($1560 PPP6M cost increase; p-value <0.001), dizziness and giddiness ($211 PPP6M cost increase; p-value = 0.009), and malaise and fatigue ($127 PPP6M cost increase; p-value <0.001). Symptoms involving the nervous and/or musculoskeletal systems and symptoms involving the head and neck also displayed significant cost increases from the pre-diagnosis to the post-diagnosis period. For the former, mean costs rose by $179 PPP6M, while for the latter, they rose by $578 PPP6M.
While medical costs increased following the development of brain metastasis, pharmacy costs decreased in the same period, from $25,549 PPP6M to $15,840 PPP6M (p-value <0.001). Chemotherapy drugs appeared to drive a portion of this decline, consistent with shifts away from these treatments ($8847 PPP6M decrease in costs; p-value <0.001). Targeted therapy drug costs also seemed to decrease (from $2093 PPP6M to $1947 PPP6M), although this decrease was not found to be statistically significant (p-value = 0.546).
Indirect costs
presents productivity loss results for patients during the pre-diagnosis and post-diagnosis periods. The proportion of patients with at least one disability day increased from 37% to 42%, although the increase was not statistically significant. Meanwhile, the proportion of patients with at least one medically-related absenteeism day decreased from 95% to 79% (p-value <0.001). The average total work loss days increased from 51.0 PPP6M to 64.7 PPP6M, an increase of more than 13.7 work days. Specifically, days on disability rose from a mean of 31.9 PPP6M to 39.4 PPP6M, an increase of 7.5 days (p-value =0.018). Similarly, medically-related absenteeism days increased from a mean of 19.1 PPP6M to 25.3 PPP6M, an increase of 6.2 days PPP6M (p-value = 0.006). Among these absenteeism days, the average number of paid sick days decreased from 5.9 PPP6M to 2.4 PPP6M (p-value <0.001), while the average number of unpaid sick days increased from 13.2 PPP6M to 22.9 PPP6M (p-value <0.001).
Table 4. Comparison of productivity loss during the pre- and post-diagnosis periods.
The costs associated with patients’ productivity loss following the development of brain metastasis are shown in , from patient, payer, and employer perspectives. Costs increased significantly from all three perspectives. For patients, salary losses increased by $2853 PPP6M, from a mean of $5675 PPP6M to $8528 PPP6M (p-value <0.001). Much of this increase was driven by unpaid sick days, which contributed $2366 PPP6M, having risen from $3141 PPP6M to $5507 PPP6M from the pre- to the post-diagnosis period (p-value <0.001). For payers, the costs from productivity losses also rose substantially from the pre-diagnosis to the post-diagnosis period. These costs from disability benefit payouts rose by $2557 PPP6M, from a mean of $5940 PPP6M to $8496 PPP6M (p-value = 0.019). For employers, the cost of patients’ productivity loss following the development of brain metastasis increased by $4570 PPP6M, from a mean of $13,085 to $17,655 PPP6M (p-value <0.001).
Table 5. Comparison of productivity loss-related costs during the pre- and post-diagnosis periods.
Discussion
This study contributes to the literature on the economic burden of brain metastasis, focusing on lung cancer patients. We found that patients’ healthcare resource use and the associated direct costs substantially increased following the development of brain metastasis. The analyses showed similar trends to those noted in other studies in the literatureCitation3,Citation27, including those found by Guérin et al.Citation28 for their narrower patient population of crizotinib-treated ALK+ NSCLC patients. Comparable to that study, this one showed a marked increase in patients’ use of general healthcare resources and corresponding costs. Some differences, however, exist in the services and costs experiencing increases following the development of brain metastasis. For this study, resource use and cost increases were concentrated in hospital-based services and day-to-day supportive care, consistent with patients’ increased need for intensive treatment and day-to-day assistance. Outpatient visits showed no significant change in resource use or costs, while pharmacy costs significantly decreased. In contrast, the ALK+ NSCLC study found significant increases in resource use and costs for outpatient care and pharmacy services, in addition to inpatient care. Aside from these differences, the ALK+ NSCLC study also found greater total cost increases following the development of brain metastasis. However, among its study patients, total costs rose by almost 280% following the development of brain metastasis, while the present study observed increases of only 23%. This variation may reflect both treatment and severity differences between the two patient populations. ALK+ NSCLC patients constitute a distinct sub-group of (younger) lung cancer patients who have a more aggressive type of cancer and often have a more advanced stage of the disease. It is important to note that, although several targeted therapies exist today for the treatment of some sub-types of lung cancer, there aren’t many targeted therapies that are specifically indicated for the treatment of brain metastases. In the current study, cohort targeted therapy costs were low compared to chemotherapy costs, and were lower in the post-diagnosis period than in the pre-diagnosis period, indicating relatively low use of targeted therapies. We speculate that this may be because life expectancy is poor for lung cancer patients with brain metastases (patient survival at 6 months ranges from 0–10% for patients with brain metastasis and more than three extracranial organs affected by metastasis to 27–52% for 0–1 sitesCitation31,Citation32). Therefore, a large percentage of patients are expected to be undergoing radiation therapy and other surgical treatmentsCitation33,Citation34, treatment with chemotherapy, or even palliative careCitation35 for those patients deemed too sick to effectively treat with existing therapies. Safe and effective therapies for most patients with brain metastasis remain an unmet need in the oncological field.
Importantly, the current study also considered the indirect costs of the disease, a topic not previously addressed in analyses of brain metastasis, despite its predictably considerable impact on patients and other stakeholders. Comparing patients before and after the development of brain metastasis revealed that patients missed substantially more days of work following the development of brain metastasis. These additional absences translated to ∼13.7 work days PPP6M, bringing patients’ total time away from work in the post-diagnosis period to ∼64.7 work days PPP6M, an absenteeism rate of ∼50%. These work absences resulted in an incremental salary loss of $2853 PPP6M to patients, bringing their total salary loss to $8528 PPP6M in the post-diagnosis period. For context, these financial hits translate to a household income reduction of 10% and a total reduction of 30%, respectively, using the US Census Bureau’s 2013 median household income for people aged 55–64, adjusted to 6 months of reportingCitation36. Although these salary losses are considerable, they likely under-state the full financial burden from indirect costs. The total impact to families is arguably greater, factoring in the effect to family caregivers, patients forced to leave the workforce, and premature mortality.
In addition to examining the impact on patients, the study found significant indirect costs to payers and employers from patients’ development of brain metastasis. Despite this lack of prior attention, indirect costs are an important economic consideration that can substantially impact cancer patients and their families. In fact, the National Institutes of Health estimated that indirect costs from lost productivity, defined as medically-related work loss and premature mortality, comprised 60% of the total costs of cancer ($263.8 billion) for 2010Citation37. For lung cancer patients, such indirect costs are particularly pertinent, as many of these patients are of working age. Salary losses from disability and/or medically-related absenteeism can place considerable financial strain and hardship on them and their families, adding to their overall burden from the disease. In this paper, we consider these illness-related salary losses to patients, as well as the indirect secondary costs to their employers and payers.
For payers, the incremental cost from disability related to brain metastasis averaged $2557 PPP6M, bringing their total disability payout in the post-diagnosis period to $8496 PPP6M. For employers, the incremental cost from lost productivity related to brain metastasis averaged $4570 PPP6M, bringing their total productivity losses to $17,655 PPP6M in the post-diagnosis period. Additional effects such as presenteeism, defined as having lower productivity at work, were not included in calculations due to lack of data. Therefore, the currently reported employer costs likely represent conservative estimates. These trends in costs were consistent with expectations of the greater severity of the disease, more intensive care needs, and reduced autonomy of patients with brain metastasis; accordingly, these patients displayed increased costs for more intensive treatment as found in hospital settings and day-to-day support services from sources like home health, extended care facilities, and hospices. Furthermore, patient health insurance coverage and disability insurance for a large proportion (44%) of patients was covered by preferred provider organization insurance plans, which typically have minimal-to-no co-pays or out-of-pocket expenses for patients, depending on intervention type; therefore, the experience of this group of patients is an under-estimate of the costs borne by patients with other types of healthcare insurance. Finally, to help halt the unsustainable increases in healthcare costsCitation38, the Affordable Care Act (ACA) was passed in 2010; its implementation is ongoing, and may affect current analyses. It is expected that, as more formerly uninsured and under-insured people gain access to affordable healthcare, the need for various interventions and services will grow, and the challenge remains in being able to offer good quality of care at an affordable price to society. Evaluating the value of an interventionCitation39 may become increasingly important in this re-designed healthcare system in order to provide a more efficient use of healthcare resources, and considering alternative solutions—such as investments in home care support in order to reduce overall medical costs—may become an important tool in curbing the current expansive trend in the cost of healthcare in the US. Studies like the current analysis may help highlight the issues, as well as the growing need for innovative care models for cancer patients.
Potential areas of future investigation on the subject of the burden of illness in patients with brain metastasis include examination of differential costs and resource use across patients, the effect of therapeutic interventions on the clinical and economic burden of brain metastasis, the per patient growth in costs over time following the development of brain metastasis, and analysis of the overall burden of brain metastasis on the family unit of patients. In addition, further investigation should be done to explore the economic burden due to extracranial metastatic disease.
Limitations
This study was subject to some limitations. First, due to data availability, indirect costs were confined to a sub-set. As previously noted, these excluded indirect costs experienced by family caregivers of patients. The burden to these individuals may have been substantial, both in terms of work days missed from caregiving, as well as from the emotional and physical toll of having a sick family member, as noted in other studies on the economic burden of cancerCitation40,Citation41. This could, in turn, result in poorer health and, for example, increased frequency of mental health visits for these caregivers, in addition to salary lossCitation42. Similarly, indirect costs due to presenteeism were also not included due to lack of data availability. As previously noted, this omission tends to under-state indirect costs to employers. Second, the study focused on patients who were active employees. This design feature may have introduced selection bias if these patients were generally healthier than the average lung cancer patient with brain metastasis. This, in turn, may limit the generalizability of results. Third, the analysis of indirect costs was limited to the period during which patients were employed. Indirect costs from premature retirement or mortality were not considered. Consequently, the characterization of the economic burden of brain metastasis is anticipated to be conservative. Fourth, patients were limited to those with disability benefits, limiting the generalizability of results to patients with comparable benefit structures. Fifth, due to small sample considerations, the study period spanned a fairly long time horizon of 14 years. If changes occurred over this time period in terms of standards of care or generosity of benefits, then the generalizability of results to the present day may be limited beyond the relative effect. Finally, general limitations of using claims data, such as potential coding inaccuracies and record incompleteness, may apply to this study.
Conclusion
This study finds evidence of a substantial economic burden associated with the development of brain metastasis in lung cancer patients. Specifically, patients incurred significantly more healthcare resource use after the development of brain metastasis, contributing to $25,579 higher medical costs PPP6M in their post-diagnosis period. Patients also missed considerably more days of work during the post-diagnosis period than their pre-diagnosis phase. These productivity losses resulted in an incremental burden of $2853 PPP6M in salary loss for patients, $2557 PPP6M in disability payments for payers, and $4570 PPP6M in productivity losses for employers.
Transparency
Declaration of funding
This study was supported by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
MS, JZ, and KC are employees of Novartis Pharmaceuticals Corporation and own stock/stock options. KD, RN, ARM, EQW, and AG are employees of Analysis Group, Inc., which has received consultancy fees from Novartis Pharmaceuticals Corporation for this project. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgment
Editing assistance was provided by Ana Bozas, PhD, an employee of Analysis Group, Inc.
References
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005;75:5-14
- Gállego Pérez-Larraya J, Hildebrand J. Brain metastases. Handb Clin Neurol 2014;121:1143-57
- Ray S, Dacosta-Byfield S, Ganguli A, et al. Comparative analysis of survival, treatment, cost and resource use among patients newly diagnosed with brain metastasis by initial primary cancer. J Neurooncol 2013;114:117-25
- Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002;249:1357-69
- Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007;12:884-98
- Lalondrelle S, Khoo V. Brain metastases. Clin Evid (Online) 2009;2009:535-7
- Seute T, Leffers P, ten Velde GPM, et al. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004;100:801-6
- Villano JL, Durbin EB, Normandeau C, et al. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol 2015;17:122-8
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78–84
- Schouten LJ, Rutten J, Huveneers HAM, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705
- Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology 1993;43:1678-83
- Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol 1992;31:268-73
- Oltean D, Dicu T, Eniu D. Brain metastases secondary to breast cancer: symptoms, prognosis and evolution. Tumori 2009;95:697-701
- Coia LR, Aaronson N, Linggood R, et al. A report of the consensus workshop panel on the treatment of brain metastases. Int J Radiat Oncol Biol Phys 1992;23:223-7
- Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol 2014;4:248
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25
- National Comprehensive Cancer Network. Central Nervous System Cancers Version 1.2015. Fort Washington, PA: National Comprehensive Cancer Network; 2015:1-120. Available at: http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Last accessed December 21, 2015]
- Bearz A, Garassino I, Tiseo M, et al. Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer 2010;68:264-8
- Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer 1999;85:1599-605
- Wong ET, Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist 2004;9:68-79
- Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31
- Wu Y-L, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol 2013;24:993-9
- De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol 2010;100:443-7
- Shaw AT, Kim D-W, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97
- Costa DB, Shaw AT, Ou S-HI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8
- Ou S-HI, Ahn JS, Petris L De, et al. Efficacy and safety of the ALK inhibitor alectinib in ALK+ non-small-cell lung cancer (NSCLC) patients who have failed prior crizotinib: an open-label, single-arm, global phase 2 study (NP28673). J Clin Oncol 2015;33: suppl; abstr 8008
- Pelletier EM, Shim B, Goodman S, et al. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat 2008;108:297-305
- Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ 2015;18:312-22
- U.S. Bureau of Labor Statistics. Paid sick leave: Number of annual days by service requirement - Data Table. Washington, DC: United States Department of Labor; 2013. Available at: http://www.bls.gov/ncs/ebs/benefits/2015/ownership/govt/table35a.htm [Last accessed December 21, 2015]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms and for defining comorbidities data in ICD-9-CM administrative. Med Care 2005;43:1130-9
- Gerdan L, Segedin B, Nagy V, et al. Brain metastasis from non-small cell lung cancer (NSCLC): prognostic importance of the number of involved extracranial organs. Strahlentherapie und Onkol Organ der Dtsch Roüntgengesellschaft 2014;190:64-7
- Gerdan L, Šegedin B, Veninga T, et al. Number of involved extracranial organs predicts survival in patients with brain metastasis from small cell lung cancer. Anticancer Res 2013;33:3887-9
- Guérin A, Sasane M, Wakelee H, et al. Treatment, overall survival, and costs in patients with ALK -positive non-small-cell lung cancer after crizotinib monotherapy. Curr Med Res Opin 2015;31:1587-97
- Kotecha R, Zimmerman A, Murphy ES, et al. Management of brain metastasis in patients with pulmonary neuroendocrine carcinomas. Technol Cancer Res Treat 2015. published online 3 June, 2015, doi: 10.1177/1533034615589033
- Kim HJ, Kim YJ, Seo M-D, et al. Patterns of palliative procedures and clinical outcomes in patients with advanced non-small cell lung cancer. Lung Cancer 2009;65:242-6
- U.S. Census Bureau. Table H-10. Age of householder: all races by median and mean income: 1967 to 2013. Washington, DC: U.S. Department of Commerce; 2015. Available at: https://www.census.gov/hhes/www/income/data/historical/household/ [Last accessed December 21, 2015]
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society, 2010. http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf. Accessed November 2, 2015
- Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population, Board on Health Care Services, Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. In: Levit L, Balogh E, Nass S, Ganz PA, eds. Washington, DC: National Academies Press, 2013. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24872984 [Last accessed December 21, 2015]
- Stabile M, Thomson S, Allin S, et al. Health care cost containment strategies used in four other high-income countries hold lessons for the United States. Health Aff (Millwood) 2013;32:643-52
- Longo CJ, Fitch M, Deber RB, et al. Financial and family burden associated with cancer treatment in Ontario, Canada. Support Care Cancer 2006;14:1077-85
- Zarogoulidou V, Panagopoulou E, Papakosta D, et al. Estimating the direct and indirect costs of lung cancer: a prospective analysis in a Greek University Pulmonary Department. J Thorac Dis 2015;7:S12-19
- Wittenberg E, Prosser LA. Disutility of illness for caregivers and families: a systematic review of the literature. Pharmacoeconomics 2013;31:489-500