Abstract
Background Dermatomyositis and polymyositis (DM/PM) are inflammatory myopathies characterized by muscle inflammation/weakness. Patients with DM/PM have a reduced quality-of-life and are at an increased risk for several comorbidities. Studies have assessed the incidence and prevalence of DM/PM; however, no study has estimated the burden of the diseases in terms of both healthcare resource utilization (HCRU) and work loss incurred by patients.
Objective To provide a comprehensive, current estimate of the annual HCRU and work loss in DM/PM patients in the US.
Methods All patients (aged 18–64 years) with a first diagnosis of DM/PM between January 1, 1998 and March 31, 2014 (‘index date’) were selected from a de-identified privately-insured administrative claims database. DM/PM patients were required to have continuous health-plan enrollment 12 months prior to and following their index date. Propensity-score (1:1) matching of DM/PM patients with non-DM/PM controls was carried out based on a logistic regression of demographic characteristics, comorbidities, costs, and HCRU to control for these confounding factors. Burden of HCRU and work loss (disability days and medically-related absenteeism) were compared between the matched DM/PM and the non-DM/PM cohorts over the 12-month period after the index date (‘outcome period’).
Results Of the 2617 DM/PM patients that met sample selection criteria, 2587 (98.9%) were matched with a non-DM/PM control. During the outcome period, DM/PM patients had significantly increased HCRU across places of service, including 44% more inpatient admissions (3.6 vs 2.5, p < 0.001), increased visits with specialists such as rheumatologists, neurologists and physical therapists, and filled 4.7 more prescriptions (32.2 vs 27.5, p < 0.001) than matched control patients. The increased HCRU led to significantly more medically-related work loss among DM/PM patients than matched controls (p < 0.001).
Conclusions DM/PM imposes a substantial increase in healthcare resource use and is associated with statistically significantly greater work loss in the first year following diagnosis.
Introduction
Dermatomyositis and polymyositis (DM/PM) are systemic autoimmune inflammatory myopathies that are characterized by chronic muscle inflammation leading to progressive muscle weaknessCitation1. While the two disorders are often grouped together due to their shared clinical features, dermatomyositis has a distinct skin involvement that distinguishes the condition from polymyositis; in 93% of such patients, the presenting feature is a skin rashCitation2. The estimated combined annual incidence of DM/PM is two cases per 100 000 persons in the USCitation3; overall prevalence may be as high as 22 cases per 100 000 personsCitation4.
Patients diagnosed with DM/PM have reduced quality-of-life and are at an increased risk for a number of comorbiditiesCitation5–10. For example, studies have estimated that DM/PM patients have up to a 7-fold increased risk of developing cancer compared with the general populationCitation6,Citation7 and that one in four DM/PM patients develop an associated connective tissue disease such as scleroderma, systemic lupus erythematosus, or rheumatoid arthritisCitation8. DM/PM patients are also at an increased risk of heart attack and stroke, and have a high prevalence of interstitial lung diseaseCitation9,Citation10. The overall mortality ratio in DM/PM patients is 3-fold higher compared with the general population, with cancer, lung and cardiac complications, and infections being the most common causes of deathsCitation11. Although the clinical burden of DM/PM has been described in recent studies, little is known about the economic burden of DM/PM. A recent economic study conducted using a US administrative database concluded that patients with newly diagnosed idiopathic inflammatory myopathies, such as DM/PM and interstitial myositis, have significantly higher use of ambulatory visits, specialist visits, and inpatient hospital stays compared to controlsCitation12. The increased resource use was associated with a 3-fold increase in annual medical costsCitation12. Another study conducted in a Canadian administrative healthcare database concluded that the health services costs in inflammatory myopathies may equal, or exceed, those of other serious diseases, such as rheumatoid arthritis and systemic sclerosisCitation13. However, no such studies have evaluated the economic burden of DM/PM alone in the US setting, in terms of both healthcare resource utilization and work loss (i.e., medically-related absenteeism and disability). The objective of this study was to provide a robust, current estimate of the healthcare resource use and medically-related work loss associated with DM/PM in the US. To accomplish this objective, the study used de-identified administrative claims records from a nationally-representative database for privately-insured populations. Resource use was estimated for DM/PM patients and a control population without DM/PM. The incremental healthcare resource use and associated work loss of DM/PM were assessed using a matched case-control study design to account for differences in age, gender, rates of underlying comorbidities, prior healthcare resource use, and prior healthcare costs.
Patients and methods
Data sources
This study used de-identified administrative claims from OptumHealth Reporting and Insights, a database containing healthcare utilization records of over 18.5 million beneficiaries (including employees, spouses, dependents, and retirees) with commercial insurance from over 80 large self-insured US-based companies. The database contains information regarding patient age, gender, enrollment history, medical diagnoses, procedures performed, dates and place of service, and payment amounts for the time period spanning January 1, 1998 to March 31, 2014. Prescription drug claims (including fill dates, national drug codes, and payment amounts) are available for all beneficiaries. Information regarding wages and work loss due to disability are available for a subset of employees.
Sample selection
Patients from the administrative claims database were divided into two mutually exclusive groups: patients with at least one diagnosis of DM/PM (n = 9219), and patients without a diagnosis of DM/PM (‘controls’) (n = 13 648 822), with DM/PM diagnosis identified by ICD-9 CM diagnosis codes 710.3 (DM) or 710.4 (PM) during the study period (1/1/1998–3/31/2014). Given the large size of the overall control population (n = 13 648 822), a 10% sample selected using a random number generator was used as a statistically valid control sample (n = 1 364 882).
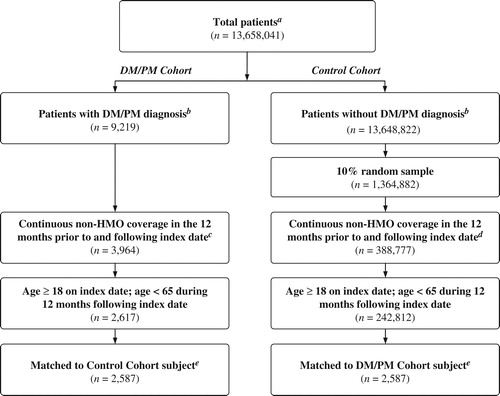
The earliest DM/PM diagnosis during the study period was designated as the ‘index date’ for patients in the DM/PM cohort. By definition, patients in the control population did not have a DM/PM diagnosis at any time before or after the index date; therefore, a random medical claim occurring during the study period was selected and assigned as the index date for these patients. Patients were required to be aged 18–64 years and continuously eligible with non-Health Maintenance Organization (HMO) coverage throughout the 12 months prior to the index date (‘baseline period’) and the 12 months following the index date (‘outcome period’) to ensure that all relevant drug and medical claims were captured. Patients aged 65 years and older were excluded from the study, as their Medicare eligibility may have limited the ability to observe all relevant drug and medical claims. After applying all of the criteria, 2617 DM/PM patients and 242 812 potential non-DM/PM control patients were included in the study ().
Figure 1. Selection of DM/PM patients and non-DM/PM controls. aTotal patient population includes all beneficiaries with at least one medical claim in OptumHealth Reporting Insights between 1/1/1998 and 3/31/2014 (‘study period’). bDM/PM diagnosis was determined by ICD-9-CM diagnosis code 710.3 or 710.4 occurring during the study period. cIndex date defined as the earliest DM/PM diagnosis occurring during the study period. dIndex date defined as a randomly selected medical claim occurring during the study period. eA greedy matching algorithm was used to match each DM/PM patient with a control patient based on propensity score. Propensity scores were estimated using a multivariate logistic regression with several covariates measured at baseline. Matched pairs were required to have the same availability of work loss data.
Propensity score matching
To account for underlying differences between the two cohorts, DM/PM patients were matched one-to-one to non-DM/PM controls using a ‘greedy’ matching methodologyCitation14 based on the likelihood of having been diagnosed with DM/PM determined by propensity scores (±1/8 standard deviation). This approach, which is commonly used in matched healthcare utilization studiesCitation15–18, selects a control for each subsequent case based on the nearest propensity score. Propensity scores were calculated for patients using a multivariate logistic regression with the following covariates measured at baseline: age, gender, region, year of index date (to account for differences in utilization and treatment patterns over time), Charlson Comorbidity IndexCitation19 (a measure of underlying disease severity), comorbid conditions common among DM/PM patients (i.e., rheumatic disease, rheumatoid arthritis, systemic lupus erythematosus, chronic obstructive pulmonary disease, diabetes without chronic complication, mild liver disease, any malignancy except neoplasm of the skin), number of medical visits (emergency department, inpatient, outpatient, other [e.g., home health, extended care, hospice], rheumatologist, neurologist, physical therapy), number of prescriptions filled, and total costs (medical costs, prescription drug costs). In order to facilitate comparisons of work loss, matched pairs were required to have the same availability of work loss data. These criteria resulted in a final matched sample of 2587 DM/PM patients and 2587 potential non-DM/PM control patients ().
Calculation of healthcare resource utilization
Total and incremental all-cause healthcare resource utilization in the 12-month outcome period was compared for DM/PM patients and non-DM/PM controls. Resource utilization was categorized by place of service (i.e., inpatient, outpatient/physician office, emergency department [ED], and other [e.g., skilled nursing facilities, home health]) in order to identify sources of differential medical care. Utilization of selected medical specialists known to care for DM/PM patients (i.e., neurologists, rheumatologists, physical therapists) was assessed. Among the DM/PM patients, healthcare utilization specifically associated with DM/PM care (i.e., for which a DM/PM diagnosis is present on the medical claim) was categorized as ‘DM/PM-related’ resource use and was summarized separately.
In addition, total and incremental work loss due to disability and medically-related absenteeism during the outcome period were estimated for the sub-set of privately-insured patients with disability and wage information available. Days of short- and long-term disability leave were obtained directly from the database; medically-related absenteeism days were estimated using medical claims occurring during the workweek. Each hospitalization day or ED visit accounted for a full day of missed work; all other visits accounted for half a day of absenteeismCitation20.
Statistical analyses
For categorical variables, statistical significance was assessed using chi-squared tests for comparisons between pre-match DM/PM patients and controls, and McNemar tests for the matched cohort. For continuous variables, statistical significance was assessed using Wilcoxon rank-sum tests (pre-match) and Wilcoxon signed-rank tests (post-match). All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
Prior to matching, DM/PM patients were statistically different (at the 0.001 significance level) from the non-DM/PM control population on every measure examined during the baseline period (i.e., 12 months prior to the index date) (). DM/PM patients were older (49.5 vs 43.4 years) and had statistically significantly higher rates of rheumatic diseases such as rheumatoid arthritis (9.9% vs 0.6%) and systemic lupus erythematosus (8.1% vs 0.2%) compared with non-DM/PM controls during the baseline period. In addition, DM/PM patients had higher rates of other comorbidities including chronic obstructive pulmonary disease (15.1% vs 6.3%) and diabetes (11.7% vs 5.5%).
Table 1. Patient characteristics, resource utilization, and healthcare costs during the 12 months prior to the index date.
Compared with the potential controls, DM/PM patients had more inpatient admissions (+136.4%), ED visits (+75.0%), and outpatient/physician office visit (+106.0%) in the baseline period. These differences resulted in baseline medical care costs among DM/PM patients that were ∼2.5-times those of the potential control population ($12,145 vs $4760).
After matching DM/PM patients with comparable controls, the 2587 pairs of DM/PM and control patients had more similar baseline demographics and comorbidities (). The age differential across cohorts was reduced from 6.1 years to 1.2 years (49.4 years for matched DM/PM patients vs 50.6 years for matched controls, p < 0.001). The matched groups had similar disease severity, as evidence by the same value for the Charlson Comorbidity Index (1.0 vs 1.0, p = 0.344), a measure which was originally 3-fold higher in the unmatched DM/PM patients compared to controls. Further, the magnitude of the healthcare utilization during the baseline period was similar for the matched DM/PM and control groups (23.6 vs 23.7 medical visits, p = 0.003; 26.0 vs 26.4 prescriptions filled, p = 0.231). Baseline medical care costs, which originally differed by more than $9000 across the unmatched groups, were similar in the matched cohorts ($14,622 vs $14,276, p = 0.115).
Direct healthcare resource use during the outcome period
After matching, the DM/PM patients used more healthcare resources (all-cause medical visits and prescriptions filled) during the 12-month outcome period compared with matched controls (p < 0.001) (). DM/PM patients had an average of 31.0 medical visits compared with 23.6 visits among matched non-DM/PM controls during the outcome period. Approximately 57% of the additional medical visits in the DM/PM cohort were directly attributable to DM/PM-related care (4.2 of the 7.4 visit differential).
Table 2. Healthcare resource utilization during the 12 months following the index date among matched DM/PM patients and controls.
DM/PM patients had 44.0% more inpatient admissions (3.6 vs 2.5), 33.3% more ED visits (0.8 vs 0.6), and 26.7% more outpatient/physician office visits (21.8 vs 17.2) compared with matched non-DM/PM controls (all comparisons, p < 0.001). Furthermore, DM/PM patients had significantly more rheumatologist (1.8 vs 0.6), neurologist (0.8 vs 0.4), and physical therapy (3.7 vs 2.6) visits compared with matched controls (p < 0.001). In addition, DM/PM patients filled on average 4.7 more prescriptions than matched non-DM/PM controls during the outcome period (32.2 vs 27.5 fills, p < 0.001).
Disability and medically-related absenteeism during the outcome period
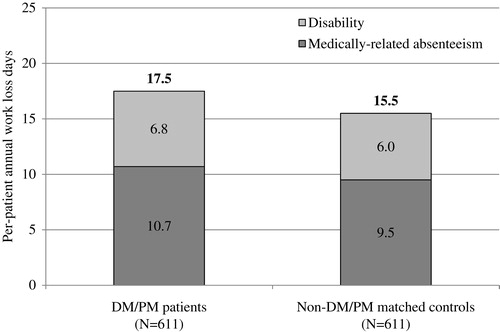
Work loss information was available for 611 of the 2587 matched pairs (). On average, DM/PM patients had 2.0 days more of work loss than matched controls (17.5 vs 15.5 days, p < 0.001). Work loss was predominantly driven by a significant increase in medically-related absenteeism (10.7 vs 9.5 days, p < 0.001). DM/PM patients also had nearly one more day of disability compared with matched controls (6.8 vs 6.0 days, p = 0.977).
Figure 2. Disability and medically-related absenteeism during the 12 months following the index date among matched DM/PM patients and controls. Analyses were limited to primary beneficiaries who had work loss data available for the entirety of the 12-month outcome period. Medically-related absenteeism was calculated using medical claims occurring during the workweek. Days with a hospitalization or emergency department visit were counted as a full day of absenteeism, and all other visits were counted as a half day of absenteeism.
Discussion
Of the nearly 14 million privately-insured beneficiaries with a medical claim available in the administrative claims database, 9219 (0.07%) were diagnosed with DM/PM between 1/1/1998 and 3/31/2014. The DM/PM patients identified for the analysis were ∼50 years of age, predominantly female, and had several additional co-morbid conditions, particularly other rheumatic diseases. Prior to matching, DM/PM patients were statistically significantly older, had higher rates of comorbidities, and had more healthcare resource use and costs than the non-DM/PM control population. However, these differences were largely eliminated after matching, with matched pairs having similar patient characteristics, allowing for more precise estimation of incremental resource use following DM/PM diagnosis. During the 12-month follow-up period, DM/PM patients had statistically significantly higher healthcare resource use (both medical visits and prescription drug fills) than matched control patients. Furthermore, this study found that, compared with non-DM/PM matched controls, DM/PM patients missed more days of work due to medically-related absenteeism, imposing an economic burden on employers.
To our knowledge, this is one of the first studies to assess the excess healthcare resource utilization and work loss burden among DM/PM patients in the US by employing robust methods to account for underlying differences between patients with and without DM/PM. While there are no published estimates of the incremental burden of DM/PM alone in the US, this study’s findings are consistent with prior research on idiopathic inflammatory myopathiesCitation12, and other similar chronic musculoskeletal diseases, such as those reported for patients with osteoporosisCitation21, arthritis and joint painCitation21, and rheumatoid arthritisCitation22.
While this study found substantial resource use among DM/PM patients, it likely understates the actual burden of DM/PM in the US. The study focuses on healthcare resource utilization in the 12 months following DM/PM diagnosis—the healthcare resource use may change (and likely increases) as the disease progresses beyond the outcome period evaluated in this study. Further, the matching process disproportionately removed DM/PM patients with relatively high healthcare resource use and control patients with relatively low healthcare resource use, as these ‘outliers’ could not be matched. While only 30 DM/PM patients could not be matched with controls, these 30 patients had substantially higher resource utilization during the outcome period than the 2587 matched DM/PM patients: 59.6 vs 31.0 medical visits and 70.8 vs 32.2 prescription fills (both p < 0.001, data not shown). These 30 unmatched DM/PM patients were, on average, 50% more costly than the matched DM/PM patients included in the analyses.
This study had additional limitations, inherent to the data used in the analysis. First, this study relied on the accuracy of diagnosis codes to identify patients with or without DM/PM and to evaluate their comorbidity profiles at baseline as well as resource use in the outcome period. Miscoding in the database could affect the results, but there is no reason to believe that coding inaccuracies in the data may have affected the DM/PM and non-DM/PM control groups differently. A future study validating the use of diagnosis codes to identify patients with DM/PM in administrative claims databases is warranted. In addition, the estimate of indirect burden of disease in this study was limited to work time lost due to disability or medical treatment measures directly or indirectly observable in the data. The true indirect burden of disease may have been under-estimated, as measures of work time lost did not include absenteeism for other reasons, such as sick time at home or reduced on-the-job productivity (i.e., ‘presenteeism’). Conversely, an over-estimation may have occurred due to the method used to approximate medically-related absenteeism, a measure not available in the data-set. The method, which has been used in previous health research studiesCitation20, assumed that medical appointments occurring during the workweek were associated with missed work time, although it is possible that the medical visits instead occurred outside of working hours. The magnitude of over- or under-estimation associated with the aforementioned limitations is unknown.
In order to create a homogenous study population which allowed the observation of all relevant healthcare utilization, this study relied on data for a population of commercially-insured non-HMO beneficiaries under the age of 65 years. However, the study was not able to account for patients under the age of 65 who were Medicare eligible. Finally, the generalizability of the results to other populations (e.g., Medicaid, Medicare, uninsured) is unknown.
Conclusions
This study is the first to use rigorous methodologies to estimate the incremental direct and indirect burden of DM/PM using recent, national administrative claims data, controlling for a broad set of underlying differences between commercially-insured DM/PM and control populations. As a result, the findings of this study help shed light on the substantial economic burden of DM/PM by quantifying the incremental healthcare resource use associated with treating these patients as well as the increase in medically-related absenteeism, which burdens both patients and employers.
Transparency
Declaration of funding
This study was funded by Mallinckrodt Pharmaceuticals, Inc., Hazelwood, MO.
Declaration of financial/other relationships
MP is an employee of Mallinckrodt Pharmaceuticals, which provided research funding to Analysis Group, Inc. (employer of JBR, AW, AL, and PG). PS was affiliated with Mallinckrodt Pharmaceuticals at the time that this study was conducted. BP is an employee of Xcenda, L.L.C. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Abstracts containing results from this analysis were presented at Academy of Managed Care Pharmacy (AMCP) Nexus, October 26–29 2015, Orlando, FL.
References
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-82
- Bohan A. Clinical presentation and diagnosis of polymyositis and dermatomyositis, in polymyositis and dermatomyositis. In: Dalakas M, ed. Stoneham, MA: Butterworth Publishers, 1988. p. 19-36
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223-43
- Bernatsky S, Joseph L, Pineau CA, et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex and regional differences. Ann Rheum Dis 2009;68:1192-6
- Regardt M, Welin Henriksson E, Alexanderson H, et al. Patients with polymyositis or dermatomyositis have reduced grip force and health-related quality of life in comparison with reference values: an observational study. Rheumatology (Oxford) 2011;50:578-85
- Barnes BE. Dermatomyositis and malignancy: a review of the literature. Ann Intern Med 1976;84:68-76
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96-100
- Hak AE, de Paepe B, de Bleecker JL, et al. Dermatomyositis and polymyositis: new treatment targets on the horizon. Neth J Med 2011;69:410-21
- Marie I, Hachulla E, Cherin P, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 2002;47:614-22
- Tisseverasinghe A, Bernatsky S, Pineau CA. Arterial events in persons with dermatomyositis and polymyositis. J Rheumatol 2009;36:1943-6
- Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012;14:275-85
- Furst DE, Amato AA, Iorga SR, et al. Medical costs and health-care resource use in patients with inflammatory myopathies in an insured population. Muscle Nerve 2012;46:496-505
- Bernatsky S, Panopalis P, Pineau CA, et al. Healthcare costs of inflammatory myopathies. J Rheumatol 2011;38:885-8
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424
- Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 2009;169:480-8
- Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med 2010;170:1979-86
- Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010;170:1968-76
- Lindenauer PK, Pekow PS, Lahti MC, et al. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010;303:2359-67
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Birnbaum HG, Barton M, Greenberg PE, et al. Direct and indirect costs of rheumatoid arthritis to an employer. J Occup Environ Med 2000;42:588-96
- Initiative, U.S.B.a.J, The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: United States Bone and Joint Initiative 2014
- Merkesdal S, Ruof J, Huelsemann JL, et al. Indirect cost assessment in patients with rheumatoid arthritis (RA): comparison of data from the health economic patient questionnaire HEQ-RA and insurance claims data. Arthritis Rheum 2005;53:234-40
