Abstract
Objectives Currently, patients with persistent moderate-to-severe house dust mite (HDM) allergic rhinitis despite use of symptom-relieving medication can be offered subcutaneously administered allergy immunotherapy (SQ SCIT; Alutard SQ) as standard care of treatment in Denmark. Recently, a HDM sublingually administered allergy immunotherapy tablet (SQ SLIT-tablet; ACARIZAX) has been developed for at-home treatment. The purpose of this analysis is to compare the costs related to treatment and administration of SQ SLIT-tablet and SQ SCIT.
Methods Assuming equal efficacy between ther SQ SLIT-tablet and SQ SCIT, the cost-minimization analysis was the most appropriate for the comparison. According to guidelines and Summary of Product Characteristics, the treatment duration of SQ SLIT-tablet is 3 years and 3–5 years for SQ SCIT. The courses of treatment vary among patients and, therefore, the costs of treatment have been calculated for an average patient with HDM respiratory allergic disease (RAD) receiving either SQ SLIT-tablet or SQ SCIT. All costs associated with allergy immunotherapy were collected, i.e., cost of medication, administration and treatment setting, and discounted according to Danish guidelines. Comprehensive univariate sensitivity analyses were carried out.
Results The treatment costs for an average patient with HDM RAD are €3094 for SQ SLIT-tablet and €3799 for SQ SCIT; however, when adding indirect costs to the calculations the total costs of the treatments are €3697 and €6717 for SQ SLIT-tablet and SQ SCIT, respectively. Therefore, if 2500 patients with HDM RAD were treated with SQ SLIT-tablet instead of SQ SCIT, it would elicit a saving to the healthcare system of ∼€1.8 million. The conclusion was robust to any changes in the sensitivity analysis.
Conclusion With regards to the cost of treating Danish patients with HDM RAD, it is clearly cost-saving to treat patients with SQ SLIT-tablet compared to SQ SCIT.
Introduction
Worldwide there is an increasing prevalence of respiratory allergic disease (RAD) including allergic rhinitis (AR) and allergic asthma (AA), with AR being the most prevalent disease affecting an average of ∼25% of the European population. Allergy towards house dust mites (HDMs) may result in symptoms affecting the entire respiratory system, thereby causing a RAD. Approximately 50% of all patients with HDM RAD have both AR and AACitation1.
The increasing prevalence in RAD is associated with an increasing burden to society, affecting the quality-of-life among patients, but also constitutes a substantial cost to the individual patient, the healthcare system, and societyCitation2,Citation3.
For patients with persistent moderate-to-severe HDM AR despite use of symptom-relieving medication, allergy immunotherapy (AIT) is the only treatment that treats the underlying disease instead of only providing short-term relief, which is associated with pharmacotherapy. Standard of care of AIT is currently subcutaneous injections (subcutaneously administered immunotherapy; SQ SCIT; Alutard SQ) with controlled doses of standardized natural allergens. During the treatment the immune system is gradually desensitized resulting in the patient becoming tolerant to the allergen previously triggering the allergic disease. Due to the treatment course of SQ SCIT the treatment is associated with inconvenience to the patient, as SQ SCIT requires many visits to the clinician and thereby lost productivity at work.
Previously, a sublingually administered immunotherapy (SLIT)-tablet has been introduced for the treatment of grass pollen AR. Recently, a similar SLIT-tablet, the SQ SLIT-tablet (ACARIZAX), has been developed for the treatment of HDM AA and AR. With a clinical development program including eight completed trials with more than 5000 patients the efficacy and a favorable safety profile of SQ SLIT-tablet have been demonstratedCitation4–11. On the contrary to the treatment course of SQ SCIT, the treatment course of SQ SLIT-tablet ensures convenience for the patient and a minor burden on the healthcare system and society as the tablet can be self-administered at home, except for the very first tablet.
Currently no head-to-head comparison of HDM SLIT-tablets and HDM SCIT products are available and, therefore, there is no direct way of comparing the efficacy of the SQ SLIT-tablet and SCIT for HDM RAD. When head-to-head trials are not available, indirect comparisons can be used based on meta-analysis. However, due to inter-study heterogeneity in HDM SCIT studies, a meta-analysis comparing the efficacy of SQ SLIT-tablets and HDM SCIT products will contain evidence which can be considered weakCitation12,Citation13. A feasible approach would be to compare HDM AIT studies with a similar study design, selection criteria and scoring of patient reported outcomes. Comparisons of outcomes for trials with similar design features may be made, as seen in a study by Mosbech et al.Citation1, where a HDM SLIT-tablet trial is compared to two HDM SCIT trials with similar selection criteriaCitation14,Citation15. Based on similarities in the trial designs, patient population and selection criteria in general, Mosbech et al.Citation1 state a comparable effect and no significant difference between the HDM SLIT-tablet and SCIT trials. Based on this approach there would be no reason to believe that the efficacy of SQ SLIT-tablets is different from the efficacy of SQ SCIT.
A cost-minimization analysis was the chosen method for this analysis, based on the assumption of similar efficacy between HDM SLIT-tablet and HDM SCIT, with the purpose of comparison of the economic consequences of treating an average patient with HDM RAD using either SQ SLIT-tablet or SQ SLIT.
Methods
Cost-minimization analysis
A cost-minimization analysis is relevant when the two healthcare technologies in comparison are equivalent in terms of efficacyCitation16. The analysis was performed according to health economic guidelinesCitation16,Citation17 as a model-based analysis developed in Microsoft Excel 2013 comparing costs related to the healthcare sector and society, as well as costs of administration for the patients including productivity lossesCitation16. Therefore, the treatment costs and costs related to administration of SQ SLIT-tablet (Dermatophagoides pteronyssinus (D. pteronyssinus) and Dermatophagoides farinae (D. farinae), 12 SQ-HDM, ALK, Denmark) were compared with the treatment costs and costs of administration of SQ SCIT (Alutard SQ, D. pteronyssinus, 100 000 SQ-U, ALK, Denmark).
Course of treatment and resource use
The SQ SLIT-tablet consists of components from the two predominant species of HDMs; D. pteronyssinus and D. farinae. To obtain the same treatment with SQ SCIT this would require two types of SQ SCIT treatments, as SQ SCIT is specific AIT treating one allergen per treatment. In Denmark, however, patients are very rarely treated for more than one HDM speciesCitation18, and the focus was, therefore, assumed to be on the costs of treatments for patients suffering for one HDM allergy onlyCitation18.
The courses of treatment differ between SQ SLIT-tablet and SQ SCIT. During the 3-year treatment with SQ SLIT-tablet, there are two initial consultations and a follow-up consultation the first year of treatment followed by two follow-up consultations every year during the remaining years of treatmentCitation18. As the patient must take the SQ SLIT-tablet every day, the number of tablets consumed through the course of a SQ SLIT-tablet treatment was calculated as the daily dose of one tablet times the average number of days per year and the compliance rate.
The injections associated with SQ SCIT are given at consultations. According to guidelines and Summary of Product Characteristics (SmPC), the 3–5-year treatment with SQ SCIT consists of an up-dosing period and a maintenance period. The up-dosing period involves a series of 15 up-dosing injections with 1-week intervals on average followed by two transition injections with 3-week intervals on average. For the maintenance period, injections are given with 8-week intervals on average. Approximately 80% of the patients are treated for 3 years, and ∼20% of the patients are treated for 5 yearsCitation18. A titration kit contains the vials necessary for up-dosing. The number of maintenance vials was calculated based on the number of maintenance visits, which were determined by the remaining number of weeks per year after the up-dosing phase and the average interval between maintenance visits.
presents an overview of the general assumptions used in the cost estimations, including assumptions on treatment duration and level of compliance.
Table 1. General model assumptions.
Consultations in relation to both SQ SLIT-tablets and SQ SCIT are carried out by general practitioners, specialists in internal medicine, allergology and otolaryngology (allergological services) in both private clinics and in hospitals on an outpatient basisCitation18. The average resource use associated with SQ SLIT-tablet and SQ SCIT treatments appear in and the applied unit cost estimates appear in .
Table 2. Average resource use for treatment of a patient with HDM RAD.
Table 3. Unit costs (€).
Data about resource use in terms of medication use, physician visits, and patient time were based on information from clinical trials and treatment guidelines in Denmark (according to the SmPC); where information was limited, medical expert opinion was acquiredCitation18. The clinical experts represented all relevant medical specialties, ensuring a representative and robust description of Danish clinical practice.
Unit costs
Market prices were applied as unit cost estimates for medicationCitation19. The fees for consultations and treatments at general practitioners and specialists in private clinics were obtained from the Danish Medical AssociationCitation20. The applied fees and charges included consumables. Charges for treatments in the hospital (outpatient charges) were obtained from the Danish Health Data Agency (Sundhedsdatastyrelsen)Citation21. Average gross wages from Statistic Denmark were applied as cost estimates for hours of lost workCitation22. Travel cost per kilometer was based on national rates for travel reimbursement in private vehiclesCitation23.
Costs were obtained in Danish kroner (DKK) but converted into Euro (€) using the average exchange rate from 2015 (€1 = 7.46 DKK). Costs were reported in 2015 prices and future costs beyond the first year were discounted to present value (PV) with 3% according to Danish guidelinesCitation24,Citation25.
Sensitivity analyses
In order to test the robustness of the results, comprehensive univariate sensitivity analyses—changing different parameters one-by-one—were performed.
Results
Costs of treatment with SQ SLIT-tablet and SQ SCIT
presents the results of the budget impact and cost-minimization analyses. For an average patient receiving AIT, the direct cost (present value) of treating HDM RAD with SQ SLIT-tablet is €3094, while the direct cost of a treatment with SQ SCIT is €3799. The total treatment cost for an average patient with HDM RAD is €3697 for treatment with SQ SLIT-tablet and €6717 for SQ SCIT. The difference is driven by both treatment costs and indirect costs. The cost of medication is lower for SQ SCIT; however, the larger amount of consultations more than outweigh this difference.
Table 4. Cost of treatments for HDM RAD with SQ SLIT-tablet and SQ SCIT (€).
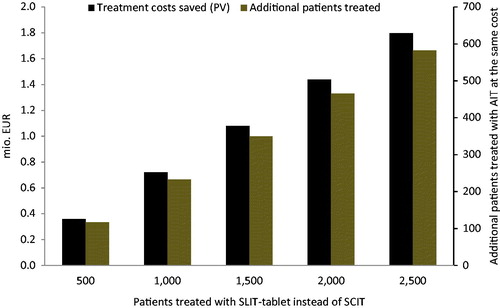
shows how the potential for saved direct treatment costs (black bars) depends on the number of patients treated with SQ SCIT. The figure also shows how the potential for treating more patients at the same cost (brown bars) depends on the number of patients treated with SQ SCIT. If, for example, 2500 patients are treated with SQ SLIT-tablet instead of SQ SCIT, the potential for reduced direct treatment costs amounts to ∼€1.8 million. The reduction corresponds to the cost of treating ∼600 additional patients with the SQ SLIT-tablet, i.e., 24% more patients.
Sensitivity analyses
The results of a series of univariate sensitivity analyses appear from . Only direct costs were included in the sensitivity analyses. Input parameters affecting the cost of treatment for both SQ SLIT-tablet and SQ SCIT were changed.
Table 5. Univariate sensitivity analysis for HDM RAD (direct treatment costs in €).
With a few exceptions, the sensitivity analyses showed that the cost of treatment for HDM RAD with SQ SLIT-tablet was lower than the cost of treatment with SQ SCIT, regardless of which parameter was changed. Due to the large number of physician visits associated with the treatment with SQ SCIT, parameters affecting the treatment setting had a relatively large impact on the SQ SCIT treatment costs.
Discussion
In this cost-minimization analysis, the economic impact of treatments with SQ SLIT-tablet vs SQ SCIT was analysed. Equal efficacy of SQ SLIT-tablet and SQ SCIT was assumed.
When including only direct costs of the treatments, the treatment costs for an average patient with HDM RAD was estimated to a potential savings of €705 for the SQ SLIT-tablet compared to SQ SCIT. The robustness of these results was confirmed by the sensitivity analyses, showing that the SQ SLIT-tablets, with a few exceptions, were cost saving. However, some of the performed sensitivity analyses must be characterized as analysis of extremes—e.g., a 100% follow-up in a hospital setting for SQ SLIT-tablet treatment is not realistic.
The difference in treatment costs between SQ SLIT-tablet and SQ SCIT implies that there is a potential for reducing the costs of treatment for HDM RAD if patients are treated with the SQ SLIT-tablet instead. Alternatively, there is a large potential for increasing the number of treated patients at unchanged costs.
As stated, allergy is associated with substantial cost to society, implying that information about the costs of a SQ SLIT-tablet vs SQ SCIT is highly relevant. The findings of this analysis is supported by a recently published reviewCitation26 in which it is reported that therapy with SLIT-tablets is associated with lower costs compared to SCIT in four out of six studies. In addition, it is also reported that SLIT-tablets and SCIT are cost saving compared to pharmacotherapy, which only provides short-term symptomatic relief.
The main driver of the cost difference between treatments with SQ SCIT and SQ SLIT-tablet in this analysis was the difference in the number of physician visits throughout the respective treatment periods. In addition, it should be noted that the treatment with SQ SLIT-tablet is a more convenient form of treatment for patients, as the number of physician visits will be reduced markedly. However, home administration may influence the compliance rate. In the analysis the compliance rate for SQ SLIT-tablet was set to 80%, which was lower than the compliance rate for SQ SCIT. Nevertheless, it should be noted that a Swedish study found compliance rates for SLIT-tablets to be high and on the same level as compliance rates for SCIT27.
This analysis had some limitations. First, the perspective of the analysis was relatively restricted as only the treatment costs, costs of administration and patients’ costs related to the administration were included. That is, this analysis does not provide a full picture of the costs of HDM RAD.
Second, costs associated with treatment of adverse events related to SQ SCIT and SQ SLIT-tablet, respectively, were not included, assuming costs related to adverse events of both treatment modalities were equal. Some studiesCitation28 indicate that the number of adverse events may be lower in SLIT-tablet therapy compared to SCIT, implying that exclusion of the costs of adverse events lead to a more conservative estimate of the saved costs associated with SQ SLIT-tablet therapy.
Third, fees and charges were applied as unit cost estimates. These may not accurately reflect the opportunity cost, which should ideally be used in such analyses. However, the fees and charges used were considered the best available proxies for the opportunity costs.
Finally, data/assumptions on treatment setting (patient distribution) and compliance were based on advice from experts, implying some degree of uncertainty for these parameters.
Conclusion
In conclusion, this cost-minimization analysis showed that treatment with SQ SLIT-tablet is a cost-saving alternative to SQ SCIT when treating patients with persistent moderate-to-severe HDM AR, despite use of symptom-relieving medication. From a healthcare perspective, the SLIT-tablet represents an efficient use of cost and resources. Further analyses may be necessary to evaluate the overall health economic consequences of implementation of the SQ SLIT-tablet instead of SQ SCIT.
Transparency
Declaration of funding
This study was supported by ALK.
Declaration of financial/other relationships
SR, CRJ, ST, and AW have received advisor/consultants fees from ALK. Incentive has received consultancy fees from ALK. JO is an employee of Incentive. JHP and JNA are employees of ALK.
Notice of correction
Please note that tables 4 and 5 have been corrected since the article was first published online (15 March 2016).
References
- Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2014;134:568-75.e7
- Poole CD, Bannister CA, Andreasen JN, Andersen JS, Currie CJ. Estimation of health-related utility (EQ-5D index) in subjects with seasonal allergic rhinoconjunctivitis to evaluate health gain associated with sublingual grass allergen immunotherapy. Health Qual Life Outcomes 2014;12:99
- Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO. Allergic rhinitis and the common cold–high cost to society. Allergy 2010;65:776-83
- U.S. National Institutes of Health. ClinicalTrials.gov. http://clinicaltrials.gov. Search: house AND dust AND mite AND allergy. Accessed September 12, 2014
- Emminger W, Smeenk F, Guzik T, et al. Abstract 417 The SQ HDM SLIT-tablet is well-tolerated in respiratory allergic disease; results from 2 DBPC phase III trials (MERIT and MITRA). Allergy 2014;69(99 Suppl):111-99
- Hernández D, Mosbech H, Dichmann R, et al. Abstract 421 SQ HDM SLIT-tablet induces significant immunomodulatory response; results from a DBPC phase III trial (MITRA). Allergy 2014;69(99 Suppl):111-99
- Horak F, Maloney J, Nelson S, et al. Abstract 1778 Onset of action of house dust mite sublingual immunotherapy tablet (SLIT-T) using an environmental exposure chamber. Allergy 2014;69(99 Suppl):573-619
- Demoly P, Decot E, Lozovskis V, et al. Abstract 415 Effective treatment of house dust mite (HDM) allergic rhinitis with the SQ HDM SLIT-tablet; results from a DBPC phase III trial (MERIT). Allergy 2014;69(99 Suppl):111-99
- Virchow J, Backer V, Kuna P, et al. Abstract 414 SQ HDM SLIT-tablet is effective in the treatment of allergic asthma; results from a DBPC phase III trial (MITRA). Allergy 2014;69(99 Suppl):111-99
- Maloney J, Prenner BM, Bernstein DI, et al. Safety of house dust mite sublingual immunotherapy standardized quality tablet in children allergic to house dust mites. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol 2016;116:59-65
- JAPIC Clinical Trials Information. Torii TO-203. JapicCTI number 121848. http://www.clinicaltrials.jp. Accessed December 2015
- Nelson HS. Subcutaneous immunotherapy versus sublingual immunotherapy: which is more effective? J Allergy Clin Immunol Pract 2014;2:144-9
- Calderon MA, Casale TB, Nelson HS, et al. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol 2013;132:1322-36
- Blumberga G, Groes L, Haugaard L, et al. Steroid-sparing effect of subcutaneous SQ-standardised specific immunotherapy in moderate and severe house dust mite allergic asthmatics. Allergy 2006;61:843-8
- Zielen S, Kardos P, Madonini E. Steroid-sparing effects with allergen-specific immunotherapy in children with asthma: a randomized controlled trial. J Allergy Clin Immunol 2010;126:942-9
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Oxford University Press, 2005. Oxford, UK
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health J Int Soc Pharmacoeconomics Outcomes Res 2014;17:5-14
- Focus group meeting conducted by ALK with participation of physicians with knowledge about treatments with SCIT and SLIT-tablets. 2014
- Lægemiddelstyrelsen. www.medicinpriser.dk. Accessed December, 2015.
- Lægeforeningen. www.laeger.dk. Accessed December, 2015
- Sundhedsdatastyrelsen. www.drg.dk. Accessed December, 2015
- Danmarks Statistik. www.statistikbanken.dk. Accessed December, 2015.
- Skat. www.skat.dk. Accessed December, 2015
- DSI Institut for Sundhedsvæsen. Standardiseret rapporteringsstruktur for sundhedsøkonomiske analyser i ansøgninger om generelt tilskud til lægemidler. Lægemiddelstyrelsen. Copenhagen 2004.
- Lægemiddelstyrelsen, for Sundhedsvæsen DI. Erfaringer med sundhedsøkonomiske analyser i ansøgninger om generelt tilskud til lægemidler. 2011
- Hankin CS, Cox L. Allergy immunotherapy: what is the evidence for cost saving? Curr Opin Allergy Clin Immunol 2014;14:363-70
- Andreasen J, Lawton S, Bæch S, Svärd M. Compliance and persistence to Grass Immunotherapy Treatment is comparable for allergy immunotherapy tablets and subcutaneous immunotherapy: a Swedish Registry Study. World Allergy Organ J 2012;5(2 Suppl):S86
- Bahceciler NN, Babayigit Hocaoglu A, Galip N. A milestone in house dust-mite-allergen immunotherapy: the new sublingual tablet S-524101 (actair). Expert Rev Vaccines 2014;13:1427-38