Abstract
Objectives Studies reporting healthcare resourse use (HRU) for melanoma, one of the most costly cancers to treat, are limited. Using consistent, robust methodology, this study estimated HRU associated with the treatment of metastatic melanoma in eight countries.
Methods Using published literature and clinician input, treatment phases were identified: active systemic treatment (pre-progression); disease progression; best supportive care (BSC)/palliative care; and terminal care. HRU elements were identified for each phase and estimates of the magnitude and frequency of use in clinical practice were obtained through country-specific Delphi panels, comprising healthcare professionals with experience in oncology (n = 8).
Results Medical oncologists are the key care providers for patients with metastatic melanoma, although in Germany dermato-oncologists also lead care. During the active systemic treatment phase, each patient was estimated to require 0.83–2 consultations with a medical oncologist/month across countries; the median number of such assessments in 3 months was highest in Canada (range = 3.5–5) and lowest in France, the Netherlands and Spain (1). Resource use during the disease progression phase was intensive and similar across countries: all patients were estimated to consult with medical oncologists and 10–40% with a radiation oncologist; up to 40% were estimated to require a brain MRI scan. During the BSC/palliative care phase, all patients were estimated to consult with medical oncologists, and most to consult with a primary care physician (40–100%).
Limitations Panelists were from centers of excellence, thus results may not reflect care within smaller hospitals; data obtained from experts may be less variable than data from broader clinical practice. Treatments for metastatic melanoma are continually emerging, thus some elements of our work could be superseded.
Conclusions HRU estimates were substantial and varied across countries for some resources. These data could be used with country-specific costs to elucidate costs for the management of metastatic melanoma.
Introduction
Melanoma is relatively rare compared with non-melanoma skin cancer, accounting for fewer than 5% of all skin cancer casesCitation1, but causes an estimated 90% of skin cancer-related deathsCitation2.
Melanoma is curable if diagnosed at an early stage; however, the disease progresses rapidly and can relapse suddenly, and is more likely than other skin cancers to spread to distant sites in the body where it can become difficult to treatCitation1,Citation3,Citation4. Approximately 20% of patients with melanoma develop metastatic disease (unresectable stage IIIB/C and stage IV)Citation5 and survival varies with the stage and site of metastasisCitation6,Citation7; even in patients with early stage metastatic melanoma (stage IIIB/C) 3 year survival is only 32%, falling to 5% for those with stage IV (M1c) metastatic melanomaCitation6. Regarding the site of mestastasis, skin, subcutaneous tissue, or distant lymph node metastases is associated with a better prognosis than lung metastases or metastases at other visceral sitesCitation7.
Although melanoma affects people of all ages, 34% of patients are younger than 55 years of ageCitation8, and patients with melanoma die an average of 20 years prematurelyCitation9. As a result, melanoma is considered to have a greater cost per death in terms of lost productivity than other cancersCitation10.
Studies reporting the direct economic burden of metastatic melanoma are limited, but suggest that the treatment of metastatic melanoma is costly because it involves a wide range of healthcare resourcesCitation11,Citation12, and that it is one of the most costly cancers to diagnose, treat, and monitorCitation11. Treatment options for metastatic melanoma have changed considerably in recent years with the approval of several new therapies since 2011Citation13. Thus, up-to-date data on healthcare resource use (HRU) at specific treatment phases throughout the management of metastatic melanoma are required to understand the economic burden of metastatic melanoma. To our knowledge, no studies to date have reported a comprehensive overview of resource use for the management of patients with metastatic melanoma.
In this study we estimate HRU associated with the treatment of metastatic melanoma, from treatment initiation to death, based on country-specific guidelines in Australia, Canada, and six European countries (France, Germany, Italy, the Netherlands, Spain, and the UK).
Methods
This study was conducted in three stages using a mixed-methods approachCitation14 to collect qualitative and quantitative data on HRU: (1) determination of the resource use structure associated with the management of metastatic melanoma; (2) estimation of HRU with Delphi (consensus) panels for elements of the resource use structure; and (3) collection of HRU data associated with terminal care and drug administration and monitoring from published literature.
The methodology was reviewed by a UK health economist with experience in such studies. The first stage was validated in each country by a local clinician involved in the treatment of metastatic melanoma.
Determination of the resource use structure associated with the management of metastatic melanoma
The resource use structure was determined to identify specific resources used from treatment initiation through to death. In this study, only resource use associated with currently used (active) systemic therapy was considered; resource use associated with adjuvant therapies, or legacy systemic chemotherapies was not explicitly part of the management that we considered. International clinical guidelines published by the European Society for Medical Oncology (ESMOCitation15) and the National Comprehensive Cancer Network (NCCNCitation16), European interdisciplinary guidelinesCitation2, national guidelinesCitation17–24, and literature published up to December 2013 on treatment patterns and resource use in metastatic melanomaCitation25–29 were used to inform the resource use structure.
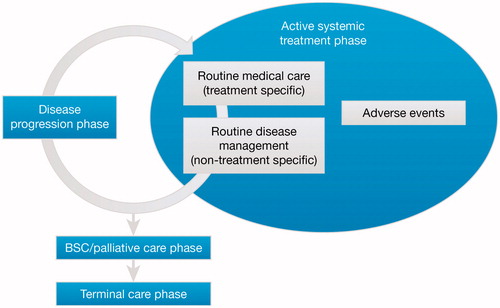
Four treatment phases were identified in the management of metastatic melanoma ():
| • | active systemic treatment (pre-progression) phase – from the start of active systemic therapy to disease progression, including adverse events; | ||||
| • | disease progression phase – from disease progression until the start of a new active systemic therapy (characterized as a transitional period between measuring disease progression with one treatment but prior to initiation of treatment with the next); | ||||
| • | best supportive care (BSC)/palliative care phase – care received after active treatment but before the last few weeks of life (terminal care); this phase can include a spectrum of resources and is assumed to last 4 months30; | ||||
| • | terminal care phase – the short period before death (assumed to be 8 weeks)Citation30. | ||||
All the clinicians involved in the panels were also active in clinical trials; however, they were made aware that the Delphi consensus panels had to reflect clinical practice outside of clinical trials, and responded to the questions accordingly. Based on feedback from clinicians it was assumed that, besides the choice of therapy and any specific healthcare resources associated with that therapy, non-treatment specific resource use during a given treatment phase would not be influenced by line of therapy; level of clinical response (stable disease vs complete/partial response); stage of disease (IIIB/C and IV M1a/M1b/M1c), or BRAF mutation status.
Healthcare resources identified for each treatment phase are shown in .
Table 1. Resource use structure for the management of metastatic melanoma.
Estimation of resource use from consensus panels
Estimates for the elements of the resource use structure were generated at Delphi consensus panels involving clinicians from each country. Delphi consensus panels are an established methodology for obtaining resource use data in oncologyCitation31–33 and other therapeutic areasCitation34. Clinical expert opinion was also used in the submission to the National Institute for Health and Care Excellence (NICE) for its assessment of ipilimumab in previously treated unresectable malignant melanomaCitation35.
Ethics approval for the conduct of these panels was granted by the Human Research Ethics Committee of the University of Technology, Sydney; all participants provided informed consent beforehand. The panels were blinded so that the name of the sponsor was not revealed to the participants or vice versa.
Development of the questionnaire for use at panels
Respondents were asked to estimate the proportion of patients who use each type of resource and, for those who do use the resource, the frequency of resource use (e.g., the number of consultations) within a given time period (i.e., one-off, 3 or 6 months).
Questions covered consultations with key health professionals (including oncologists, general practitioners [GPs], surgeons, and dermatologists), monitoring, hospital visits, surgical and other procedures (such as radiotherapy), and hospitalization for adverse events (AEs). For AE-related resource use, the proportion of patients requiring hospitalization for treatment-related grade 3 and 4 AEs of interest was included; no other resource use relating to the management of AEs was collected. Non-treatment specific resource use for patients with BRAF-mutated and BRAF wild-type disease were not considered to differ.
The panel in Australia was conducted as a pilot to test the validity, comprehensibility, and completeness of the questions and testing methods. Minor revisions were made following the pilot:
| • | data for palliative care for countries other than Australia include palliative care nurses as well as palliative care physicians; | ||||
| • | for resource use associated with routine disease monitoring, a question relating to the number of consultations with a radiation oncologist was included – in Australia each patient was assumed to receive a single consultation with a radiation oncologist to plan radiotherapy; | ||||
| • | data on stereotactic radiosurgery and neurosurgery for brain metastases were collected in countries other than Australia. | ||||
Panelist recruitment
Each panel compromised health professionals involved in the treatment of patients with metastatic melanoma. Eligibility criteria included: board-certified or specialized in oncology (clinical or medical oncology) or dermato-oncology (Germany); at least 5 years’ experience in this role after completion of training (note that, for the Netherlands, a clinician with more than 2 years’ experience, but fewer than 5 years’, was included in order to meet the required sample size); use of pharmaceutical therapy in the treatment of metastatic melanoma; treatment of at least 10 patients with metastatic melanoma per year; and able to speak and write English. Panelists were recruited from different geographic regions within each country in order to capture intra-country variability. The sample size is within the range used in recently published panels in oncologyCitation31–33.
Panel conduct
One-day panel meetings were held between August and December 2013 in each country; panels were conducted according to established published procedures for Delphi panelsCitation36,Citation37. The process was explained to the participants at the start of the panel, and examples of the voting process were presented to demonstrate the method.
The panelists were presented with an overview of the management of metastatic melanoma that was specific to their country and then the questionnaire was presented systematically, one question at a time. Numeric data were collected using a televoting system that allowed each participant to record their response anonymously. For each question, a two-round voting process was used to obtain a point estimate and measure of variability for the respondents’ estimates. For the first vote, each panelist recorded the most plausible value for the parameter based on their own clinical experience. The range of responses were then shown to the panel, who discussed their responses, led by a moderator, to focus on generating a single point estimate. A second vote was then held unless consensus was achieved after the first vote, or if the participants indicated that differences between responses reflected variation in clinical practice and their responses would not change with a second vote. A two-round voting approach was considered adequate as most changes in response occur in the transition from the first to second voteCitation38.
A face-to-face panel was not possible in the Netherlands. Instead, a modified two-round voting process was used: the questionnaire was sent to the panelists to complete individually and return within a week in order to capture a first vote; the second vote was then captured during a moderated teleconference.
Data analyses
Data were anonymized prior to analysis. The median from the reported data points after the first vote (if consensus was achieved) or the second vote was used as the point estimate for that parameter. This methodology is consensus-based and is intended to reduce variability between respondents. The minimum and maximum values from the first vote were also reported to provide an estimate of uncertainty around the point estimate for each parameter.
Data are expressed as the median monthly (or one-off) resource use, which is the proportion of patients using a specific resource multiplied by the level of use for that resource.
Collection of healthcare resource use data associated with terminal care and drug administration and monitoring from published literature
Data on resource use associated with drug administration and treatment-specific monitoring requirements were obtained from the Summaries of Product Characteristics; these data are not reported here.
Resources associated with terminal care are assumed to be non-disease-specificCitation39. Terminal care is likely to be organized differently across countries and within a given healthcare system, encompassing formal and informal care. Estimates of resource use could not be identified in the published literature. However, it was assumed that all patients would require these healthcare resources, but terminal care costs would not be expected to vary for different active treatmentsCitation30; thus, terminal care was not considered further in this study.
Results
Panelist demographics
The majority of panels comprised eight specialists; the panel in Germany comprised nine specialists; the panels in the Netherlands and the UK comprised seven specialists each. The majority of panelists were medical oncologists (52, 83%); others were dermato-oncologists (seven, Germany), surgeons (one each in Italy and the Netherlands), an oncology nurse (Australia), and a pathologist (Canada), all of whom were selected for their specific experience in managing patients with metastatic melanoma. The pathologist did not treat patients and did not answer questions on pharmaceutical treatment, but did answer questions on non-treatment specific resource use.
Panelists were mainly from specialist oncology centers or university teaching hospitals (57, 90%); the remaining six panelists were from community hospitals. Overall, the panelists had between 2–38 years’ experience in treating patients with metastatic melanoma (medians: Australia 17; Canada 9; France 20; Germany 14; Italy 10; Netherlands 6; Spain 15; UK 13); the number of patients treated in the year before the panel ranged from 5–300.
Management of metastatic melanoma
In each country, panelists agreed with the overview of the management of metastatic melanoma presented for their country. The treatments recommended by guidelines differ depending on whether the melanoma is associated with a mutation in the BRAF gene (BRAF-mutated) or not (BRAF wild-type)Citation40. At the time of the panel, the predominant treatments used for BRAF-mutated disease were similar in all countries: vemurafenib for first line, ipilimumab for second line, and dacarbazine for third line; for BRAF wild-type disease, dacarbazine and ipilimumab are recommended for first- and second-line treatments, respectively, in all the countries, whereas third-line treatment differed (no treatment, paclitaxel plus carboplatin, or fotemustine). As the treatment landscape for melanoma is continually evolving, panelists were asked if they expected the emergence of new therapies to alter the management of melanoma, organization of healthcare resources, or resource use. They confirmed that they did not expect the overall patterns of resource use to change in the coming years; however, differences in AE profiles for new therapies might result in some difference in resource use.
Panelists confirmed that, besides the choice of therapy and any specific healthcare resources associated with that therapy, non-treatment specific resource use during a given treatment phase would not be influenced by line of therapy; level of clinical response (stable disease vs complete/partial response); stage of disease (IIIB/C and IV M1a/M1b/M1c); or BRAF mutation status.
Active systemic treatment (pre-progression) phase
The active systemic treatment (pre-progression) phase includes HRU for both routine medical care that is specific to particular treatments (treatment-related) and routine disease management that applies to all patients and is not treatment specific ().
Medical care during active systemic treatment
Consultations (specific to treatment): All patients were estimated to consult with a medical oncologist during treatment; each patient was estimated to require 0.83–2 consultations per month. In all countries except France, patients (range of point estimates = 10–100%) were also estimated to require consultation with a dermatologist during treatment with vemurafenib (0.03–0.67 consultations per month). The proportion of patients estimated to consult a dermatologist was lowest in the UK, as patients are often referred directly to a plastic surgeon. In Germany, dermatologists are often consulted for a second opinion, even though dermato-oncologists are the key providers of care. Patients in Canada, Germany, and the Netherlands were also expected to visit a dermatologist while receiving ipilimumab (5–10% of patients) or dacarbazine (3.5–25% in Canada and Germany only), but visits are generally infrequent, resulting in low resource use (0.02–0.07 visits overall for ipilimumab; 0.01–0.17 for dacarbazine).
The number of times a medical oncologist would be expected to monitor for disease progression and response to treatment as part of scheduled patient visits differed between the countries. Across treatments, the median number of assessments in 3 months was highest for Canada (range of point estimates = 3.5–5) and lowest for France, the Netherlands, and Spain (point estimate = 1). This assessment does not include visits for tests, etc., that are captured elsewhere.
Adverse events (specific to treatment): The estimated hospitalization rate for patients with treatment-related grade 3 and 4 AEs of interest varied within and between countries. Generally, the proportion of patients expected to be hospitalized was lower for grade 3 than for grade 4 AEs; this proportion was below 50% for several grade 3 AEs (including rash [0–25%], elevated liver enzymes [1–20%], anemia [0–40%], and endocrine disorders [0–50%]). Other grade 3 AEs estimated to require hospitalization in a substantial proportion of patients included febrile neutropenia (20–95%), dyspnea (30–80%), hypertension (0–70%), vomiting (2.5–90%), and deep vein thrombosis (5–90%). For some grade 3 AEs, up to 100% of patients were hospitalized in some countries (colitis [0–100%], diarrhea [20–100%], cellulitis [20–100%], and dehydration [30–100%]). Grade 4 AEs with the highest point estimates of hospitalization rates included colitis (100%), dyspnea (100%), diarrhea (95–100%), cellulitis (95–100%), and dehydration (90–100%). Among the grade 4 AEs, point estimates had the widest range for anemia and elevated liver enzymes (22.5–100% and 5–100%, respectively).
Routine disease management during active systemic treatment phase
The median monthly resource use associated with routine disease management during the active systemic treatment (pre-progression) phase was generally similar across countries for many different resources (). Of the range of resources used, it was estimated that a high proportion of patients required a whole-body CT scan (range of point estimates = 87.5–100%) and that many would have a GP consultation (range of point estimates = 15–90%). Panelists reported that inpatient stay was not usually needed during routine management of melanoma, but that day hospital visits may be required. During the pre-progression phase, brain MRI scans were not common, possibly because of the expense and time involved.
Table 2. Resource use associated with routine disease management during the active systemic treatment (pre-progression) phase.
Disease progression phase
Median estimates of resource use associated with disease progression did not differ markedly across countries (). Consultations are a resource intensive element at this phase of the disease and all patients were expected to consult with a medical oncologist. Regarding hospitalization, 5–18% of patients were expected to have an inpatient stay, with a median length of stay of 3–6.5 days. Overall, 10–40% of patients were estimated to get radiotherapy, with an average number of fractions of 2–5, except in Germany, where it was 15 fractions. Some patients (range of point estimates = 7.5–40%) were expected to require a brain MRI scan during this phase.
Table 3. Resource use during the disease progression phase.
Additional resource use for the management of brain metastases varied greatly between countries: patients with brain metastases may receive whole-brain radiotherapy, stereotactic radiosurgery, or neurosurgery, depending on the availability of expertise; whole-brain radiotherapy was the dominant resource use.
BSC/palliative care phase
Median monthly resource use associated with the BSC/palliative care phase varied across countries, particularly for palliative and hospice care (), as resource use during this phase of the disease depends on how care delivery is organized. The proportion of patients expected to require hospice care ranged from 10% in Italy to 50% in Australia (Canada 15%; France 30%; Germany 20%; the Netherlands 25%; Spain 22.5%, UK 40%).
Table 4. Resource use during the BSC phase.
As with the other phases of the disease, consultations were a frequent resource use: all patients expected to see a medical oncologist, and most patients (range of point estimates = 40–100%) were expected to consult a GP. Inpatient stays (range of point estimates = 20–80% of patients) were also a frequent resource use.
Discussion
This study has estimated HRU in the treatment of patients with metastatic melanoma using Delphi consensus panel studies to generate new data. Delphi panels are an established methodology for obtaining resource use data in oncologyCitation31–33 and other therapeutic areasCitation34, and use of expert opinion is recognized and accepted by health technology assessment (HTA) agenciesCitation35. To our knowledge, this study represents the most comprehensive overview of resource use for patients with metastatic melanoma. At the time of our study, ipilimumab and vemurafenib were only newly reimbursed or undergoing reimbursement assessment, and these treatments and dacarbazine were identified as the most commonly used therapies for the patient group of interest. Each panel was asked about ipilimumab, vemurafenib, and dacarbazine, as well as some other less commonly used therapies (temozolomide, fotemustine, paclitaxel, and interferon); however, panelists did not expect resource use to change with the emergence of new therapies, thus our estimates of non-treatment specific resource use will be applicable to new treatments.
The results of the study show that a considerable number of different resources are required, some common across all treatment phases (e.g., GP consultation) and some specific to a particular phase (e.g., home aide visit/hospice care in the BSC/palliative care phase). Although the elements of resource use were similar across countries, the magnitude and frequency of use of some resources may vary between different healthcare systems and because of geographic distribution of services within a country. For example, in Italy and the Netherlands, BSC/palliative care is a resource use to the healthcare system, and panelists reported that care may also be provided in the home by non-healthcare professionals; in the UK, hospices are often run by charitable organizations and are not a resource use to the healthcare system.
The data generated in this study could be used with country-specific costs to elucidate the cost of each element of the resource use structure. As we have shown, treatment of metastatic melanoma is associated with HRU in some expensive areas, such as hospitalizations, whole-body CT, and specialist consultations; thus, the cost of HRU will be an important consideration alongside drug costs in the overall assessment of the economic burden of metastatic melanoma. As a resource use study, this study did not include drug use, the costs of which can obscure costs associated with other direct medical resource useCitation41, particularly for newer treatments in the early stage of the disease. Costs can also depend on whether the disease is BRAF wild-type or BRAF-mutated, the latter accounting for 40–50% of melanoma casesCitation42. Drug options and treatment duration differs according to BRAF status: of the treatment regimens approved since 2011, vemurafenib, dabrafenib, trametinib, and the combination of dabrafenib and trametinib are approved for BRAF-mutated disease only, whereas ipilimumab, nivolumab, and pembrolizumab are approved for both BRAF-mutated and BRAF wild-type melanomaCitation13. Actual costs for resource use estimates are not presented in this study, as such costs can quickly become out of date. Also, as the study incorporated data from eight countries, calculating a single cost would involve a mixture of currencies and exchange rates. Instead, the resource use data presented in our study can be adapted to different situations and used to estimate costs as required.
Our study showed that a range of resources are used intensively at the point of disease progression, which suggests that achieving a durable complete response/remission has the potential to avoid these healthcare resources and delaying or preventing progression will inevitably delay or prevent this resource use. Total medical costs per patient are higher with disease progression and at later stages (i.e., stage IV) of diseaseCitation43, particularly if active therapies continue to be used; thus, prevention of progression in terms of developing metastases such as visceral brain metastases would be expected to result in a substantial reduction in HRU overall.
There are some potential limitations of this study. Panelists were from centers of excellence, which should ensure that the results reflect the care provided to the majority of patients; however, the results may not reflect the care provided in smaller hospitals or clinics. Furthermore, solicitation of expert opinion only may result in less variability in resource use data than would be observed in broader clinical practice, where clinicians with less experience may be involved in treatment. Another limitation is that the survey has not fully evaluated the impact on resource use of AEs: limited data were collected in this study. Finally, the treatment landscape for melanoma is continually evolving and this can mean some elements of our work could be superseded; to address this, the resource use structure is split into treatment-specific elements (e.g., drug administration and monitoring) and elements common to all treatments (e.g., palliative care/BSC) to increase the generalizability of the results. The study, therefore, provides a framework to cost the management of metastatic melanoma with existing/emerging treatments, assuming non-treatment specific resources do not change. From these results, it should be possible to calculate the burden of melanoma and compare this with the burden for other cancers on a country-by-country basis when the methodology is comparable.
While recognizing that this study does not present data based on patient records, the data do reflect variations in clinical practice through the ranges of resource use estimates presented, based on clinicians’ expert opinion of clinical practice. As such, the study provides the basis for future work to compare with resource use in real-world practice; such comparisons would need to be country-specific to reflect differences in clinical practice in each country.
Conclusions
This study has estimated the HRU for managing patients with metastatic melanoma across eight countries, using a consistent, robust methodology that has been accepted by HTA agencies. The estimates of HRU were substantial and varied across countries for some resources, reflecting differences in healthcare systems and organization of care. Over time, HRU will also be likely to evolve according to BRAF mutation status and line of therapy, as more products are reimbursed and used to manage metatstatic melanoma; treatments that produce durable complete response and prevent disease progression to advanced stages can be evaluated within this framework to estimate non-treatment specific HRU and combined with treatment-specific information to demonstrate cost savings to healthcare systems.
Transparency
Declaration of funding
This study was sponsored by Amgen Inc., Thousand Oaks, CA.
Declaration of financial/other relationships
BB and ZZ are employees of Amgen Inc. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Dr Katerina Bilitou for study co-ordination and Professor Deborah Saltman for panel moderation and co-ordination of ethics approval, on behalf of Amgen Inc. and PRMA Consulting. The authors also wish to thank Dr Becky Bradley who provided medical writing services on behalf of Amgen Inc. and PRMA Consulting.
References
- American Cancer Society. Cancer Facts & Figures 2011. Atlanta: American Cancer Society, 2011. http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2011. Accessed June 14, 2015
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-Update 2012. Eur J Cancer 2012;48:2375-90
- Cancer research UK. Melanoma skin cancer. 2015. http://www.cancerresearchuk.org/about-cancer/type/melanoma/treatment/melanoma-statistics-and-outlook. Accessed May 14, 2015
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84
- EMA. European Public Assessment Report for Yervoy (ipilimumab). London: EMA, 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002213/WC500157027.pdf. Accessed April 28, 2015
- Song X, Zhao Z, Barber B, et al. Overall survival in patients with metastatic melanoma. Curr Med Res Opin 2015;31:987-91
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206
- International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. Lyon: International Agency for Research on Cancer, 2013. http://www.iarc.fr/. Accessed May 20, 2015
- Ekwueme DU, Guy GP, Jr., Li C, et al. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity-United States, 2000 to 2006. J Am Acad Dermatol 2011;65(5 Suppl 1):S133-43
- Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer 2015;136:E136-45.
- Alexandrescu DT. Melanoma costs: a dynamic model comparing estimated overall costs of various clinical stages. Dermatol Online J 2009;15:1
- Davis KL, Mitra D, Kotapati S, et al. Direct economic burden of high-risk and metastatic melanoma in the elderly: evidence from the SEER-medicare linked database. Appl Health Econ Health Policy 2009;7:31-41
- Marzuka A, Huang L, Theodosakis N, et al. Melanoma treatments: advances and mechanisms. J Cell Physiol 2015;230:2326-33
- Wisdom J, Creswell JW. Mixed methods: integrating quantitative and qualitative data collection and analysis while studying patient-centered medical home models. Rockville, MD: Agency for Healthcare Research and Quality, 2013. https://pcmh.ahrq.gov/sites/default/files/attachments/MixedMethods_032513comp.pdf. Accessed October 13, 2015
- Dummer R, Hauschild A, Guggenheim M, et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(7 Suppl):vii86-91
- Coit DG, Andtbacka R, Anker CJ, et al. Melanoma, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2013;11:395-407
- AIOM. Linee guida melanoma. 2013. http://www.aiom.it/C_Common/Download.asp?file=/$Site$/Attivita_Scientifica/Linee_Guida/2013/Melanoma_v6_24.09.13.pdf. Accessed June 14, 2015
- Alberta Health Services. Systemic therapy for unresectable stage III or metastatic cutaneous melona, version 2. 2013. http://www.albertahealthservices.ca/hp/if-hp-cancer-guide-cu012-systemic-therapy.pdf. Accessed June 14, 2015
- British Columbia Cancer Agency. Melonama guideline, recurrent and/or metastatic disease. 2013. http://www.bccancer.bc.ca/health-professionals/professional-resources/cancer-management-guidelines/skin/melanoma#Recurrent-and-or-Metastatic-Disease. Accessed October 13, 2015
- Cancer Care Ontario. Systemic Adjuvant Therapy for patients at high risk for recurrent melanoma, Toronto: Cancer Care Ontario, 2013. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=34373. Accessed October 13, 2015
- IKNL. Melanoom, Landelijke richtlijn, Versie: 2.0. 2012. http://www.oncoline.nl/melanoom. Accessed October 13, 2015
- Marsden JR, Newton-Bishop JA, Burrows L, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J Plast Reconstr Aesthet Surg 2010;63:1401-19
- NHMRC, Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. 2008. http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp111.pdf. Accessed October 13, 2015
- SEOM. Melanoma Madrid: SEOM, 2013. http://www.seom.org/en/informacion-sobre-el-cancer/info-tipos-cancer/melanoma. Accessed October 13, 2015
- Bedane C, Leccia MT, Sassolas B, et al. Treatment patterns and outcomes in patients with advanced melanoma in France. Curr Med Res Opin 2013;29:1297-305
- Johnston K, Levy AR, Lorigan P, et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: results from a retrospective, longitudinal survey (MELODY study). Eur J Cancer 2012;48:2175-82
- Lebbe C, Lorigan P, Ascierto P, et al. Treatment patterns and outcomes among patients diagnosed with unresectable stage III or IV melanoma in Europe: a retrospective, longitudinal survey (MELODY study). Eur J Cancer 2012;48:3205-14
- Lorigan P, Marples M, Harries M, et al. Treatment patterns, outcomes, and resource utilisation of patients with metastatic melanoma in the UK: the MELODY study. Br J Dermatol 2014;170:87-95
- Maio M, Ascierto P, Testori A, et al. The cost of unresectable stage III or stage IV melanoma in Italy. J Exp Clin Cancer Res 2012;31:91
- NICE, Liverpool Reviews and Implementation Group. Ipilimumab for previously treated unresectable malignant melanoma Liverpool: NICE, 2011. https://www.nice.org.uk/guidance/ta268/documents/melanoma-stage-iii-or-iv-ipilimumab-evidence-review-group-report3. Accessed April 28, 2015
- Asseburg C, Frank M, Kohne CH, et al. Cost-effectiveness of targeted therapy with cetuximab in patients with K-ras wild-type colorectal cancer presenting with initially unresectable metastases limited to the liver in a German setting. Clin Ther 2011;33:482-97
- Isla D, Gonzalez-Rojas N, Nieves D, et al. Treatment patterns, use of resources, and costs of advanced non-small-cell lung cancer patients in Spain: results from a Delphi panel. Clin Transl Oncol 2011;13:460-71
- Kim K, Hernlund E, Hernadi Z, et al. Treatment patterns, health care utilization, and costs of ovarian cancer in Central and Eastern Europe using a Delphi panel based on a retrospective chart review. Int J Gynecol Cancer 2013;23:823-32
- Lee D, Gladwell D, Batty AJ, et al. The cost effectiveness of licensed oromucosal midazolam (Buccolam(®)) for the treatment of children experiencing acute epileptic seizures: an approach when trial evidence is limited. Paediatr Drugs 2013;15:151-62
- NICE. Methods guide: assessing cost impact. London: NICE, 2011. http://www.nice.org.uk/Media/Default/About/what-we-do/Into-practice/Costing_Manual_update_050811.pdf. Accessed April 28, 2015
- Gordon TJ. The delphi method. In Future Res Methodol version 3.0. Eds. Glenn JC, Gordon TJ. Washington DC, 2009.
- Hsu C, Sandford B. The Delphi technique: making sense of consensus. Pract Assess Res Eval 2007;12:1-8
- Van Zolingen SJ, Klaassen CA. Selection processes in a Delphi study about key qualifications in Senior Secondary Vocational Education. Technol Forecast Soc Change 2003;70:317-40
- Addicott R, Dewar S. Improving choice at end of life: a descriptive analysis of the impact and costs of the Marie Curie delivering choice programme in Lincolnshire. London: King's Fund, 2008. http://www.kingsfund.org.uk/sites/files/kf/improving-choice-end-of-life-descriptive-analysis-impact-costs-marie-curie-choice-programme-lincolnshire-rachael-addicot-steve-dewar-april-2008.pdf. Accessed October 13, 2015
- NCCN. Clinical practice guidelines in oncology: melanoma. Version 2. Fort Washington PA: NCCN, 2013. www.nccn.com. Accessed September 30, 2013
- Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol 2007;25:180-6
- Colombino M, Capone M, Lissia A, et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J Clin Oncol 2012;30:2522-9
- Farr AM, Zhao Z, Song X, et al. Medical Costs by disease stage in Medicare patients with melanoma. Value Health 2014;3:A78