Abstract
Objectives Cost-effectiveness of febuxostat compared with allopurinol in the treatment of hyperuricemia in patients with gout.
Methods Costs, clinical outcomes, and QALYs were estimated using a Markov model. Febuxostat 80 mg and 120 mg sequentially, used as first line and second line therapy, was compared with allopurinol 300 mg. Patients switched to the next treatment in the sequence according to a dichotomous response vs no response (target serum urate level < 6 mg/dl outcome) after 3 months of active treatment. A 3% discount rate and 5-year time horizon were applied. Perspective: National Health System.
Results The addition of febuxostat to any therapeutic strategy was an efficient option, with incremental cost-effectiveness ratios (ICER) compared with allopurinol 300 mg ranging from €5268–€9737.
Conclusions Febuxostat is a cost-effective treatment in Spain for the management of hyperuricemia in gout patients, with ICERs far below accepted Spanish efficiency thresholds (30 000€/QALY).
Introduction
Gout is a disease caused by the nucleation and deposition of monosodium urate crystals (MSUCs) in the tissues, mainly in the musculoskeletal and subcutaneous. MSUCs deposition is conditioned by sustained hyperuricemia (considered as the solubility threshold of serum urate (sUA) over 6.8 mg/dl), which leads to super saturationCitation1.
As any storage disease, it is conceptually a chronic process, although clinical manifestations vary from acute flares, asymptomatic periods, and persistent complaints derived from chronic synovitis and joint structural damage related to tophi. In addition, gout is associated with a wide range of comorbidities including cardiovascular disease, renal disease, and the metabolic syndromeCitation2–7.
Hyperuricemia and gout are frequent conditions in the adult populationCitation8. The prevalence of gout in Western countries ranges between 0.5–1%, and rises with age to exceed 3% in persons aged ≥65 yearsCitation9. In Spain, it has been estimated to be 0.2% in males aged 18–54 years and 0.5% in males aged ≥65 years, suggesting a figure of more than 200 000 people affectedCitation10. Despite of this, data on the economic impact in Spain are limited, and proper control of hyperuricemia in patients with gout did not reach target in over 50% of patients previously to the release of febuxostatCitation11.
Febuxostat is a non-purine selective xanthine oxidase inhibitor, which is approved by the European Medicines Agency (EMA), the Food and Drug Administration (FDA), and the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan for the treatment of hyperuricemia in situations when urate deposition has already occurred (including a history of or the presence of tophus and/or gouty arthritis).
The cost-effectiveness of febuxostat vs allopurinol in the treatment of hyperuricemia was evaluated by the National Institute for Health and Clinical Excellence (NICE)Citation12 and the Scottish Medicines Consortium (SMC)Citation13 in 2008 and 2010, respectively. Recently, Beard et al.Citation14 have reported on the cost-effectiveness model presented to the SMC and concluded that febuxostat 80 mg followed by 120 mg is highly cost-effective in reducing sUA levels, especially when used as second line treatment after allopurinol.
The aim of this study was to determine the cost-effectiveness of different treatment sequences for the treatment of hyperuricemia in gout patients in the Spanish setting, not including indirect costs, and focused on febuxostat vs allopurinol 300 mg alone as first line treatment, thereby complementing the study by Beard et al.Citation14 in which no-urate lowering therapies (ULT) was used as a reference comparator.
Methods
This cost-effectiveness analysis adapted a pharmacoeconomic model in which the effects and costs over time of different treatment sequences for hyperuricemia were compared in the Spanish setting.
Study population and comparators
The characteristics of the cohort of patients considered in the base case were intended to reflect the population of the APEXCitation15 and FACTCitation16 studies: adult patients with gout and sUA ≥8 mg/dl to be treated with allopurinol 300 mg/day or febuxostat 80 or 120 mg/day. Supplementary analyses were made for patients with mild-to-moderate renal function impairment (in whom it is recommended to be treated with lower doses of allopurinol), and patients in whom allopurinol was not an appropriate treatment, due either to intolerance or lack of response. Benzbromarone was not included in the analysis, since its use in Spain is restricted to severe gout in patients with failure or intolerance to allopurinol, and is only prescribed by rheumatology and nephrology specialists.
Different treatment sequences, in addition to febuxostat vs allopurinol as first line treatment, were compared (). Treatment sequences including second line therapies were included in order to reflect standard practice of treatment in Spain, where patients may be switched to an alternative option once the current treatment no longer provides clinical benefit. Thus, treatment sequences consist of an initial dose of febuxostat 80 mg once daily, which was increased to 120 mg once daily if required, allopurinol 300 mg once daily (with dose reduction in the supplementary analysis in patients with mild or moderate renal failure [30–89 ml/min]), and no treatment (NT) for patients intolerant to allopurinol or who have discontinued active treatment (last step of the therapeutic sequence).
Table 1. Types of analyses and strategies compared.
Type of analysis
The results were expressed as incremental cost-effectiveness ratios (ICER):
where CostS1-S2-S3 and CostS0 are the costs associated with the different strategies, S0 is the reference, and EffectivenessS1-S2-S3 and EffectivenessS0 are the clinical consequences, expressed in quality-adjusted life years (QALY), of the different strategies, with S0 as the reference. Thus, the ICER expresses the incremental cost per QALY of each strategy (S1–S3) relative to S0.
The time horizon of the base case was 5 years, and a discount rate of 3% was applied to account for future costs and effects occurring after the first year, as recommended in Spanish health technology evaluation guidelinesCitation17,Citation18. The analysis was conducted from the perspective of the Spanish National Health System (NHS) and, therefore, only direct medical costs were considered.
Model description
The Markov model, which was adapted to the Spanish setting, was based on cycles of 3 months, reflecting the period in which the therapeutic response to the initial treatments and whether to continue with the same ULT or switch to a new one should be assessed. shows the structure of the model, distinguishing between the initial treatment (first 3 months with each ULT of the sequence) and maintenance therapy (long-term, represented by subsequent Markov cycles).
Figure 1. Diagram of model (adapted from Beard et al.Citation14). Tx, treatment.
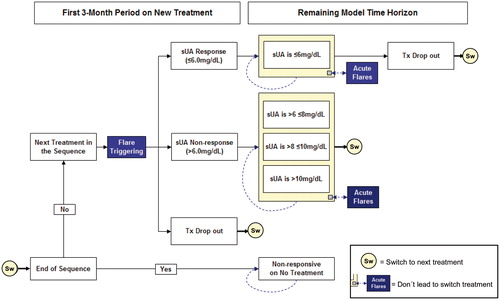
The cohort of patients started the model receiving the first ULT of each sequence (initial treatment) and were assigned, according to the response to such treatment, to the following health states (based on sUA levels): responders (sUA1: ≤6 mg/dL), non-responders (sUA2: >6 mg/dL and ≤8 mg/dL, sUA3: >8 mg/dL and ≤10 mg/dL, sUA4: >10 mg/dL).
Patients who responded (sUA1), were assumed to remain on the same health state unless failure and treatment discontinuation, defined as treatment drop-out. When patients did not achieve an adequate response based on sUA levels (non-responders: sUA2, sUA3, or sUA4), or did achieve a response (responders: sUA1), but suffered treatment drop-out, they moved to the next treatment in the sequence (Sw), with the response (initial treatment) being determined again according to sUA levels for the new ULT, as described above.
Patients at the end of the treatment sequence were distributed across the sUA levels associated with the no-treatment health state. In each cycle of the model, the probability of experiencing acute gout flares at any sUA level (assuming a duration of 1 week) was considered. Acute gout flares did not involve a change of ULT, but did have consequences in terms of cost and reduced quality-of-life.
The analysis included general mortality in order to calculate the long-term results in terms of QALYs. The direct risk of mortality related to gout was not included in the model, assuming, conservatively, that hyperuricemia had no impact on patient survival, despite increased risk of mortality has been recently reported as associated to both severity and the highest levels of sUA in patients with gout compared to the general populationCitation19.
Clinical data
Febuxostat has demonstrated its efficacy and safety in the treatment of hyperuricemia in patients with gout in the randomized, controlled, double-blind phase 3 APEXCitation15, FACTCitation16, and CONFIRMSCitation20 trials, and the long-term FOCUSCitation21 and EXCELCitation22 studies (5 and 3 years, respectively).
The clinical efficacy of febuxostat 80 mg/120 mg and allopurinol 300 mg once daily, defined as the reduction in sUA levels achieved, was obtained from aggregated data from the APEXCitation15 and FACTCitation16 controlled clinical trials (). Efficacy data for the supplementary analysis in patients with chronic kidney disease (CKD) were obtained from the CONFIRMS controlled clinical trialCitation20, which included a sub-set of patients with mild-to-moderate renal impairment (creatinine clearance 60–89 ml/min and 30–59 ml/min, respectively) who received a lower dose of allopurinol.
Table 2. Efficacy of treatments for hyperuricemia.
Drop-outs from treatment with either allopurinol or febuxostat by any cause, defined in the clinical trials as ‘premature discontinuation’, were included in the model. Drop-out rates were obtained from the pooled data of the APEX and FACT studiesCitation15,Citation16 for the first year of ULT and from the EXCEL long-term, open-label extension studyCitation22 for subsequent maintenance treatment. shows the drop-out probabilities for the first year and subsequent years for each treatment.
Table 3. Discontinuation rates.
The rate of acute gout flares was obtained from the APEX and FACT studiesCitation15,Citation16 for the initial treatment (first cycle of 3 months). In clinical practice, the initiation of ULT is commonly associated with increases in gout flares, for which prophylaxis for 6 months is nowadays recommendedCitation2,Citation23. Since the prophylaxis period was only 8 weeks in the APEX and FACT studiesCitation15,Citation16, an adjustment was made in the flare rate observed in the APEX and FACT studies, according to the reduction in the risk of flare with and without prophylaxis observed at 12 weeks in the study by Borstad et al.Citation24 to estimate the rate of acute gout flares when prophylaxis was extended. As a result, the adjusted probability of suffering a gout flare in the first 3 months of treatment was 0.51 for allopurinol 300 mg, 0.63 and 0.87 for febuxostat 80 mg and 120 mg, respectively, and 0.25 for no treatment.
For cycles subsequent to the first 3 months of treatment, the probability of having a gout flare depended on sUA levels, which were obtained from a study by Institute of Medical Science (IMS) Europe of 140 patients in the UK, Germany, and FranceCitation25. For each health state in the model, the probabilities of experiencing flare were 0.65 for sUA1, 0.73 for sUA2, 0.80 for sUA3, and 0.86 for sUA4.
Utilities and costs
Utility values were used to represent the impact on the quality-of-life associated with a specific health state on a scale from 0 (death) to 1 (perfect health). Quality-of-life data were obtained from the EQ-5D questionnaire administered in the IMS Europe studyCitation25 which found a mean value for patients with gout of 0.710 (95% CI = 0.638–0.736). Utility values were assigned to each health state using a multivariate analysis that evaluated the impact of sUA levels on the EQ-5D scores. The utility values assigned to each health state were 0.7463 for sUA1, 0.7120 for sUA2, 0.6777 for sUA3, and 0.6435 for sUA4. A reduction in utility (disutility) of 0.0097 was assigned to acute gout flares, assuming a 7-day quality-of-life effectCitation25.
The drug costs of ULT were expressed as the retail price plus value added tax (RP + VAT)Citation26, taking into account the corresponding deductions according to Royal Decree-Law 8/2010Citation27. The resulting daily cost for each ULT is shown in . The cost of an acute gout flare was estimated from the use of resources reported in the observational study by Sicras-Mainar et al.Citation28, to which reported Spanish tariff unit costs were assignedCitation29 ().
Table 4. Drug costs.
Table 5. Cost of an acute gout flare.
Sensitivity analysis
To evaluate the uncertainty associated with some model parameters, and to further determine the robustness of the results, deterministic and probabilistic sensitivity analyses were developed.
In the deterministic sensitivity analyses, variables were modified individually with respect to the base case (univariate), in order to identify variables with the greatest impact on the results of the model. The results at different time horizons (1, 2, 10 years and lifetime) were analyzed, the response threshold to the ULT was modified (≤5 and 7 mg/dL), the discount rate for costs and effects was modified (0% and 5%), and the baseline value of the utilities and the cost of gout flares was modified by ±20%.
In the probabilistic sensitivity analysis, 1000 simulations were performed using a Monte-Carlo second-order simulationCitation30, simultaneously changing the parameters of reductions in sUA, treatment discontinuation, and annual rate (after one year) of gout flares (beta distribution), the flare rates during the first cycle (log-normal distribution), and the healthcare cost (gamma distribution).
Results
Primary analysis (base case)
shows the clinical results (expressed as QALYs, the number of acute gout flares, and the days with sUA ≤6 mg/dL) and economic results for each of the sequences (strategies S0–S3). also shows the incremental output (in QALYs and costs) of the S1–S3 strategies with respect to the S0 strategy. All strategies that included the use of febuxostat 80 mg/120 mg were cost-effective with respect to the use of first-line allopurinol 300 mg alone, using the cost-effectiveness threshold of €30 000 per QALY commonly accepted in SpainCitation31.
Table 6. Results of the primary analysis.
Supplementary analyses
The results of the analyses made in patients with CKD and those intolerant to allopurinol are in line with those obtained in the primary analysis (), showing that febuxostat was a cost-effective option compared with first-line allopurinol. In a cohort of patients with mild-to-moderate CKD, typically treated with lower doses of allopurinol (100–200 mg/day), the ICER resulting from the comparison of the first-line strategies (S1 vs S0) was €12,106 per QALY gained. For sequential strategies vs first-line allopurinol 300 mg alone (S2 and S3 vs S0) the ICERs were €5358 and €7708 per QALY gained, respectively.
In a cohort of patients in whom allopurinol was not a suitable treatment due to intolerance or inadequate response, the ICER for febuxostat 80/120 mg compared with no treatment was €6348 per QALY gained.
Sensitivity analysis
shows the results of the deterministic sensitivity analysis.
Table 7. Results of univariate deterministic sensitivity analysis.
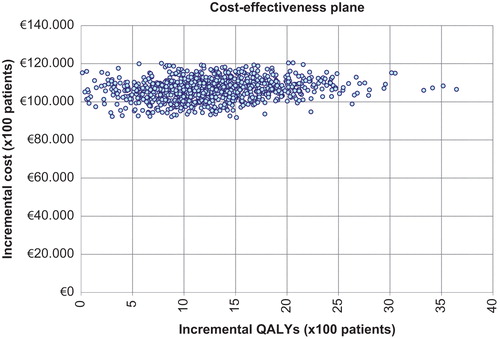
shows the results of the probabilistic sensitivity analysis using an incremental cost-effectiveness plane to compare the S0 and S1 strategies. The ordinate axis represents the incremental cost of S1 compared with S0, and the horizontal axis the incremental effect of S1 compared with S0 expressed in QALYs. All simulations are located in the first quadrant, representing greater effectivity and a greater cost of S1 compared with S0. Using the above-mentioned threshold of €30,000/QALYCitation31, 96.2% of simulations showed S1 as a cost-effective option compared with S0.
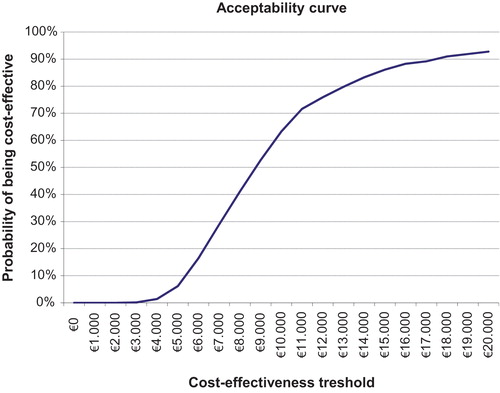
shows the probabilistic sensitivity analysis results in the form of an acceptability curve for S1 compared with S0 at different cost-effectiveness thresholds. For the above-mentioned threshold of €30,000/QALY, the probability of S1 being cost-effective compared with S0 was 100%: this fell to 92.8% and 63.3% when the acceptability threshold was lowered to €20,000 and €10,000 per QALY gained, respectively.
Discussion
Gout has a substantial economic impact in terms of both direct healthcare costs (medical visits, drugs, etc.) and indirect costs such as lost productivityCitation32,Citation33. A Spanish population-based study of 3130 patients with gout that aimed to determine the use of resources and the economic impact of patients with gout found that the total cost of the patients included in the study amounted to 7 million euros, of which 96.9% were direct health costs and 3.1% non-healthcare costs (lost productivity)Citation28. In addition, gout has a high impact on the quality-of-life of patients, which may be due both to direct musculoskeletal manifestations and to associated comorbiditiesCitation2,Citation34.
The results obtained in this analysis, the first to determine the cost-effectiveness of febuxostat in Spain, show that initiating treatment for hyperuricemia with febuxostat instead of allopurinol is a cost-effective option for the NHS.
Several economic evaluations of the treatment of hyperuricemia in gout with febuxostat and allopurinol have been publishedCitation14,Citation35–40, including the study by Beard et al.Citation14 describing the Markov model presented to the SMC, which has been adapted to the Spanish setting in the present analysis. These economic evaluations differ in the time horizons used, but in models using longer horizonsCitation14,Citation35,Citation36,Citation38,Citation40, even despite the higher acquisition cost of febuxostat, it was a cost-effective option for both first-lineCitation35,Citation36,Citation40 and second-line treatmentCitation14,Citation38 when costs and effects are modeled in the long-term.
The model has limitations, some of which are inherent to this type of pharmacoeconomic models, which have structural rigidity that could make correct representation of clinical reality difficult.
For example, this type of model usually uses long-term extrapolations of data from studies of limited duration. The 28 and 52 weeks duration of the APEX and FACT studies, respectively, provide significant information about reductions in sUA levels, but not on the long-term clinical outcomes, reflected in reductions of gout flares that are associated to improvement in quality-of-lifeCitation41. Therefore, in the model, the reduction in sUA levels occurs at the beginning of each ULT (based on the evidence of the APEX and FACT studies), assuming that this reduction is maintained over time if the patient continues treatment correctly.
For the drop-out rate beyond the first year (for which the pooled results of the APEX and FACT studies were used), it was also necessary to extrapolate from the results of a study of limited duration. Using the results of the 3-years study EXCEL, the annual probability of discontinuation was estimated and, therefore, applied constant from the second year onwards. In this respect, discontinuation was over-estimated, since the EXCEL study found that treatment abandonment fell over time and, in our model, the annual probability calculated was the same for each year. The CONFIRMS study found slightly-lower rates of drop-out than the pooled results of the APEX and FACT studies, and also favorable to febuxostat 80 mg (the study did not consider febuxostat 120 mg) over allopurinol. Therefore, the analysis is conservative in considering higher drop-out rates during the first year of treatment. Neither were the costs associated with the adverse events of febuxostat and allopurinol included in the model, since clinical evidence suggests no significant difference in the adverse events rate between the two treatments. However, the treatment discontinuation due to adverse effects itself is recorded within the variable ‘drop-out’.
Another limitation was due to the adaptation to the Spanish setting: the lack of a national cost database. This made it necessary to estimate the cost associated with an acute gout flare according to the use of resources in primary and specialist care reported in the observational study by Sicras-Mainar et al.Citation28. Importantly from the clinical practice point of view, other costs associated with the management of hyperuricemia and gout were not included in the analysis, such as follow-up visits or laboratory tests for escalating doses of allopurinol as recommendedCitation23. Since no evidence is available on possible differences in the use of these resources according to sUA levels, it is not possible to establish differences in the cost of disease management for each health state (sUA1, sUA2, sUA3, and sUA4). Therefore, if the non-drug management cost is the same for all health states, this is irrelevant in incremental terms, given that the clinical benefits of febuxostat are due to a reduction in sUA levels. As the analysis was made from the Spanish National Health System perspective, indirect costs due to the lost productivity associated with hyperuricemia were not included. The indirect costs associated with gout flares are considerable. The study by Brook et al.Citation32 estimated the indirect costs of the active population suffering gout as €2883, while a recent study by Spaetgens et al.Citation42 estimated the cost of work absenteeism due to gout as €4982. The study by Sicras-Mainar et al.Citation28 reported lower indirect costs associated with gout flares, this difference might be due to their conservative information source for the unit cost (the minimum wage instead of average wage cost) or the registration of job loss by the insurers (temporary job loss might not be registered). In any case, given the reduction in sUA levels observed in patients treated with febuxostat, which lead to a reduction in gout flares in the medium- and long-term, if indirect costs had been included in the analysis, the ICER of febuxostat vs allopurinol as first-line treatment would have been lower.
To overcome the uncertainty associated with all these limitations, the assumptions made and the values finally considered in the analysis were validated by a clinical expert, in addition to the deterministic and probabilistic sensitivity analyses developed.
In conclusion, and, even though it only considers direct costs, this analysis confirms and supplements the results of the study by Beard et al.Citation14, and shows that febuxostat 80 mg to 120 mg daily is a cost-effective option in the treatment of hyperuricemia of gout in the Spanish setting, not only when both ULT are used sequentially, but also when febuxostat is considered as first-line treatment.
Further economic evaluations should be developed in order to include indirect costs, or if relevant price changes befall.
Transparency
Declaration of funding
The present study was supported by Menarini Group, who market Febuxostat (Adenuric®) in Spain.
Declaration of financial/other relationships
FP received fees as advisor/speaker from AstraZeneca, Menarini, and Cymabay. DC received financing from Menarini for the pharmacoeconomic analysis and drafting the manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheumatism 2004;51:321-5
- Spanish Society of Rheumatology (SER). Clinical practice guidelines for management of gout. Madrid, Spain: Spanish Society of Rheumatology (SER), 2013. www.ser.es/practicaClinica/Guias_practica_clinica/Guias_finalizadas.php. Accessed April 20, 2015
- Vazquez-Mellado J, Alvarez Hernandez E, Burgos-Vargas R. Primary prevention in rheumatology: the importance of hyperuricemia. Best Pract Res Clin Rheumatol 2004;18:111-24
- Wortmann RL. Gout and hyperuricemia. Curr Opin Rheumatol 2002;14:281-6
- Baker JF, Krishnan E, Chen L, et al. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med 2005;118:816-26
- Kim KY, Ralph Schumacher H, Hunsche E, et al. A literature review of the epidemiology and treatment of acute gout. Clin Ther 2003;25:1593-617
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811-21
- Smith EU, Diaz-Torne C, Perez-Ruiz F, et al. Epidemiology of gout: an update. Best Pract Res Clin Rheumatol 2010;24:811-27
- Mikuls TR, Saag KG. New insights into gout epidemiology. Curr Opin Rheumatol 2006;18:199-203
- Carmona L. Epidemiología de las enfermedades reumáticas. Manual SER de las enfermedades reumáticas. 4th edn. Madrid: Editorial Panamericana, 2004
- Perez-Ruiz F, Carmona L, Yebenes MJ, et al. An audit of the variability of diagnosis and management of gout in the rheumatology setting: The Gout Evaluation and Management Study. J Clin Rheumatol 2011;17:349-55
- National Institute for Health and Clinical Excellence (NICE). Final appraisal determination. Febuxostat for the management of hyperuricaemia in people with gout. NICE, 2008. London (UK). https://www.nice.org.uk/guidance/ta164. Accessed April 20, 2015
- Scottish Medicines Consortium (SMC). Febuxostat 80 mg and 120 mg Tablets (Adenuric) SMC No. 637/10. 2010. Glasgow (UK). http://www.scottishmedicines.org.uk/files/advice/febuxostat-Adenuric-FINAL-August-2010.pdf. Accessed April 20, 2015
- Beard SM, von Scheele BG, Nuki G, et al. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ 2014;15:453-63
- Schumacher HR, Jr., Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008;59:1540-8
- Becker MA, Schumacher HR, Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450-61
- Lopez Bastida J, Oliva J, Antoñanzas F, et al. [A proposed guideline for economic evaluation of health technologies]. Gac Sanit 2010;24:154-70. Spanish
- Puig-Junoy J, Oliva-Moreno J, Trapero-Bertrán M, et al. Guía y recomendaciones para la realización y presentación de evaluaciones económicas y análisis de impacto presupuestario de medicamentos en el ámbito del CatSalut. Generalitat de Catalunya. Barcelona: Departament de Salut. Servei Català de la Salut, 2014
- Perez-Ruiz F, Martinez-Indart L, Carmona L, et al. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis 2014;73:177-82
- Becker MA, MacDonald P, Chefo S, et al. Baseline (BL) characteristics of gout subjects influence Urate-Lowering (UL) efficacy during febuxostat and allopurinol treatment [abstract]. Arthritis Rheum 2009;60(10 Suppl):704
- Schumacher HR, Jr, Becker MA, Lloyd E, et al. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology (Oxford) 2009;48:188-94
- Becker MA, Schumacher HR, MacDonald PA, et al. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 2009;36:1273-82
- Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006;65:1312-24
- Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis J Rheumatol 2004;31:2429-32
- Institute of Medical Science World Publications Ltd. A health economic assessment of febuxostat in the management of gout. Data on File. Version No. 1.0. 2007. London (UK)
- General Council of the Association of Official Pharmacists. General Council of the Association of Official Pharmacists Database: Bot PLUS 2.0. Madrid (Spain). https://botplusweb.portalfarma.com/. Accessed April 22, 2015
- Royal Decree-Law 8/2010. Extraordinary measures to reduce the public deficit. Amended by the Royal Decree-Law 9/2011. Madrid (Spain). http://www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. Accessed April 2015
- Sicras-Mainar A, Navarro-Artieda R, Ibáñez-Nolla J. Resource use and economic impact of patients with gout: a multicenter, population-wide study. Reumatol Clin 2013;9:94-100
- Gisbert R, Brosa M. Healthcare cost database eSALUD [Internet]. Barcelona: Oblikue Consulting, 2015. www.oblikue.com. Accessed April 20, 2015
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000;17:479-500
- Sacristán JA, Oliva J, Del Llano J, et al. What is an efficient health technology in Spain? Gac Sanit 2002;16:334-43
- Brook RA, Kleinman NL, Patel PA, et al. The economic burden of gout on an employed population Curr Med Res Opin 2006;22:1381-9
- Khanna PP, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual Life Outcomes 2012;10:117
- Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology 2007;46:1441-4
- Ipsen UK. Febuxostat for the treatment of gout: single technology appraisal submission to the National Institute for Health and Clinical Excellence. 2008. London (UK). http://www.nice.org.uk/guidance/ta164/documents/manufacturer-ipsen-submission2. Accessed January 2015
- Redding L, Hornberger J, Cowens W, et al. Cost-effectiveness of febuxostat in managing hyperuricemia in gout patients in Canada. Poster presented at: Canadian Agency for Drugs and Technologies in Health Symposium; April 3-5, 2011; Vancouver, British Columbia, Canada. https://www.cadth.ca/poster-presentations-0. Accessed April 2015
- Meltzer M, Pizzi LT, Jutkowitz E. Payer decision-making with limited comparative and cost-effectiveness data: the case of new pharmacological treatments for gout. Evid Based Med 2012;17:105-8
- Jutkowitz E, Choi HK, Pizzi LT, et al. Cost-effectiveness of allopurinol and febuxostat for the management of gout. Ann Intern Med 2014;161:617-26
- Smolen LJ, Wielage RC, Gahn JC, et al. The cost-effectiveness of Febuxostat in the management of patients with gout. J Manag Care Pharm 2014;20(4-a Suppl):S52. M18EM [Abstract]. http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=17840. Accessed April 20, 2015
- Gandhi PK, Gentry WM, Ma Q, et al. Cost-effectiveness analysis of allopurinol versus febuxostat in chronic gout patients: a U.S. payer perspective. J Manag Care Spec Pharm 2015;21:165-75
- Khanna PP, Perez-Ruiz F, Maranian P, et al. Long-term therapy for chronic gout results in clinically important improvements in the health-related quality of life: short form-36 is responsive to change in chronic gout. Rheumatology (Oxford) 2011;50:740-5
- Spaetgens B, Wijnands JM, van Durme C, et al. Cost of illness and determinants of costs among patients with gout. J Rheumatol 2015;42:335-44