Abstract
Background: Multiple sclerosis (MS) causes significant disability and diminished quality-of-life. Delayed-release dimethyl fumarate (DMF; also known as gastro-resistant DMF) is a new oral treatment for relapsing-remitting MS (RRMS) approved in the US, Australia, Canada, and Europe. Objectives: A cost-effectiveness model was developed to compare the health economic impact of DMF against other disease-modifying therapies (DMTs) as first-line RRMS treatment from a Canadian Ministry of Health perspective. Methods: A Markov cohort model was developed to simulate patients’ progression through health states based on the Kurtzke Expanded Disability Status Scale (EDSS) over a life-time horizon. Patients entered the model based on a distribution of baseline EDSS scores, from which they could progress to higher or regress to lower EDSS state, or remain in the same state. Relapses could occur at any EDSS score. Results from a mixed-treatment comparison were used to inform model inputs for disease progression and relapse rates per treatment. Costs included direct medical costs stratified by EDSS score. Utilities were accrued based on time spent in each EDSS state. Results: Compared with glatiramer acetate, DMF yielded 0.528 incremental quality-adjusted life-years (QALYs) at an incremental cost of $23 338 Canadian dollars (CAD), resulting in an incremental cost-effectiveness ratio (ICER) of CAD $44 118/QALY. The ICER for DMF compared with Rebif 44 mcg was CAD $10 672. Results were consistent across a wide range of one-way and probabilistic sensitivity analyses. Conclusions: Based on traditional cost-effectiveness thresholds in Canada (CAD $50 000–60 000), DMF can be considered a cost-effective option compared to other first-line DMTs.
Background
Multiple sclerosis (MS), the most common inflammatory disorder of the central nervous system (CNS), is a progressive disease characterized by immune-mediated demyelination of nerve fibres and disability associated with accumulation of CNS lesionsCitation1. The degree of disability experienced by patients with MS is variable—some experience little disability throughout their lifetimes, whereas upwards of 60% experience ambulatory problems within 20 years of disease onset. The disabling effects of MS can have major negative effects on patients’ quality-of-life and increase financial costs for the healthcare systemCitation2. The prevalence of MS in Canada (132.5 cases per 100 000 individuals) is among the highest in the world, ranking fifth behind Hungary, Slovenia, Germany and the USCitation3. In Canada, the 20–25-year average age of MS onset is considerably younger than that of the 29.2-year worldwide average, making the disease a particular burden on young Canadian adultsCitation3.
Individuals with relapsing-remitting multiple sclerosis (RRMS) experience periods of acute relapse alternating with complete or partial remissionCitation4. Patients with the RRMS sub-type often progress to secondary progressive multiple sclerosis (SPMS) over time; in a British Columbia, Canada study on treated population, over half of RRMS patients converted to SPMS within a median span of 19 yearsCitation5. With SPMS, the initial relapses characteristic of RRMS are replaced by irreversible CNS damage and progressive disability, even during remission cyclesCitation4,Citation6. Accumulating evidence suggests that early treatment of RRMS with therapies that control disease activity may postpone progression of MS and lead to better long-term patient outcomesCitation7,Citation8.
The progression of disability associated with MS can be followed using the Kurtzke Expanded Disability Status Scale (EDSS), which employs results of neurological examinations to assign disability status to one of 20 categories based on scores ranging from 0 (normal neurological exam) to 10 (death)Citation9,Citation10.
Although MS is currently incurable, treatment options include disease-modifying therapies (DMTs) that can lower the frequency/duration of relapses, reduce the number of new lesions detected by magnetic resonance imaging (MRI) and provide symptomatic reliefCitation11–15; however, due to safety and efficacy limitations, the need continues for novel MS treatments with improved benefit-risk profiles.
Delayed-release dimethyl fumarate (DMF; also known as gastro-resistant DMF), a twice-daily oral treatment, was approved in Canada in April 2013 as TECFIDERA for first-line treatment of adults with RRMS. DMF has been studied in patients with RRMS in DEFINE and CONFIRM, two phase 3 clinical studies (ClinicalTrials.gov identifiers NCT00420212 and NCT00451451, respectively). The primary objectives of both DEFINE (proportion of patients with relapse at two years)Citation16 and CONFIRM (reduced annualized relapse rate at 2 years)Citation17 were met, with DMF 240 mg BID, the approved dosage, demonstrating favorable benefit-risk profiles in both studiesCitation16,Citation17.
Results of a study using a budget impact model demonstrated that introduction of DMF for treatment of RRMS in Canada increased drug acquisition costs by C$602/patient/year that were partially offset by reduced costs associated with relapses of C$192/patient/year. This analysis did not consider the positive impact of DMF on quality-of-life of both patients and caretakers that may be associated with the improved relapse rates seen with this treatmentCitation18.
The current study compares the health economics aspects of DMF with other DMTs as first-line treatment for RRMS using a cost-effectiveness model developed from the perspective of the Canadian Ministry of Health.
Methods
Model design
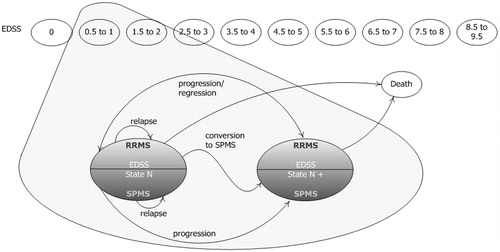
A cohort-based Markov model was developed to simulate patients’ progression through a series of health states, based on the Kurtzke EDSS over a life-time horizon (model base case uses a time horizon of 20 years)Citation9. The clinical course of MS was modeled by using 21 health states, including EDSS 0–9 for RRMS patients, EDSS 0–9 for SPMS patients, and death. Patients entered the model based on a distribution of baseline EDSS scores, as defined by DMF clinical trial data (DEFINE and CONFIRM)Citation16,Citation17. From this state, patients could transition to higher (progress) or lower (regress) EDSS states, remain in the same state, or have a disease relapse while in an RRMS state (). Patients could also progress to SPMS, which is characterized by fewer relapses and faster disease progression than RRMSCitation4,Citation6. Patients who progressed to SPMS entered the next stage of disease severity, as defined by EDSS. Once they became SPMS patients, they could either progress to a higher EDSS state or remain in their current state. Results from a mixed-treatment comparison were used to inform model inputs for disease progression and relapse rates per treatment; the probabilities of progression, regression or relapse were dependent on EDSS score and RRMS or SPMS status. Transitions between the health states occurred in 1-year cycles.
Figure 1. Model schematicCitation15.
Treatment options within the model (intervention and comparators) included glatiramer acetate and Rebif 44 mcg, which were selected as they are more commonly prescribed and are most similar to Tecfidera in anticipated use. These treatments acted in the model to delay the progression of disease and reduce relapse frequency in patients with RRMS. Patients could discontinue treatment due to conversion to SPMS, an EDSS score of 7 or higher or other reasons. These discontinuation rules are in line with clinical guidelines in CanadaCitation19. After discontinuation, patients received standard of care treatment until death or the end of the model time horizon. The mortality rate was dependent upon age, gender and EDSS score. Costs included direct medical costs stratified by EDSS score, along with relapse, adverse events (AEs) and treatment-related costs. Treatment-related costs were dependent on the DMT received and time spent on treatment. Utilities were accrued based on time spent in each EDSS state, adjusted for disutilities associated with relapses, AEs and caregiver burden. A 5% discount rate was applied.
Baseline population
Data for baseline population characteristics were derived from pooled analysis of the intent to treat population of relevant DMF clinical trials (CONFIRM and DEFINE)Citation16,Citation17. The average starting age of the patient cohort is 37.8 years old. There are 2.5-times more females than males in the baseline cohort. Due to the exclusion criteria of the trials, all patients have EDSS scores below 6 (). Unless otherwise noted, the dosing for all DMF treatment in the model is 240 mg twice daily.
Table 1. Baseline patient EDSS distribution.
Disease
Natural history: Disease progression and regression
To fully evaluate disease natural history, three independent transition matrices are used in the model:
Transition matrix of movement between EDSS states within RRMS (EDSS 0–9);
Transition matrix of RRMS to SPMS (EDSS 1–9); and
Transition matrix of movement between EDSS states within SPMS (EDSS 1–9).
Disease natural history (no DMT) was modeled via transition matrices between EDSS states. Transition probabilities for EDSS states up to (and including) 7 were derived from the DMF clinical trials. Transition probabilities derived from the London Ontario dataset were used for EDSS states 8–9 because of the limited number of observations beyond EDSS 8 in the DMF clinical trials. For patients with RRMS, EDSS scores can increase, decrease or stay the same. Due to the nature of SPMS, a patient’s EDSS score can only increase or stay the same.
The London Ontario database contains data from the London Multiple Sclerosis Clinic (London Health Sciences Centre, Ontario, Canada), established in 1972, which provides long-term care for patients with multiple sclerosis from its referral area of southwest Ontario. The clinic and database characteristics have been extensively outlinedCitation20–23. Patients were evaluated annually or semi-annually, regardless of clinical course. Disability was assessed using the Disability Status ScaleCitation24. Data collection was performed through separate research charts containing data forms completed at patient visits, with the observation period ending in 2000. Transition matrices estimated from this dataset have been used in previous submissions to the National Institute for Health and Care Excellence (NICE)Citation25,Citation26 ().
Table 2. Probability of transitioning between RRMS states.
The probability of converting from RRMS to SPMS in each cycle was dependent on current EDSS (). These probabilities, as well as EDSS transition matrix for patients with SPMS, were based upon data from the London Ontario dataset and were used in the Natalizumab NICE submissionCitation26 ().
Table 3. Probability of conversation to SPMS.
Table 4. Probability of transitioning between SPMS states.
Natural history: Annualized relapse rates
Relapse was defined as ‘new or recurrent neurologic symptoms not associated with fever or infection, lasting at least 24 hours, and accompanied by new objective neurological findings upon examination by the examining neurologist’Citation17. Annualized natural history relapse rates per person per year were sourced from pooled baseline data from the DMF trials, which documented the annual relapse rate in the 12 months before enrolment in the studies.
The resulting annualized relapse rates (ARRs) used in the model are shown in . Because patients with high EDSS scores are more likely to have SPMS rather than RRMSCitation27, the ARR for EDSS ≥6 is slightly lower than for EDSS <6. These data will be used in the model as the relapse rates that patients would experience if they were untreated with any DMT. However, in actual clinical trials, patients may have been subject to DMTs already before the trials started. Consequently, the placebo arm is not entirely naive to DMT. We acknowledge this as a data limitation to our study.
Table 5. Annualized relapse rate (ARR) by EDSS.
Mortality
Mortality rates are used to estimate the number of patients who die over the time horizon considered in the model. Mortality rates in patients with MS have been shown to be significantly higher than the general population, including suicide as a significant cause of deathCitation28–31. The model adjusted for the elevated mortality due to MS by applying an EDSS-dependent relative risk on the probability of death for general population. The relative risk data were extrapolated from published literatureCitation29, shown in .
Table 6. Relative risk.
Economic burden of the disease
The model takes into account two different types of natural history costs: costs associated with disability progression through EDSS states and an average cost associated with relapse.
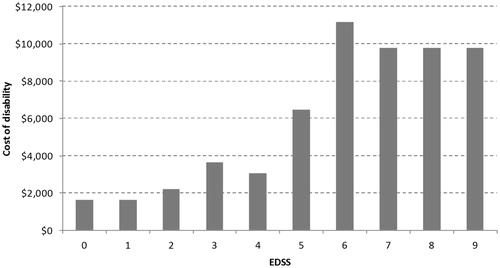
Cost-associated disability is dependent on EDSS states and was sourced from published papers (). Cost values for EDSS 0–6 were from Grima et al.Citation32 while those for EDSS 7–9 were sourced from Karampampa et al.Citation33. The cost mainly includes outpatient expenses like emergency room visits, physicians assessments, optometrist, laboratory tests and diagnostic imaging. The cost of home-based nursing care was also covered. It was assumed that cost of disability is associated only with EDSS and not with gender or type of MS (RRMS or SPMS).
The excess cost attributable to relapses was calculated as the difference in the mean annual cost (excluding the cost of MS treatments) between RRMS patients with EDSS < 5 who experienced relapse(s) and those who did notCitation33. The average cost of relapses was estimated by dividing the excess cost by the number of relapses.
Treatment
Treatment efficacy
Treatment efficacy was expressed in terms of reduced relapse rate and slower disability progression. It was assumed in the base case that treatment has no impact on the probability of converting from RRMS to SPMS or disability regression. Efficacy data was derived from a systematic literature review and mixed treatment comparison (unpublished). It was calculated as a relative risk compared to placebo (for disability progression) or relative rate compared to placebo (for ARR) ().
Table 7. Treatment efficacy on disability progression and relapse.
Confirmed disability progression was sustained for 3 months at 24 months (relative risk compared to placebo);
Annualized relapse rate (relative rate compared to placebo).
In the model base case, treatment efficacy was assumed to be constant over time. Since NICE has recommended including treatment waning effect in the appraisal of various DMTs for MS, including fingolimodCitation25 and teriflunomideCitation34, the model can accommodate various scenarios of treatment waning effect, including 50% waning for Year 3+, 100% waning for Year 3+, and a linear decrease of treatment efficacy for 10 years. The waning of treatment efficacy influenced both disease progression and relapse. In accordance with current treatment guidelines in Canada, all DMTs will be stopped when EDSS has progressed over 7 or upon conversion to SPMSCitation35. Based on clinical trial data, annual treatment discontinuation due to other causes were set to be 10.9%, 9.2%, and 14.6% for DMF, glatiramer acetate, and rebif 44 mcg, respectively.
Treatment-related cost
Treatment-related costs include drug acquisition and administration cost, as well as costs related to AEs. Drug acquisition cost was estimated by Biogen using commercial confidential information. Administration cost covers nursing and specialist hours and lab tests accompanying the treatment. These data were obtained from the schedule of benefits of the Canadian health insurance system and adjusted for inflation where necessaryCitation36. Costing for AEs was conducted by considering both serious and non-serious events. The weight of the average cost of AEs was calculated by multiplying incidence rates with cost per event for both types.
Health-related Quality-of-Life (HRQoL)
HRQoL calculation involves the following three aspects:
Utility by EDSS states, MS status (RRMS or SPMS), and relapse status;
AE disutility; and
Disutility for carers.
Utility weights for EDSS states were derived from the DMF clinical trial data (CONFIRM and DEFINE), by pooling observations for each EDSS state (0–9) and calculating the mean EQ-5D index score for each state. The resulting base case values are shown in .
Table 8. Base case utility scores per EDSS state.
To calculate quality-of-life impact over one annual cycle per AE, the disutility per event was multiplied by the event duration (in years). The treatment-specific incidence of adverse events (serious and non-serious) is then used to calculate the disutility of each treatment per cycle. Clinical expert opinion was sought to validate all the values used in the model and to provide estimates where data was missing.
Disutility for caregivers of MS patients is accepted as a valid treatment benefitCitation37 and, thus, is included in the model base case. In the NICE submission for natalizumab, maximum disutility for a caregiver of a person with MS was assumed to be 0.14, which was based on a mean caregiver utility of 0.84 quoted in the NICE assessment of treatment for Alzheimer’s diseaseCitation26. The assumption was made due to insufficient data on the effect of MS on the QoL of caregivers.
Results
Base case outcome
Base case outcomes over 20 years are presented in . A discount rate of 5% was applied to both health and economic outcomes. Relapse cost is accounted for as part of total EDSS state cost. All currencies are reported in 2013 Canadian dollars.
Table 9. Base case results (discounted).
The higher treatment cost of DMF is partially offset by lower EDSS state costs. DMF is cost-effective at a threshold of CAD50 000 compared to both GA or Rebif 44 mcg, with an ICER of CAD44 118 and CAD10 672, respectively.
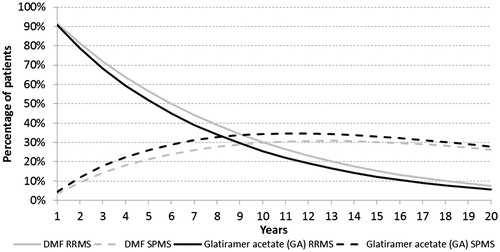
The percentage of patients in RRMS and SPMS states over time are shown in . The comparison between DMF and Rebif 44 mcg is similar. In general, patients are converting to SPMS or dying as time advances. The population undergoing DMF treatment has higher percentage of RRMS and lower proportion with SPMS, indicating its effectiveness in preventing patients from progressing to the more severe form of MS.
Sensitivity analyses
Both deterministic and probabilistic sensitivity analyses were conducted. For deterministic sensitivity analysis (DSA), wherever applicable the upper and lower bounds of inputs were determined with either 95% confidence interval or alternative data sources. When neither such data was available, inputs were varied by ±25% of their base case. For the treatment waning effect, we explored the scenario of 50% waning after 3 years compared with no waning effect in the base case. While the default utility values were derived from clinical trials of DMF, utilities used in natalizumab NICE HTA submission were studied as an alternative data sourceCitation26. Moreover, the efficacy of DMF on disease progression and relapse was adjusted according to 95% confidence intervals from the same unpublished mixed treated comparison.
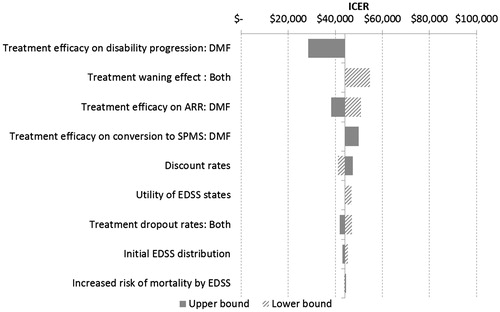
The results of DSA are presented in a tornado diagram in which variables are displayed in descending order based on their influence on model outcomes, as shown in . DSA for DMF compared with Rebif 44 mcg resulted in similar outcomes. The outcomes of all scenarios were ranked by their average deviation in ICER from the base case. In other words, scenarios at the top of the tornado diagram can influence ICER to a larger extent compared to scenarios at the bottom of the diagram.
DMF’s efficacy on disability progression is the most influential input. Changing it to upper 95% confidence interval (CI) led to an ICER of CAD28 474 per QALY. If lower 95% CI was used, the DMF arm’s cost is higher by CAD17 224 and its QALY is lower by 0.032 when compared to GA, resulting in an ICER of CAD538 000 (not shown in the tornado diagram). Other influential inputs include the following:
Waning effect of all treatments: 50% waning for Year 3+ vs no waning effect (default);
Treatment efficacy on ARR;
Treatment efficacy on conversion to SPMS: relative risk 0.75 vs no efficacy (default);
Discount rates; and
Utility of EDSS states.
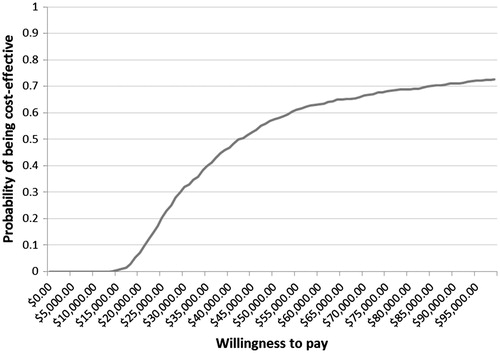
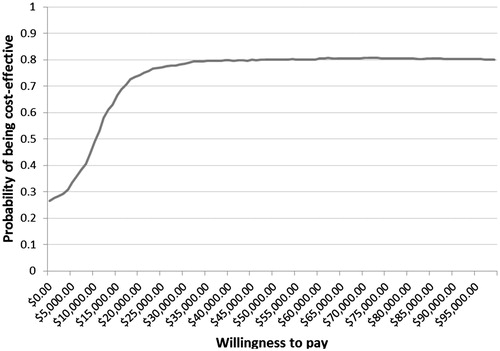
Probabilistic sensitivity analysis was also conducted to evaluate the robustness of model predictions under real world variations. The model randomly generated cost inputs following gamma distribution, utility, and discontinuation inputs following beta distribution, and relapse and efficacy inputs following log-normal distribution. At a willingness-to-pay threshold of CAD50000, DMF has a probability of 0.58 and 0.80 of being cost-effective compared to glatiramer acetate (GA) and Rebif 44 mcg, respectively, as shown in and .
Discussion
The objective of this paper is to evaluate the cost-effectiveness of DMF compared with other existing first line DMTs. Rebif 44 mcg is considered a high efficacy interferon and GA has been the standard of care in the US and Canada.
Many published studies have modeled the cost-effectiveness of existing DMTs such as GA and Rebif 44 mcg compared with best supportive care with different conclusionsCitation38–45. Many studies found these two agents had a favorable cost-effectiveness profile. For example, Darba et al.Citation42 reported GA and interferon beta-1a to have cost-effectiveness ratios of between €13,000–€22,000 over 10 years from a Spanish National Health Services perspective. On the other hand, Chilcott et al.Citation38 reported considerably different ICERs from a UK NHS perspective at £97,636 and £71,732 for glatiramer acetate and Rebif 44 mcg, respectively. Nonetheless, these two treatments have been widely adopted in the US and Canada and, thus, make appropriate comparators for DMF, which our study shows is cost-effective when compared with either GA or Rebif 44 mcg. RRMS patients will slowly transition into SPMS patients or die over time; DR-DMT slows that progression. Due to the indirect benefit of slower EDSS progression, the DMF arm had more patients alive and fewer patients with SPMS. This means DMF generates considerable health benefit to justify its higher acquisition cost compared with the best existing first-line DMTs from the perspective of Canadian payers.
DMTs have a direct impact on ARR and disability progression. In addition, because ARR is dependent on EDSS states, slower disability progression will indirectly influence ARR. The base case results show that EDSS state cost (including cost of relapse) is the biggest driver for the economic burden of MS, followed by the cost of DMTs (including administration cost).
Not surprisingly, inputs related to treatment efficacy and disability are most influential. Treatment efficacies on disability, relapse and conversion to SPMS have all been shown to have a large impact on model predictions. Since treatment waning effect and dropout rates can negatively impact treatment efficacy for the entire time horizon of the model, these two variables also have significant control over model outcomes. Among other influential input variables are initial EDSS distribution and utility values by EDSS states, both of which are relevant to the level of disability of the patient.
The robustness of model predictions was supported by probabilistic sensitivity analysis results. For DMF compared with GA, the probability of being cost-effective is 0.58 at CAD50 000 and 0.63 at CAD60 000. The probability of DMF being cost-effective compared with Rebif 44 mcg is 0.80 at both CAD50 000 and CAD60 000. This is likely due to the higher cost of Rebif 44 mcg relative to GA.
Limitations
There are still some limitations of this model. First, more alternative scenarios could be tested on treatment waning effect considering its significant impact on model outcomes. Currently the model base case assumes no waning effect. The model allows a few alternative assumptions like 50% waning for Year 3 and above, 100% waning for Year 3 and above, and linear decrease over 10 years. However, these scenarios are somewhat arbitrary, and more research on a real world treatment waning scenario can certainly benefit the robustness of the model.
Second, the EDSS transition matrix and AE incidence were derived from clinical trials with relatively short time horizon (∼2 years). Since the model studies a period of 20 years, more evidence is needed to support the pattern of disability progression over longer terms. In addition, some of the AEs may happen only in the short-term, and have been/will be managed through careful dose titration. Furthermore, some of the events listed may have led to treatment discontinuation. Thus, while these events were observed in the trial, it is likely that the future incidence of such events may diminish, as patients who are susceptible to those events discontinue therapy.
Third, due to data availability, not all inputs were based on Canadian sources. For instance, initial EDSS distribution and utility by EDSS were based on pooled patient data in the multinational CONFIRM and DEFINE trials. Because both trials have an inclusion criteria of EDSS less than or equal to 5.0Citation16,Citation17, average EDSS of the baseline population in the model is lower than the actual prevalent population in Canada. This is likely to cause an under-estimation of treatment benefits in the model.
Conclusion
Based on traditional cost-effectiveness thresholds in Canada (CAD50 000–60 000), DMF can be considered a cost-effective option compared to other first-line DMTs. This conclusion was supported by probabilistic sensitivity analysis results. Deterministic sensitivity analysis indicated that the initial EDSS distribution, treatment effect, treatment costs, utility values by EDSS, and discontinuation rates are the influential factors in determining the cost-effectiveness of DMTs.
Transparency
Declaration of funding
Funding for this study was provided by Biogen.
Declaration of financial/other relationships
WS and AK were employed at Evidera, a company that received funding from Biogen to assist with the conduct of this analysis and to provide medical writing support. CV received funding from Biogen for the analysis that supports the content of the manuscript. BD was an employee of Biogen for the majority of time in which this work was completed; however, he is currently employed at Evidera. SS is an employee of Biogen. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
DMF, delayed-release DMF (also known as gastro-resistant DMF).
Acknowledgements
The authors would like to thank Andrew Lee and Jim Lewin from Biogen for coordinating the editing and production of this manuscript. Their help has made the process of writing this paper much more efficient. We also like to thank Tanya Stezhka, Managing Editor of the Journal of Medical Economics for her generous accommodation of our requests during the publishing process.
References
- Bevan CJ, Cree BA. Fulminant demyelinating diseases of the central nervous system. Semin Neurol 2015;35:656-66
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 2010;9:A387-94
- World Health Organization. Atlas of multiple sclerosis resources in the world. Geneva, Switzerland: World Health Organization, 2008. http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf. Accessed September 16, 2013
- Hurwitz BJ. The diagnosis of multiple sclerosis and the clinical subtypes. Ann Indian Acad Neurol 2009;12:226-30
- Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult Scler 2008;14:314-24
- Zuvich RL, McCauley JL, Pericak-Vance MA, et al. Genetics and pathogenesis of multiple sclerosis. Semin Immunol 2009;21:328-33
- Goodin DS, Bates D. Treatment of early multiple sclerosis: the value of treatment initiation after a first clinical episode. Mult Scler 2009;15:1175-82
- Bar-Or A, Hutchinson M, Gold R, et al. Long-term efficacy of delayed-release dimethyl fumarate for relapsing-remitting multiple sclerosis according to prior therapy: Integrated analysis of the DEFINE, CONFIRM, and ENDORSE studies. Neurol 2015;84(14 Suppl):P7.234
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52
- Multiple Sclerosis Trust. A to Z of MS: Expanded Disability Status Scale (EDSS). 29 October 2013. Letchworth Garden City, UK: Multiple Sclerosis Trust, 2013. http://www.mstrust.org.uk/atoz/edss.jsp. Accessed November 2013
- Goldenberg MM. Multiple sclerosis review. Pharm Therapeut 2012;37:175-84
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655-61
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268-76
- PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352:1498-504
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107
- Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics 2008;26:617-27
- Multiple Sclerosis Society of Canada, Disease-modifying therapies, Toronto, Canada: Multiple Sclerosis Society of Canada, 2016. https://mssociety.ca/about-ms/treatments/disease-modifying-therapies-dmts. Accessed March 30, 2016
- Cottrell DA, Kremenchutzky M, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 1999;122:625-39
- Dorman E, Kansal AR, Sarda S, The budget impact of introducing delayed-release dimethyl fumarate for treatment of relapse-remitting multiple sclerosis in Canada, 2015;18:1085-91
- Kremenchutzky M, Rice GP, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 2006;129:584-94
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 1989;112:1419-28
- Kurtzke JF. Neurologic impairment in multiple sclerosis and the disability status scale. Acta Neurol Scand 1970;46:493-512
- NICE. Fingolimod for the treatment of highly active relapsing-remitting multiple sclerosis.NICE technology appraisal 254 (TA254). London: National Institute for Health and Care Excellence (NICE), 2012. http://www.nice.org.uk/guidance/TA254. Accessed October 11, 2013
- NICE. Natalizumab for the treatment of adults with highly active relapsing-remitting multiple sclerosis (manufacturer submission to NICE). NICE technology appraisal 127 (TA127). London: National Institute for Health and Care Excellence (NICE), 2007. http://www.nice.org.uk/TA127. Accessed October 11, 2013
- Sampat MP, Berger AM, Healy BC, et al. Regional white matter atrophy–based classification of multiple sclerosis in cross-sectional and longitudinal data. Am J Neuroradiol 2009;30:1731-9
- Bronnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 2004;127:844-50
- Pokorski RJ. Long-term survival experience of patients with multiple sclerosis. J Insur Med 1997;29:101-6
- Sadovnick AD, Ebers GC, Wilson RW, et al. Life expectancy in patients attending multiple sclerosis clinics. Neurology 1992;42:991-4
- Sumelahti ML, Tienari PJ, Wikstrom J, et al. Survival of multiple sclerosis in Finland between 1964 and 1993. Mult Scler 2002;8:350-5
- Grima DT, Torrance GW, Francis G, et al. Cost and health related quality of life consequences of multiple sclerosis. Mult Scler 2000;6:91-8
- Karampampa K, Gustavsson A, Miltenburger C, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. J Popul Ther Clin Pharmacol 2012;19:e11-25
- NICE. Teriflunomide for treating relapsing-remitting multiple sclerosis. NICE technology appraisal 303 (TA303). London: National Institute for Health and Care Excellence (NICE), 2014. http://www.nice.org.uk/guidance/ta303. Accessed March, 2014
- Freedman MS, Patry DG, Grand'Maison F, et al. Treatment optimization in multiple sclerosis. Can J Neurol Sci 2004;31:157-68
- Ontario Ministry of Health and Long-term Care. Ontario Health Insurance (OHIP) Schedule of Benefits and Fees. Ontario, Canada: Ontario Ministry of Health and Long-term Care, 2013. http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html. Accessed December 3, 2013
- Loveman E, Green C, Kirby J, et al. The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer's disease. Health Technol Assess 2006;10:iii–iv, ix–xi, 1-160
- Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ 2003;326:522; discussion 522
- Nuijten MJ, Hutton J. Cost-effectiveness analysis of interferon beta in multiple sclerosis: a Markov process analysis. Value Health 2002;5:44-54
- Prosser LA, Kuntz KM, Bar-Or A, et al. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 2004;7:554-68
- Rubio-Terres C, Aristegui Ruiz I, Medina Redondo F, et al. Cost-utility analisys of multiple sclerosis treatment with glatiramer acetate or interferon beta in Spain. Farm Hosp 2003;27:159-16
- Darba J, Kaskens L, Sanchez-de la Rosa R, Cost-effectiveness of glatiramer acetate and interferon beta-1a for relapsing-remitting multiple sclerosis, based on the CombiRx study, J Med Econ 2014;17:215-22
- Brown MG, Murray TJ, SKetris IS, et al. Cost-effectiveness of interferon beta-1b in slowing multiple sclerosis disability progression. First estimates. Int J Technol Assess Health Care 2000;16:751-67
- Parkin D, Jacoby A, McNamee P, et al. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life, J Neurol Neurosurg Psychiatry 2000;68:144-9
- Tappenden P, McCabe C, Chilcott J, et al. Cost-effectiveness of disease modifying therapies in the management of multiple sclerosis for the Medicare Population. Value Health 2009;12:657-65
- Rudick R, Kappos L, Polman C, et al. Long-term outcomes in natalizumab-treated patients who were free of disease activity over the 2-year affirm study. Amsterdam: 5th Joint Triennial Congress of The European and Americas Committees for Treatment and Research in Multiple Sclerosis (ACTRIMS /ECTRIMS), 2011; Abstract no.:P513
- Arnason B, Connor P, Knappertz K, et al. Depression incidence and course in multiple sclerosis patients treated with interferon beta-1b and glatiramer acetate during the BEYOND trial. J Neurol Sci 2009;285:S211
- Bornstein MB, Miller A, Slagle S, et al. A pilot trial of cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med 1987;317:408-14
- Fox R, Miller D, Phillips T, et al. Baseline characteristics of patients enrolled in a randomized, multicenter, placebo-controlled and active comparator trial evaluating efficacy and safety of BG-12 in relapsing-remitting multiple sclerosis: the CONFIRM trial. Ontario, Canada: American Academy of Neurology (AAN) 62nd Annual Meeting, 2010; Abstract no.:P06
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing- remitting multiple sclerosis: Results of a phase III multicenter, double- blind, placebo-controlled trial. Neurology 1995;45:1268-76
- Gold R, Kappos L, Bar-Or A, et al. Clinical efficacy of BG-12, an oral therapy, in relapsing-remitting multiple sclerosis: data from the phase 3 DEFINE trial. Amsterdam:5th Joint Triennial Congress of The European and Americas Committees for Treatment and Research in Multiple Sclerosis (ACTRIMS /ECTRIMS), 2011; Abstract no.:95
- Von Rosenstiel P, Hohlfeld R, Calabresi P, et al. Clinical outcomes in subgroups of patients treated with fingolimod (FTY720) or placebo: 24-month results from FREEDOMS. Mult Scler 2010;16: S143
- PRISM study group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998; 352: 1498-1504
- Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFN(beta)2-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72: 1976-83
- Calabrese M, Bernardi V, Atzori M, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Mult Scler 2011;18:418-24
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurol Scand 2006;113:283-7
- Johnson KP, Brooks BR, Ford CC, et al. Glatiramer acetate (Copaxone): Comparison of continuous versus delayed therapy in a six-year organized multiple sclerosis trial. Mult Scler 2003;9:585-91
- Panitch H, Goodin D, Francis G, et al. Benefits of high-dose, high-frequency interferon beta-1a in relapsing-remitting multiple sclerosis are sustained to 16 months: Final comparative results of the EVIDENCE trial. J Neurol Sci 2005;239:67-74
- Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993;43:662-7
- De Stefano N, Sormani MP, Stubinski B, et al. Efficacy and safety of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis: Further outcomes from the IMPROVE study. J Neurol Sci 2011; doi
- Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: Results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002;359:1453-60
- Knobler RL, Greenstein JI, Johnson KP, et al. Systemic recombinant human interferon-(beta) treatment of relapsing-remitting multiple sclerosis: Pilot study analysis and six-year follow-up. J Interferon Res 1993;13:333-40
- Simon JH, Lull J, Jacobs LD, et al. A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon (beta)-1a. Neurology 2000;55:185-92
- Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008;7:903-14
- Khatri B, Barkhof F, Comi G, et al. Oral fingolimod (FTY720) reduces the rate of relapses that require steroid intervention or hospitalization compared with intramuscular interferon-1a: results from a phase III study (TRANSFORMS) in multiple sclerosis. Ontario, Canada: American Academy of Neurology (AAN) 62nd Annual Meeting, 2010; Abstract no.:P06