Abstract
Objective Ranibizumab, an anti-vascular endothelial growth factor designed for ocular use, has been deemed cost-effective in multiple indications by several Health Technology Assessment bodies. This study assessed the cost-effectiveness of ranibizumab monotherapy or combination therapy (ranibizumab plus laser photocoagulation) compared with laser monotherapy for the treatment of visual impairment due to diabetic macular edema (DME).
Methods A Markov model was developed in which patients moved between health states defined by best-corrected visual acuity (BCVA) intervals and an absorbing ‘death’ state. The population of interest was patients with DME due to type 1 or type 2 diabetes mellitus. Baseline characteristics were based on those of participants in the RESTORE study. Main outputs were costs (in 2013 CA$) and health outcomes (in quality-adjusted life-years [QALYs]) and the incremental cost-effectiveness ratio (ICER) was calculated. This cost-utility analysis was conducted from healthcare system and societal perspectives in Quebec.
Results From a healthcare system perspective, the ICERs for ranibizumab monotherapy and combination therapy vs laser monotherapy were CA$24 494 and CA$36 414 per QALY gained, respectively. The incremental costs per year without legal blindness for ranibizumab monotherapy and combination therapy vs laser monotherapy were CA$15 822 and CA$20 616, respectively. Based on the generally accepted Canadian ICER threshold of CA$50 000 per QALY gained, ranibizumab monotherapy and combination therapy were found to be cost-effective compared with laser monotherapy. From a societal perspective, ranibizumab monotherapy and combination therapy provided greater benefits at lower costs than laser monotherapy (ranibizumab therapy dominated laser therapy).
Conclusions Ranibizumab monotherapy and combination therapy resulted in increased quality-adjusted survival and time without legal blindness and lower costs from a societal perspective compared with laser monotherapy.
Introduction
Diabetic macular edema (DME) is an ophthalmological complication of diabetes mellitus. It develops in a sub-set of patients with diabetic retinopathy, which is a leading cause of visual impairment and blindness worldwideCitation1. DME is characterized by thickening of retinal tissue as a result of fluid leaking from blood capillaries—when this affects the center of the macula, it can lead to visual impairment and, if left untreated, blindness. Approximately 6.8% of patients with diabetes mellitus have DMECitation1.
Ranibizumab (Lucentis®, Novartis Pharma AG, Basel, Switzerland) is an anti-vascular endothelial growth factor designed specifically for ocular use. It is approved for the treatment of visual impairment in patients with DME, wet age-related macular degeneration (wAMD), macular edema following retinal vein occlusion (RVO), and myopic choroidal neovascularization. Ranibizumab has been shown to improve best-corrected visual acuity (BCVA) in patients with visual impairment due to DME in a number of randomized controlled trials (RCTs). The pivotal ‘Ranibizumab Monotherapy or Combined with Laser vs Laser Monotherapy for Diabetic Macular Edema (RESTORE)’ phase 3 study compared the clinical benefit and safety of ranibizumab monotherapy and ranibizumab plus laser photocoagulation with that of laser monotherapy in patients with visual impairment due to DME. It consisted of a 12-month, randomized, double-masked control phase followed by a 24-month, open-label extensionCitation2,Citation3. The RESTORE study found that ranibizumab treatment was associated with rapid improvements in BCVA that were sustained to month 36Citation2,Citation3. Additional RCTs, including the Diabetic Retinopathy Clinical Research Network (DRCR.net) trial, which compared ranibizumab or triamcinolone combined with laser photocoagulation vs laser photocoagulation aloneCitation4,Citation5, support the results of the RESTORE studyCitation4–10. Ranibizumab has been deemed cost-effective in multiple indications by several Health Technology Assessment (HTA) bodies, including the National Institute for Health and Care Excellence in the UK and the Scottish Medicines Consortium. With the inclusion of wider societal costs and benefits becoming more common in economic analysis, additional analyses of the cost-effectiveness of ranibizumab are needed.
The aim of this study was to evaluate the cost-effectiveness of 36 months of ranibizumab monotherapy or combination therapy (ranibizumab plus laser photocoagulation) compared with that of laser monotherapy for the treatment of visual impairment due to DME. Clinical outcomes were based on the RESTORE trial, in which patients were treated with the ranibizumab posology that is currently approved in Canada.
The study investigated both healthcare system and societal perspectives (including productivity costs) in Quebec. A lifetime time horizon was used based on Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines, which state that a lifetime time horizon should be used as a default, and justification should be provided when a shorter time horizon is usedCitation11. Other cost-effectiveness analyses of ranibizumab have used a lifetime time horizonCitation12,Citation13.
Patients and methods
Model structure
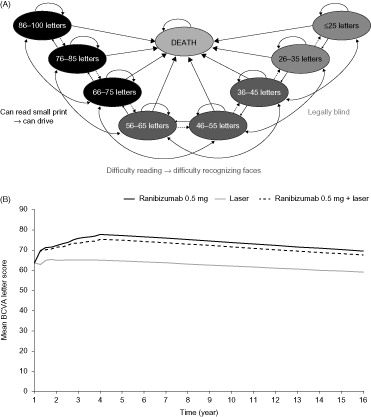
A Markov model was developed in 2013 to predict the long-term costs and health outcomes following 3 years of ranibizumab treatment for visual impairment due to DME. In the Markov structure, patients cycled between eight health states, defined by BCVA intervals ranging from 25 letters or fewer to 86–100 letters (Snellen equivalents 20/10 to 20/20) in the treated eye, and a ninth, absorbing, ‘death’ state (). Patients moved between states at the end of each 3-month cycle based on transition probabilities (Supplementary Table 1)Citation13.
Patients
The population of interest was patients with DME due to type 1 or type 2 diabetes mellitus. Baseline characteristics of patients in each treatment arm were based on those of participants in the RESTORE study (). The mean (standard deviation [SD]) BCVAs were 64.8 (10.1), 63.4 (10.0), and 62.4 (11.1) letters in the ranibizumab monotherapy, combination therapy, and laser monotherapy groups, respectively. The mean age was 63.3 years, and similar in each group; 40.2% of patients were treated in their better-seeing eye (BSE) and 22% of patients had bilateral disease. BCVA at the start of treatment was assumed based on baseline visual acuity scores in the RESTORE study (Supplementary Table 2).
Table 1. Baseline characteristics of the RESTORE study population.
Withdrawal rates
A proportion of patients were assumed to drop out in each cycle over years 1–3, with treatment-group-specific withdrawal rates determined by data from the RESTORE study. Withdrawal rates were dependent on treatment group, and not on health state. Patients withdrawing from ranibizumab arms were assumed to continue treatment with laser monotherapy. Owing to low withdrawal rates in RESTORE (11.7–12.7%), after withdrawing, patients were assumed to have the same transition probabilities as those continuing treatment, but to only incur the costs associated with laser monotherapy.
Mortality assumptions
All-cause mortality values were obtained using 5-year incremental data from Statistics CanadaCitation14, which were smoothed to generate annual rates for use in the model. Age-specific data on risk of death due to diabetes mellitus were obtained from the Public Health Agency of Canada and applied to the mortality data from Statistics CanadaCitation15. The relative risk of death due to DME was obtained from a US study of patients with type 2 diabetes mellitus and clinically significant macular edemaCitation16.
Adverse events
Adverse events were rare and similar across treatment arms in the RESTORE study; therefore, they were not included in the model.
Utilities
Utility values specific to DME are not available in the literature. Therefore, the utility values used in the model were based on validated data from studies of other ocular diseases (Supplementary Table 1). In this analysis, utility values associated with each BCVA health state were determined separately for patients treated in their BSE and for those treated in their worse-seeing eye (WSE). Quality of life in people with visual impairment is associated primarily with vision in the BSE, and utility values are generally lower in patients whose affected eye is their BSECitation17. For patients with bilateral disease, BSE utilities were used. BSE utility values were obtained from a simulation study of visual impairment associated with wAMD, adjusted for ageCitation18. WSE utility values were obtained from a multiple regression analysis of Canadian patients with RVO, under the assumption that the correlation between utility values and BCVA in the WSE of patients with DME is comparable to that in patients with RVO and AMDCitation19.
Treatment costs
Treatment costs were calculated from the perspective of the healthcare system in Quebec using unit costs of treatment, administration, and monitoring, multiplied by resource use (). It was assumed that all patients were only treated for the first 3 years of the model given the lack of accurate evidence on the clinical effects of both ranibizumab and laser beyond that point in time. The frequency of treatment was based on that in the RESTORE study for all treatment arms in year 1Citation2. In years 2 and 3, treatment frequencies for ranibizumab monotherapy and combination therapy were based on the RESTORE extension study, and the number of laser photocoagulation sessions was based on DRCR.net trial data. (In the RESTORE study extension phase, all patients received ranibizumab plus laser photocoagulation, whereas the 36-month DRCR.net trial reported data for laser photocoagulationCitation3,Citation5,Citation20.) In this model, patients receiving ranibizumab were assumed to be monitored monthly, in line with label recommendations. Based on clinical adviser input, for patients receiving laser monotherapy and for those not receiving treatment, it was assumed that individuals with a BCVA of less than 46 letters would have three monitoring visits per year, while those with a BCVA of 46 letters or higher would have five monitoring visits per year.
Table 2. Treatment costs and resource use.
Based on the ranibizumab indication (for patients with visual impairment due to DME) and label recommendations, it was assumed that patients with no visual impairment (those transitioning into health states with a BCVA of over 75 letters) would cease to receive treatment, and that treatment would only resume if patients transitioned into health states with a BCVA of 75 letters or under.
Costs of visual impairment
The healthcare utilization costs of visual impairment used in the model were derived from an observational study of the burden of illness of wAMD performed at five Canadian retinal clinics, and were broken down according to BCVA ()Citation21. Productivity losses associated with each health state, largely comprising caregiver time to help with daily activities, were calculated using data from the literatureCitation21–45. These costs of lost productivity were added to the direct costs for each health state when a societal perspective was taken. In the absence of data found on the costs associated with the WSE, it was conservatively assumed that patients treated only in their WSE did not incur costs associated with visual impairment or productivity losses.
Table 3. Mean annual healthcare utilization costs associated with visual impairment, according to BCVA.
Economic analysis
The main outcomes from the model were lifetime costs (in 2013 CA$) and total health outcomes (in quality-adjusted life-years [QALYs]). Costs and health outcomes were discounted at 5% per year in accordance with the recommendation from the CADTH guidelines, and a lifetime time horizon was assumed. The incremental cost-effectiveness ratio (ICER) was calculated as the ratio of the mean incremental cost to the mean number of QALYs gained for ranibizumab monotherapy or combination therapy compared with laser monotherapy. Costs per year without legal blindness were also calculated.
Sensitivity analyses
Deterministic sensitivity analyses were used to test the impact of parameter uncertainty on the results, including the proportion of patients with bilateral DME, the number of injections received, the treatment stopping rule (see Methods section) and the time horizon (Supplementary Table 3). Probabilistic sensitivity analyses using a Monte Carlo simulation were undertaken to assess the joint parameter uncertainty in the model (Supplementary Table 4).
Results
Health outcomes
The model reproduced the sustained improvement in BCVA seen in the RESTORE study with ranibizumab monotherapy and combination therapy compared with laser monotherapy. After treatment, BCVA progressed according to natural decline as described by the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR)Citation46,Citation47. The difference in BCVA observed between patients treated with ranibizumab and those receiving laser monotherapy was maintained over time (). This sustained improvement in BCVA was accompanied by a progressive reduction in the need for re-treatment from year 1 to year 2 to year 3. Compared with laser monotherapy, ranibizumab monotherapy was associated with gains of 0.62 years without legal blindness and 0.40 QALYs. The corresponding gains for combination therapy were 0.56 years without legal blindness and 0.32 QALYs.
Costs
From a healthcare system perspective, the lifetime costs of ranibizumab monotherapy and combination therapy were CA$25 233 and CA$26 854, respectively, and the cost of laser monotherapy was CA$15 383. From a societal perspective, lifetime costs associated with ranibizumab monotherapy and combination therapy were CA$60 200 and CA$68 078, respectively; laser monotherapy was associated with a cost of CA$79 193. The higher cost associated with laser monotherapy is a result of poorer BCVA in this group, which is associated with an increased cost of productivity losses.
Cost-effectiveness
From a healthcare system perspective, the ICERs for ranibizumab monotherapy and combination therapy vs laser monotherapy were CA$24 494 and CA$36 414 per QALY gained, respectively (). The incremental costs per year gained without legal blindness for ranibizumab monotherapy and combination therapy vs laser monotherapy were CA$15 822 and CA$20 616, respectively. Based on the generally accepted Canadian ICER threshold of CA$50 000 per QALY gained, ranibizumab monotherapy and combination therapy were cost-effective compared with laser monotherapy. From a societal perspective, ranibizumab monotherapy and combination therapy dominated laser monotherapy, providing greater benefits at a lower cost.
Table 4. Cost-effectiveness results for reference case.
Sensitivity analyses
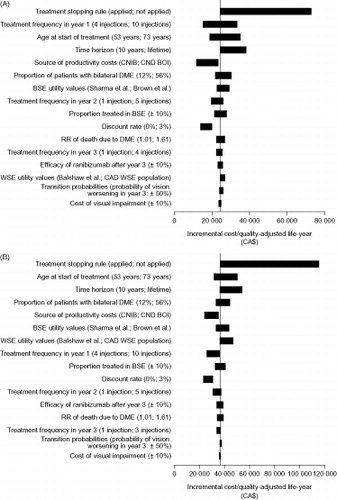
Deterministic sensitivity analyses demonstrated that the model is robust. Ranibizumab monotherapy and combination therapy remained cost-effective compared with laser monotherapy when key model assumptions were adjusted. From a healthcare system perspective, the only change to the model parameters that resulted in an ICER of more than CA$50 000 per QALY gained with ranibizumab monotherapy was the removal of the assumption that patients stop treatment if BCVA is higher than 75 letters (the treatment stopping rule). This scenario led to an ICER of CA$72 989 per QALY. The only changes that led to an ICER of more than $50 000 per QALY gained with combination therapy were the removal of the treatment stopping rule (CA$115 357 per QALY) and reduction of the time horizon to 10 years (CA$54 252 per QALY) (). From a societal perspective, ranibizumab monotherapy dominated laser monotherapy in all investigated scenarios, and combination therapy dominated laser monotherapy in all scenarios except for the one in which the treatment stopping rule was removed; this scenario led to an ICER of CA$40 873 per QALY gained (data not shown).
Figure 2. Tornado plots for one-way sensitivity analyses of (A) ranibizumab monotherapy vs laser monotherapy, and (B) combination therapy (ranibizumab and laser photocoagulation) vs laser monotherapy (healthcare system perspective). BSE, better seeing eye; CDN BOI, Canadian DME Burden of Illness Study; CNIB, Canadian National Institute for the Blind; DME, diabetic macular edema; RR, relative risk; WSE, worse-seeing eye.
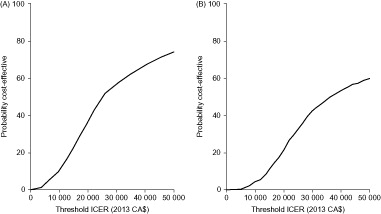
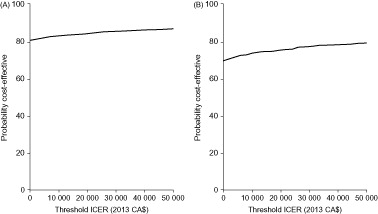
Probabilistic sensitivity analysis demonstrated that ranibizumab monotherapy and combination therapy are likely to be cost-effective compared with laser monotherapy from both healthcare system and societal perspectives. From a healthcare system perspective, the probabilities of ranibizumab monotherapy and combination therapy being cost-effective, given an ICER acceptability threshold of CA$50 000 per QALY, were 74% and 60%, respectively (). From a societal perspective, the equivalent probabilities of ranibizumab monotherapy and combination therapy being cost-effective were 87% and 79%, respectively ().
Discussion
Ranibizumab monotherapy and combination therapy resulted in increased time without legal blindness and increased quality-adjusted survival compared with laser monotherapy, and in lower costs from a societal perspective. Deterministic sensitivity analyses demonstrated that ranibizumab monotherapy and combination therapy remained cost-effective compared with laser monotherapy when key model assumptions were adjusted.
Ranibizumab monotherapy appears to be more effective and less expensive than combination therapy. The improvement in QALYs gained and years without blindness in the ranibizumab monotherapy group can be explained by the RESTORE trial results, which report that patients receiving ranibizumab monotherapy achieved slightly greater letter gains than those receiving combination therapyCitation2,Citation3.
Three previous studies have investigated the cost-effectiveness of ranibizumab compared with laser monotherapy in the treatment of DME. Dewan et al.Citation48 estimated an incremental cost of $5943 (2010 USD) per letter gained in visual acuity for ranibizumab combination therapy vs laser monotherapy over a time horizon of 2 years. As discussed, Mitchell et al.Citation13 presented an earlier version of the model reported in this study, in which outcomes were simulated from a UK healthcare system perspective using a 15-year time horizon based on available data. The ICERs for ranibizumab monotherapy and combination therapy vs laser monotherapy were £24 028 and £36 106 per QALY gained, respectively (2010 GBP). The final study by Stein et al.Citation49 found that the ICER for ranibizumab combination therapy vs laser monotherapy was $89 903 per QALY gained (2013 USD) over a 25-year time horizon, which suggests that it would be cost-effective at a willingness-to-pay threshold of USD $100 000, if patients receive fewer than 0.45 injections annually after year 2. The higher cost of ranibizumab combination therapy reported by Stein et al.Citation49 can be explained by the fact that their model adopted a US perspective; therefore, the costs are likely to be substantially different.
The present analysis builds on previous models that included clinical trial data of only 12 monthsCitation13 by including clinical trial data up to 36 months, taking into account patients treated in their WSE, their BSE or both eyes, and using a lifetime time horizon. The inclusion of wider societal costs and benefits is becoming more common in economic analysis, and the present study is the first analysis of ranibizumab for the treatment of visual impairment due to DME to take into account productivity losses. Patient baseline data and input variables related to treatment frequency and efficacy were based on individual patient records taken from large clinical trials, and are likely to be broadly applicable to clinical practice. One limitation of this and previous studies is the lack of utility value estimates specific to DME. However, our base-case analysis used utility values derived from studies of other conditions associated with a loss of vision, which are likely to be relevant to the DME population and, in addition, the DME utility values are consistent across the treatment arms. This was more appropriate than using EQ-5D utility scores, which have been shown to be extremely insensitive to changes in visual acuityCitation50,Citation51.
A potential limitation of this study is the choice of comparator group. Laser photocoagulation was selected as the comparator because, at the time of the study, it was the only licensed treatment for DME in Canada other than ranibizumab. Aflibercept and dexamethasone intravitreal implants were not reimbursed in Canada at the time this analysis was conducted and, therefore, were excluded from the analysis. The ranibizumab dose used in the current analysis was 0.5 mg pro re nata based on the RESTORE studyCitation2. A more recent study, the DRCR.net trial, has compared the efficacy of ranibizumab, aflibercept, and bevacizumabCitation52. Results show that, in patients with initial visual acuity 20/40 or better, there were no differences in efficacy among the three treatments, whereas in those with visual acuity 20/50 or worse, mean improvement was greatest with aflibercept and not significantly different between ranibizumab and bevacizumab. However, the DRCR.net trialCitation52 used a lower dose of ranibizumab (0.3 mg) using the pro re nata posology than either the RESTORE studyCitation2 or a previous DRCR.net trialCitation4, both of which showed greater efficacy of ranibizumab 0.5 mg (either as monotherapy or combined with laser therapy) than with laser therapy alone. Comparison with these other therapeutic options would be a useful direction for future research.
Conclusions
The analysis confirmed that ranibizumab as monotherapy or in combination with laser photocoagulation for the treatment of DME with visual impairment is associated with increased time without legal blindness and increased quality-adjusted survival compared with laser monotherapy. The results demonstrate the continued cost-effectiveness of ranibizumab compared with laser monotherapy over 36 months. When a healthcare system perspective was taken, ranibizumab monotherapy and combination therapy were cost-effective compared with laser monotherapy and, when productivity losses were taken into account, they both dominated laser monotherapy. These results are robust and the ICERs are below commonly accepted cost-effectiveness thresholds in Canada.
Transparency
Declaration of funding
This study was funded by Novartis Pharma AG.
Declaration of financial and other relationships
JH is an employee of Optum, Burlington, ON, Canada. MB is an employee of Novartis Pharmaceuticals Canada Inc., Dorval, QC, Canada. AF is an employee of Novartis Pharma AG, Basel, Switzerland. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_file.docx
Download MS Word (46.3 KB)Acknowledgments
The authors would like to thank Megan Pickering, Novartis Pharmaceuticals Canada Inc., for her contribution to the study. Editorial support for the preparation and revision of drafts under the direction of the authors was provided by Sophie Shina, Oxford PharmaGenesis, and funded by Novartis Pharma AG.
References
- Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615–25
- Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121:1045–53
- Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–77
- Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609–14
- Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010;33:2399–405
- Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801
- Nguyen QD, Shah SM, Heier JS, et al. Primary end point (Six Months) results of the ranibizumab for edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2009;116:2175–81
- Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010;117:2146–51
- Ohji M, Ishibashi T, Sr., and REVEAL study group. Efficacy and safety of ranibizumab 0.5 mg as monotherapy or adjunctive to laser versus laser monotherapy in Asian patients with visual impairment due to diabetic macular edema: 12-month results of the REVEAL Study. Invest Ophthalmol Vis Sci 2012;53:ARVO E-abstract 4664
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 3rd edn, Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006. https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed January 4, 2016
- Regnier SA, Malcolm W, Haig J, et al. Cost-effectiveness of ranibizumab versus aflibercept in the treatment of visual impairment due to diabetic macular edema: a UK healthcare perspective. Clinicoecon Outcomes Res 2015;7:235–47
- Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol 2012;96:688–93
- Statistics Canada. Table 102-0504 - Deaths and mortality rates, by age group and sex, Canada, provinces and territories, annual, CANSIM (database). Canada: Statistics Canada; 2008–2012. http://cansim2.statcan.gc.ca/cgi-win/cnsmcgi.exe?Lang=E&CNSM-Fi=CII/CII_1-eng.htm. Accessed June 22 2013
- Public Health Agency of Canada. Diabetes data. http://www.phac-aspc.gc.ca/ccdpccpcmc/ndss-snsd/english/diabetes_data/index-eng.php. Accessed December 1, 2011. (Link no longer accessible - data on file)
- Hirai FE, Knudtson MD, Klein BE, et al. Clinically significant macular edema and survival in type 1 and type 2 diabetes. Am J Ophthalmol 2008;145:700–6
- Avis au Ministre de L’institut National D’excellence En Santé Et En Services Sociaux. OZURDEXMC – Œdème maculaire consécutif à l’occlusion de la veine centrale de la rétine. 2012 https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Fevrier_2012/_Avis_ministre_innovateurs201202.pdf. Accessed January 19, 2016
- Czoski-Murray C, Carlton J, Brazier J, et al. Valuing condition-specific health states using simulation contact lenses. Value Health 2009;12:793–9
- Balshaw RF, Gonder J, Ferreira A, et al. Evaluation of health utility in patients with retinal vein occlusion. Value Health 2012;15:A572
- Diabetic Retinopathy Clinical Research Network, Beck RW, Edwards AR, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009;127:245–51
- Cruess A, Zlateva G, Xu X, et al. Burden of illness of neovascular age-related macular degeneration in Canada. Can J Ophthalmol 2007;42:836–43
- Access Economics Pty Limited. Access Economics Pty for the CNIB and the Canadian Ophthalmological Society 'The cost of vision loss in Canada. 2009. http://www.cnib.ca/eng/cnib%20document%20library/research/covl_full_report.pdf'. Accessed January 19, 2015
- Brown MM, Brown GC, Stein JD, et al. Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol 2005;40:277–87
- Chou SL, Misajon R, Gallo J, et al. Measurement of indirect costs for people with vision impairment. Clin Experiment Ophthalmol 2003;31:336–40
- Cruess AF, Zlateva G, Xu X, et al. Economic burden of bilateral neovascular age-related macular degeneration: multi-country observational study. Pharmacoeconomics 2008;26:57–73
- Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol 2006;124:726–32
- Frick KD, Basilion EV, Hanson CL, et al. Estimating the burden and economic impact of trachomatous visual loss. Ophthalmic Epidemiol 2003;10:121–32
- Frick KD, Gower EW, Kempen JH, et al. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 2007;125:544–50
- Gupta OP, Brown GC, Brown MM. Age-related macular degeneration: the costs to society and the patient. Curr Opin Ophthalmol 2007;18:201–5
- Keeffe JE, Chou SL, Lamoureux EL. The cost of care for people with impaired vision in Australia. Arch Ophthalmol 2009;127:1377–81
- Klein R, Klein BE, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 1997;104:7–21
- Lafuma A, Brezin A, Fagnani F, et al. Nonmedical economic consequences attributable to visual impairment: a nation-wide approach in France. Eur J Health Econ 2006;7:158–64
- Lamoureux EL, Chou SL, Larizza MF, et al. The reliability of data collection periods of personal costs associated with vision impairment. Ophthalmic Epidemiol 2006;13:121–6
- Lotery A, Xu X, Zlatava G, et al. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol 2007;91:1303–7
- McClure ME, Hart PM, Jackson AJ, et al. Macular degeneration: do conventional measurements of impaired visual function equate with visual disability? Br J Ophthalmol 2000;84:244–50
- Roberts CB, Hiratsuka Y, Yamada M, et al. Economic cost of visual impairment in Japan. Arch Ophthalmol 2010;128:766–71
- Ruiz-Moreno JM, Coco RM, Garcia-Arumi J, et al. Burden of illness of bilateral neovascular age-related macular degeneration in Spain. Curr Med Res Opin 2008;24:2103–11
- Schmier JK, Halpern MT, Covert D, et al. Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina 2006;26:1056–62
- Smith AF. The economic impact of ophthalmic services for persons with diabetes in the Canadian Province of Nova Scotia: 1993-1996. Ophthalmic Epidemiol 2001;8:13–25
- Smith TS, Frick KD, Holden BA, et al. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ 2009;87:431–7
- Taylor HR, Pezzullo ML, Keeffe JE. The economic impact and cost of visual impairment in Australia. Br J Ophthalmol 2006;90:272–5
- Thygesen J, Aagren M, Arnavielle S, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin 2008;24:1763–70
- Wong EY, Chou SL, Lamoureux EL, et al. Personal costs of visual impairment by different eye diseases and severity of visual loss. Ophthalmic Epidemiol 2008;15:339–44
- Schmier JK, Halpern MT, Covert DW, et al. Impact of visual impairment on service and device use by individuals with age-related macular degeneration (AMD). Disabil Rehabil 2006;28:1331–7
- Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–30
- Moss SE, Klein R, Klein BE. The incidence of vision loss in a diabetic population. Ophthalmology 1988;95:1340–8
- Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology 1994;101:1061–70
- Dewan V, Lambert D, Edler J, et al. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2012;119:1679–84
- Stein JD, Newman-Casey PA, Kim DD, et al. Cost-effectiveness of various interventions for newly diagnosed diabetic macular edema. Ophthalmology 2013;120:1835–42
- Malkin AG, Goldstein JE, Perlmutter MS, et al. Responsiveness of the EQ-5D to the effects of low vision rehabilitation. Optom Vis Sci 2013;90:799–805
- Kay S, Ferreira A. Mapping the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) to EQ-5D utility scores. Ophthalmic Epidemiol 2014;21:66–78
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193–203
- Régie de l'assurance maladie du Québec. Liste de médicaments. 2013. http://www.ramq.gouv.qc.ca/en/regie/legal-publications/Pages/list-medications.aspx. Accessed January 19, 2015
- Régie de l'assurance maladie du Québec. Manuel des médecins spécialistes, MAJ 84. http://collections.banq.qc.ca/ark:/52327/bs2272062. Accessed January 19, 2015
- Brown MM, Brown GC, Sharma S, et al. Utility values and diabetic retinopathy. Am J Ophthalmol 1999;128:324–30
- Sharma S, Brown GC, Brown MM, et al. Converting visual acuity to utilities. Can J Ophthalmol 2000;35:267–72
- Lachaine J, Beauchemin C, Mathurin K. Productivity losses related to vision impairment. A literature review and estimation of costs to include in the Lucentis® pharmacoeconomic model. 2011