Abstract
Aims: Up to 30% of insulin-treated type 2 diabetes patients are unable to achieve HbA1c targets despite optimization of insulin multiple daily injections (MDI). For these patients the use of continuous subcutaneous insulin infusion (CSII) represents a useful but under-utilized alternative. The aim of the present analysis was to examine the cost-effectiveness of initiating CSII in type 2 diabetes patients failing to achieve good glycemic control on MDI in the Netherlands. Methods: Long-term projections were made using the IMS CORE Diabetes Model. Clinical input data were sourced from the OpT2mise trial. The analysis was performed over a lifetime time horizon. The discount rates applied to future costs and clinical outcomes were 4% and 1.5% per annum, respectively. Results: CSII was associated with improved quality-adjusted life expectancy compared with MDI (9.38 quality-adjusted life years [QALYs] vs 8.95 QALYs, respectively). The breakdown of costs indicated that ∼50% of costs were attributable to diabetes-related complications. Higher acquisition costs of CSII vs MDI were partially offset by the reduction in complications. The ICER was estimated at EUR 62,895 per QALY gained and EUR 60,474 per QALY gained when indirect costs were included. Conclusions: In the Netherlands, CSII represents a cost-effective option in patients with type 2 diabetes who continue to have poorly-controlled HbA1c despite optimization of MDI. Since the ICER falls below the willingness-to-pay threshold of EUR 80,000 per QALY gained, CSII is likely to represent good-value for money in the treatment of poorly-controlled T2D patients compared with MDI.
Introduction
Recent prevalence figures estimate that among adults aged 30–70 years the prevalence of known diabetes is 4.9%, increasing to 6.5% when undiagnosed diabetes is included, and that 90% of diabetes cases in the Netherlands are type 2 diabetesCitation1. Additionally, crude prevalence rates in the Netherlands are higher among people classified as obese (16%) and amongst ethnic minorities including Turkish (5.6%) and Moroccan (8.0%) immigrant populationsCitation2. However, these figures may be an under-estimate as screening programs estimate that up to 1% of the Dutch population may have undiagnosed diabetesCitation3. Type 2 diabetes is associated with a notable clinical and economic burden as patients require frequent monitoring and are at elevated risk for costly long-term disease-related complications including renal and cardiovascular disease. In the Netherlands, the annual mean (SD) per patient direct costs for a patient with type 2 diabetes are EUR 2607 (8678), with indirect costs estimated at EUR 1328 (4840) per patient per year further compounding the economic burden of the conditionCitation4.
Patients with type 2 diabetes are typically treated with a stepwise algorithm starting with diet and exercise alone then in combination with one or more antidiabetic agents (OADs). Insulin is normally initiated when optimal glycemic control can no longer be achieved using OADs alone. Insulin use in type 2 diabetes is typically initially in the form of once daily administration of a long-acting basal insulin. Current guidelines state that those patients failing to respond to intensification of basal insulin therapy should be switched to basal bolus MDI therapyCitation5. Nevertheless, a substantial proportion of patients continue to demonstrate sub-optimal glycemic control despite optimization of therapyCitation6. For such patients the use of continuous subcutaneous insulin infusion (CSII) may help them to achieve improved glycemic control. There is a large body of clinical evidence supporting the use of CSII in patients with type 1 diabetes; however, the use of CSII in type 2 diabetes is less well studied.
The recent OpT2mise randomized controlled trial investigated the efficacy and safety of using CSII in patients with type 2 diabetes who were unable to achieve good glycemic control, despite optimization of MDICitation7. OpT2mise is the largest trial conducted to date of CSII vs MDI in type 2 diabetes and included a total of 331 patients with a mean baseline HbA1c of 9.0% (75 mmol/mol). At 6 months, patients in the CSII group had a significantly better improvement in HbA1c than those on MDI (HbA1c change of −1.1% for CSII vs −0.4% for MDI; between group difference [95% CI] = −0.7% [−0.9 to −0.4%]; p < 0.0001). Recently published 12 month data have also shown the reduction in HbA1c was durable over 1 yearCitation8. Patients in the CSII group also had a significantly lower daily insulin dose (97 U/day for CSII and 122 U/day for MDI; p < 0.0001) and a mean (SD) decrease in basal insulin use of 5.6 (18.7) U/day, whereas patients in the MDI group had a mean increase of 7.0 (29.2) U/day leading to a mean difference of −12.6 U/day; p < 0.0001) as well as demonstrating a clinically significant reduction in the amount of time spent in hyperglycemia. Several smaller studies have also shown improved HbA1c profiles with CSII vs MDI in type 2 diabetes patientsCitation9–11 as well as patient preference for CSII due to ease of useCitation12. Despite the advantages of CSII relative to MDI for type 2 diabetes patients struggling to achieve good glycemic control, CSII is infrequently used in type 2 diabetes. There are potential barriers to the widespread uptake of insulin pumps among patients with type 2 diabetes; one of the key barriers is the lack of knowledge and experience with CSII among primary care physicians who care for the majority of type 2 diabetes patients as insulin pump therapy is typically exclusively confined to secondary and tertiary care environments. Additionally, there is a lack of cost-effectiveness studies in type 2 diabetesCitation13.
The use of CSII relative to MDI has been shown to be cost-effective in patients with type 1 diabetes in several settings but, to date, cost-effectiveness studies in patients with type 2 diabetes are lackingCitation14–19. The aim of the current analysis was to examine the cost-effectiveness of CSII compared with MDI in patients with type 2 diabetes unable to achieve good glycemic control with MDI, based on the clinical input data from the recently published OpT2mise trial.
Materials and methods
Model description
The analysis was performed using the IMS CORE Diabetes Model (CDM; IMS Health, Basel, Switzerland), which is a validated non-product specific policy analysis tool for cost-effectiveness analyses of type 1 and type 2 diabetes. Structurally, the model is based on a series of inter-dependent sub-models that simulate diabetes-related complications (angina, myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, diabetic retinopathy, macula edema, cataract, hypoglycemia, ketoacidosis, lactic acidosis, depression, edema, nephropathy and end-stage renal disease, neuropathy, foot ulcer and amputation, and non-specific mortality). The sub-models have a semi-Markov structure and use time, state, time-in-state, and diabetes type dependent probabilities derived from published sources to simulate disease progression. Monte Carlo simulation using tracker variables is used to overcome the memory-less properties of the standard Markov model, and allows for interconnectivity and interaction between individual sub-models. For each simulation performed here, a simulated cohort of 1000 patients was run through the model 1000 times using first order Monte Carlo simulation. Long-term outcomes included total direct costs, life expectancy, quality-adjusted life expectancy and time to onset of complications. A more comprehensive description of the CDM structure and validation has been previously published by Palmer and colleaguesCitation20–22.
Simulation cohort and treatment effect
Cohort characteristics and treatment effects were sourced from the OpT2mise study of CSII use in patients with type 2 diabetesCitation7. The mean (SD) age of the simulated cohort was 56 (9.6) years, the mean duration of diabetes was 15 (8) years and the mean HbA1c at baseline was 9.0% (75 mmol/mol) (). For the CSII group a HbA1c reduction of −1.1% was used, compared with −0.4% for the MDI group, in line with the findings of the OpT2mise study. In terms of the prevalence of diabetes-related complications at baseline, a weighted average of complication rates from the two arms of the OpT2mise study was used for the simulation cohort. Major hypoglycemic event rates in the simulation were 1.2 per 100 patient years for the MDI group and zero for the CSII group, again based on the findings of the OpT2mise study. No other treatment effects were applied in the modeling simulations.
Table 1. Baseline characteristics of simulation cohort (from Reznik et al.Citation7).
Costs and utilities
Only the incremental cost of CSII relative to MDI was captured in the treatment costs used in the analysis as it was assumed that other management costs (i.e. costs associated with concomitant medications such as anti-hypertensives, statins, and aspirin, as well as procedures such as routine foot exams and routine screening for nephropathy) were equal in both arms. Incremental treatment costs for the CSII arm included costs for the current Medtronic insulin pump and infusion set on the market, differences in insulin use between arms and the costs of insulin pens and needles in the MDI arm, costs for which were sourced from official Dutch tariffs ()Citation23–27 (Appendix). Direct medical costs for the management/treatment of diabetes-related complications were sourced from published literature and, where necessary, inflated to 2013 EUR using the consumer price index from the Dutch Centraal Bureau voor de Statistiek. Indirect costs (i.e. lost productivity) for sensitivity analyses were based on the human capital approach using average salaries for the Netherlands (from the Centraal Bureau voor de Statistiek) and days off work for diabetes-related complications from a study conducted in DenmarkCitation28. Health state utilities were sourced from a published review by Beaudet et al.Citation29 and no utility benefit specific to either treatment arm (e.g. potential reduced fear of hypoglycemia with CSII) was applied.
Table 2. Complication and disease management costs (2013 EUR).
Discount rate and time horizon
The analysis was performed over a lifetime time horizon. Future costs were discounted at 4% per annum and clinical outcomes at 1.5% per annum in line with recommendations for the Dutch settingCitation30.
Sensitivity analysis
A series of one-way sensitivity analyses were performed to determine key drivers of outcomes. As the base case analysis was performed from a third party-payer perspective (including direct costs only) a sensitivity analysis was performed from the societal perspective in which both direct and indirect costs due to lost productivity were captured. Sensitivity analyses were also performed around complication costs by increasing and decreasing complication costs by 20% relative to the base case and also around training costs. Training costs associated with first time use of CSII were not included in the base case; therefore, a sensitivity analysis was performed in which CSII training costs were included in the first year costs. The influence of baseline HbA1c was explored by altering baseline HbA1c to 8.5% (69 mmol/mol), 9.5% (80 mmol/mol) and 10.0% (86 mmol/mol), compared with 9.0% (75 mmol/mol) in the base case. Additionally, sensitivity analyses were performed around discount rates (using 0%, 4%, and 6% per annum for both future costs and clinical outcomes) and around time horizon (using time horizons of 5, 10, and 20 years).
Results
In the base case analysis the use of CSII relative to MDI was associated with improved quality-adjusted life expectancy (9.38 quality-adjusted life years [QALYs] for CSII vs 8.95 QALYs for MDI; difference =0.43 QALYs) and higher direct lifetime costs (EUR 115,839 vs EUR 88,788; difference = EUR 27,051 (), which resulted in an incremental cost-effectiveness ratio (ICER) of EUR 62,895 per QALY gained. Analysis of the cost-effectiveness acceptability curve () showed that at a willingness to pay threshold of EUR 80,000 per QALY gainedCitation31,Citation32 CSII had a probability of >70% of being considered cost-effective. The higher direct costs for the CSII group were largely driven by higher lifetime treatment costs (EUR 58,024 vs EUR 20,237), but these were partly mitigated by lower complication costs owing to a reduced incidence of long-term diabetes-related complications over patients’ lifetimes. Differences in the cost of long-term renal complications were particularly pronounced, with mean costs in the CSII arm being almost EUR 10,000 per patient lower than in the MDI arm. The use of CSII also resulted in long-term cost savings in terms of direct costs associated with ulcer, amputation and neuropathy and ophthalmic complications (). When the analysis was conducted from a societal perspective the ICER was reduced to EUR 60,474 per QALY gained.
Figure 1. Cost-effectiveness acceptability curve for CSII vs MDI in patients with type 2 diabetes in the Netherlands.
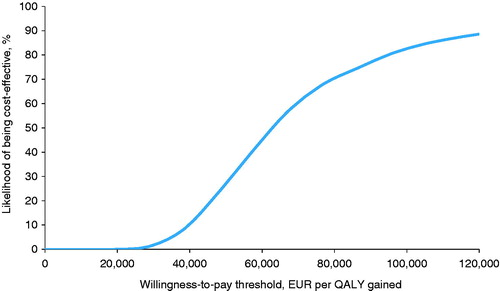
Table 3. Summary of base case analysis.
Findings from the base case analysis also showed that CSII was associated with a delay in the onset of diabetes-related complications compared with MDI (). The longest mean delays in onset were reported for gross proteinuria and macular edema, the onset of which were delayed for 1.2 years with CSII compared with MDI. The onset of neuropathy and end-stage renal disease were also delayed by ≥1 year with CSII.
One-way sensitivity analyses were performed to establish key drivers of cost-effectiveness. Baseline HbA1c strongly influenced cost-effectiveness, with CSII being most cost-effective in patients with the poorest glycemic control at baseline (). For patients with a mean baseline HbA1c of 8.5% the ICER increased to EUR 161,936 per QALY gained as the incremental QALY gain for the CSII arm was reduced to 0.20 QALYs (compared with 0.43 QALYs in the base case analysis). However, for patients with a baseline HbA1c of 9.5% (80 mmol/mol) the ICER was EUR 35,837 per QALY gained, which decreased further to EUR 18,610 per QALY gained for patients with baseline HbA1c of 10% (86 mmol/mol).
Table 4. Results of one-way sensitivity analyses.
The ICER was also influenced by time horizon () and additionally when potential training costs associated with the initial switch to CSII in the first year were also captured the ICER increased to EUR 72,008 per QALY gained. Changes in complication costs had minimal influence on the overall findings of the analysis.
Discussion
For type 2 diabetes patients in the Netherlands, who are insulin resistant and have sub-optimal glycemic control despite optimized MDI, switching to CSII was projected to be a cost-effective treatment option. CSII was associated with higher quality-adjusted life expectancy and higher direct costs compared with MDI. In the base case analysis, where the mean baseline HbA1c was 9.0% (75 mmol/mol), the ICER was EUR 62,895 per QALY gained, which decreased to EUR 18,610 per QALY gained in the sensitivity analysis in which a baseline HbA1c of 10% (86 mmol/mol) was assumed. In the Netherlands a willingness-to-pay threshold of up to a maximum of EUR 80,000 per QALY gained is usedCitation31–33.
Improvements in glycemic control associated with CSII use led to a reduced incidence of diabetes-related complications and a longer life expectancy compared with MDI. Consequently, the findings of the present analysis show that using the Dutch willingness-to-pay threshold of EUR 80,000 per QALY gained CSII can be considered cost-effective in patients who are unable to achieve good glycemic control on MDI, thereby representing good value for money in the Dutch setting. However, it should be noted that willingness-to-pay thresholds, as well as treatment and management costs, vary between settings, which limits the generalizability of the findings of the present analysis across settings.
Clinical input data for the present analysis were based on the findings of the recently published OpT2mise trial; prior to this clinical data relating to the use of CSII in type 2 diabetes were largely limited to small-scale studies. Two trials reported no significant difference in glycemic control between CSII and MDI, with both groups demonstrating similar improvements in glycemic controlCitation12,Citation34, although patients in one of these studies expressed a preference for CSII due to convenience, flexibility and ease of useCitation12. In contrast, three other studies reported significantly better improvements in HbA1c with CSII than with MDICitation9–11. In particular, the findings from previous studies suggest that patients with the poorest glycemic control at baseline, despite optimized MDI, may derive the greatest clinical benefit from the use of CSIICitation10,Citation11,Citation35. Long-term follow-up from one of these studies also suggest that the improvements reported with CSII may be maintained for up to 6 yearsCitation35.
A limitation of the OpT2mise trial as well as several other CSII vs MDI trials is that adherence to dosing and timing of insulin administration were not assessed. However, in the OpT2mise trial, there was a 2-month run in phase prior to randomization, which was designed in order to optimize injection treatment and during which insulin dose was intensified (using a standardized protocol that allowed changes in both basal and bolus dosing). During the lead-in phase patients were also encouraged to check blood glucose levels at least 3-times per day, and patients with <2.5 measurements per day were excluded prior to randomization, as such only patients with relatively good adherence were randomized. Additionally, in the OpT2mise trial patients, the distribution of motivated and adherent patients is likely to have been similar between the two treatment arms. The convenient nature of CSII means that it may be beneficial to patients with a history of poor adherence, but also in the MDI arms of trials it is possible that poor glycemic control could for some patients be at least partly attributable to poor self-management and poorer adherence to MDI dosing and timing. Consequently there may be differences between prescribed insulin dose and actual insulin used due to missed or poorly timed dosing or dose adjustments, which in turn may have implications in terms of the cost of insulin used. The potential for poor adherence and the economic consequences of this is a parameter that was not captured and, therefore, represents a limitation of the current analysis. Additionally, adherence within the clinical trial environment may differ to that in routine clinical practice, which has implications for both MDI and CSII. The clinical trial environment typically consists of motivated patients who receive frequent follow-up and monitoring. In contrast, a broader spectrum of patients is found in routine practice, including those with co-morbid conditions that may preclude entry into a clinical trial. This may limit the generalizability of adherence reported from clinical trials.
Collectively, the findings of clinical studies suggest that the treatment benefit associated with switching to CSII for patients failing to achieve glycemic targets on MDI may be influenced by several factors, including most notably baseline HbA1c. In the current analysis, the findings of sensitivity analyses showed that CSII was projected to be most cost-effective in patients with baseline HbA1c ≥ 10%. Moreover, the results from this sensitivity analysis are likely to be conservative as, in the absence of sub-group analysis according to baseline HbA1c in OpT2mise, it is based on the treatment effect (HbA1c decrease) reported for the whole population (with a mean HbA1c of 9.0% [75 mmol/mol] at baseline), and does not account for the likelihood that the magnitude of the treatment effect may be larger in patients with worse glycemic control at baseline, as observed in a previous study by Reznik et al.Citation35. Additionally, the OpT2mise trial was conducted over a period of 6 months and it is possible that patients continuing to demonstrate poor glycemic control after 6 months would have insulin treatment intensified, or supplemented with oral antidiabetic agents, in particular GLP-1 receptor agonists. However, for patients in the Netherlands, the approach of adding a GLP-1 receptor agonist or SGLT-2 inhibitor is not reimbursed. It is feasible that treatment intensification in the MDI arm would have led to improved glycemic control and a subsequent reduction in long-term complications, but also increased pharmacy costs, both of which may have influenced the results of the cost-effectiveness analysis. The long-term evolution and/or intensification of treatment in the MDI arm was, therefore, not taken into account and as such represents a limitation of the present analysis. Additionally, in long-term analyses individual treatment goals also require adjustment over time as patients become older and other co-morbid conditions develop; however, this was not factored into this analysis.
Poor glycemic control is an important risk factor for costly long-term diabetes-related complications including renal and cardiovascular disease, ophthalmic complications, neuropathy and diabetic foot complications. In the Dutch Zwolle Outpatient Diabetes project Integrating Available Care (ZODIAC), study patients with HbA1c >9% (>75 mmol/mol) had a hazard ratio (95% confidence interval) of 2.26 (1.39–3.67) for all-cause mortality and 3.13 (1.62–6.05) for cardiovascular mortality compared with those with HbA1c 6.5–7.0% (48–53 mmol/mol)Citation36. Similarly, evidence from a large-scale epidemiologic study in Sweden showed that each 1% increase in baseline HbA1c is associated with a 10% increase in risk for fatal or non-fatal cardiovascular diseaseCitation37. Hence, the benefits of improving HbA1c and reducing long-term complication rates through use of CSII relative to MDI in type 2 diabetes, should not be neglected.
Although data specific to the Dutch setting are lacking, the US Centers for Disease Control and Prevention report that over the period 2003–2006, 13% of diabetes patients (including those treated with insulin, OADs and diet/lifestyle intervention) had HbA1c >9.0% (>75 mmol/mol)Citation38. For patients in the 20–44 year age group this figure was substantially higher, at 25%; although some of the patients within this group may be able to achieve target glycemic levels with introduction or intensification of insulin therapy, some patients still fail to achieve HbA1c targets despite optimization of MDI. This patient group represents the segment where the greatest clinical and economic benefits of switching from MDI to CSII are likely to be observed.
The use of CSII in patients with type 2 diabetes is an area that is not covered in much detail in national and international guidelines, with the most recent guidance from the American Diabetes Association simply stating that CSII represents an alternative to basal-bolus insulin therapy in type 2 diabetesCitation39. However, guidelines from France, Germany, and Latin America as well as the recent consensus statement from the American Association of Clinical Endocrinologists/American College of Endocrinology are more specific and state that CSII represents an appropriate treatment option for some type 2 diabetes patients, in particular those who are unable to achieve optimal glycemic control with MDI alone, have severe insulin resistance or who have an erratic lifestyleCitation40–43. Patients with very high insulin requirements are also mentioned as being good candidates for CSII in several guidelinesCitation40,Citation42. A key limitation of the current analysis is that it does not consider cost-effectiveness exclusively within this insulin-resistant population. However, based on the results of the current analysis CSII is likely to be cost-effective in this sub-group. The clinical input data for the analysis were sourced from the OpT2mise trial, in which patients were required to have a daily insulin dose of 0.5–1.8 U/kg at screening, but which excluded patients with insulin use >220 U per day; future clinical trials in patients with very high insulin requirements may facilitate analysis specifically in this patient group.
Reimbursement policies for CSII in type 2 diabetes vary substantially across settings; however, in some countries, such as France, CSII is reimbursed for both type 1 and type 2 diabetes patients; CSII is also reimbursed in type 2 diabetes by the majority of private payers in the US for example. There is a paucity of cost-effectiveness studies on CSII in type 2 diabetes, which are necessary to inform payer decisions in many settings. To date, cost-effectiveness studies of CSII have almost exclusively focused on type 1 diabetes, the results of which have largely shown CSII to be cost-effective relative to MDICitation14–19. In addition, one US-based break-even analysis reported that, for patients in the top tenth percentile of insulin and other drug costs, the initial cost of the insulin pump was offset in under 3 years due to reduced insulin doseCitation44. A second US-based retrospective analysis of claims data by the same authors showed that patients who switched from MDI to CSII had savings in total insulin costs of USD 657–1011 per yearCitation45.
The analysis presented here is the first to examine the cost-effectiveness of CSII vs MDI in type 2 diabetes over a lifetime time horizon. The results show that, for patients in the Netherlands who are unable to achieve good glycemic control with MDI, the use of CSII is associated with substantial clinical benefits and notably delays the time to onset of all major diabetes-related complications considered in this analysis. The use of CSII for people with type 2 diabetes who are unable to achieve good glycemic control with MDI thus results in improved clinical outcomes and long-term cost savings due to reduced incidence of complications and as such represents good value for money.
Transparency
Declaration of funding
This analysis was funded by Medtronic International Sàrl.
Declaration of financial/other relationships
ED and SR are current employees of HEVA HEOR, which has received consulting fees from Medtronic. JSP and WV are current employees of Ossian Health Economics and Communications, which has received consulting fees from Medtronic. SP and BdB are current employees of Medtronic, who are manufacturers of insulin pumps. YR and HdV have previously received consulting fees/honoraria from Medtronic. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to acknowledge Medtronic International Sàrl for the funding supporting the analysis and manuscript.
References
- Rijksinstituut voor Volkgezondheid en Milieu. National Public Health Compass. How common is diabetes and how many people die from it? http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/endocriene-voedings-en-stofwisselingsziekten-en-immuniteitsstoornissen/diabetes-mellitus/omvang/. Accessed September 1, 2015
- Ujcic-Voortman JK, Schram MT, Jacobs-van der Bruggen MA, et al. Diabetes prevalence and risk factors among ethnic minorities. Eur J Public Health 2009;19:511-15
- Rutten GEHM, Janssen PGH. Screening for type 2 diabetes – The ADDITION Netherlands Study. Eur Endocrinol 2009;5:32-7
- van der Heijden AA, de Bruijne MC, Feenstra TL, et al. Resource use and costs of type 2 diabetes patients receiving managed or protocolized primary care: a controlled clinical trial. BMC Health Serv Res 2014;14:280
- Inzucchi SE, Bergenstal RM, Buse JB, et al., Management of hyperglycaemia in type 2 diabetes 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2015;38:140-9
- Hoerger TJ, Segel JE, Gregg EW, et al. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81-6
- Reznik Y, Cohen O, Aronson R, et al; OpT2mise Study Group. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 2014;384:1265-72
- Aronson R, Reznik Y, Conget I, et al; OpT2mise Study Group. Sustained efficacy of insulin pump therapy, compared with multiple daily injections, in type 2 diabetes: 12-month data from the OpT2mise randomized trial. Diabetes Obes Metab 2016 [Epub ahead of print]
- Noh YH, Lee SM, Kim EJ, et al. Improvement of cardiovascular risk factors in patients with type 2 diabetes after long-term continuous subcutaneous insulin infusion. Diabetes Metab Res Rev 2008;24:384-91
- Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res 2007;39:224-9
- Wainstein J, Metzger M, Boaz M, et al. Insulin pump therapy vs. multiple daily injections in obese Type 2 diabetic patients. Diabet Med 2005;22:1037-46
- Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care 2003;26:2598-603
- Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther 2010;12(1 Suppl):S17-S21
- St Charles ME, Sadri H, Minshall ME, et al. Health economic comparison between continuous subcutaneous insulin infusion and multiple daily injections of insulin for the treatment of adult type 1 diabetes in Canada. Clin Ther 2009;31:657-67
- St Charles M, Lynch P, Graham C, et al. A cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: a third-party US payer perspective. Value Health 2009;12:674-86
- Cohen N, Minshall ME, Sharon-Nash L, et al. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin: economic comparison in adult and adolescent type 1 diabetes mellitus in Australia. Pharmacoeconomics 2007;25:881-97
- Conget Donlo I, Serrano Contreras D, Rodríguez Barrios JM, et al. [Cost-utility analysis of insulin pumps compared to multiple daily doses of insulin in patients with type 1 diabetes mellitus in Spain]. Rev Esp Salud Publica 2006;80:679-95
- Roze S, Valentine WJ, Zakrzewska KE, et al. Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med 2005;22:1239-45
- Roze S, Smith-Palmer J, Valentine W, et al. Cost-effectiveness of continuous subcutaneous insulin infusion versus multiple daily injections of insulin in Type 1 diabetes: a systematic review. Diabet Med 2015;32:1415-24
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(1 Suppl):S5-S26
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(1 Suppl):S27-S40
- McEwan P, Foos V, Palmer JL, et al. Validation of the IMS CORE Diabetes Model. Value Health 2014;17:714-24
- de Vries FM, Denig P, Visser ST, et al. Cost-effectiveness of statins for primary prevention in patients newly diagnosed with type 2 diabetes in the Netherlands. Value Health 2014;17:223-30
- Eefting F, Nathoe H, van Dijk D, et al. Randomized comparison between stenting and off-pump bypass surgery in patients referred for angioplasty. Circulation 2003;108:2870-6
- Nederlands Huisarten Genootschap. Utrecht, The Netherlands. https://www.nhg.org/. Accessed July 2, 2015
- Nederlandse Zorgautoriteit. Tarieven en prestaties. Utrecht, The Netherlands. http://www.nza.nl/regelgeving/tarieven/. Accessed July 2, 2015
- Redekop WK, McDonnell J, Verboom P, et al. The cost effectiveness of Apligraf treatment of diabetic foot ulcers. Pharmacoeconomics 2003;21:1171-83
- Sørensen J, Ploug UJ. The cost of diabetes-related complications: registry-based analysis of days absent from work. Econ Res Int 2013;2013:1-8
- Beaudet A, Clegg J, Thuresson PO, et al. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462-70
- College voor zorgverzekeringen. Guidelines for pharmacoeconomic research, updated version College voor zorgverzekeringen. 2006. http://www.ispor.org/peguidelines/source/HTAGuidelinesNLupdated2006.pdf. Accessed May 21, 2015
- RVZ. Zinnige en duurzame zorg (Sensible and sustainable Care). Zoetermeer. Raad voor de Volksgezondheid en Zorg (Council for Public Health and Health Care); 2006. Report nr. 06/06
- RVZ. Rechtvaardige en duurzame zorg (Justified and sustainable care). Den Haag. Raad voor de Volksgezondheid en Zorg (Council for Public Health and Health Care); 2007. Report nr. 07/04. ISBN 978-90-5732-183-2
- Zorginstituut Nederland. Rapport kosteneffectiviteit in de praktijk. https://www.zorginstituutnederland.nl/binaries/content/documents/zinl-www/documenten/publicaties/rapporten-en-standpunten/2015/1506-kosteneffectiviteit-in-de-praktijk/1506-kosteneffectiviteit-in-de-praktijk/Kosteneffectiviteit+in+de+praktijk.pdf. Accessed 7 October, 2015
- Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 2005;28:1568-73
- Reznik Y, Morera J, Rod A, et al. Efficacy of continuous subcutaneous insulin infusion in type 2 diabetes mellitus: a survey on a cohort of 102 patients with prolonged follow-up. Diabetes Technol Ther 2010;12:931-6
- Landman GW, van Hateren KJ, Kleefstra N, et al. The relationship between glycaemic control and mortality in patients with type 2 diabetes in general practice (ZODIAC-11). Br J Gen Pract 2010;60:172-5
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 2010;268:471-82
- United States Centers for Disease Control and Prevention. Diabetes Public Health Resource. Percentage with A1c >9% among adults with diagnosed diabetes, by age, United States, 1988–1994 to 1999–2006. http://www.cdc.gov/diabetes/statistics/a1c/a1c_pct3_by_agegrp.htm. Accessed May 29, 2015
- American Diabetes Association. Standards of medical care in diabetes 2015. Diabetes Care 2015:38(1 Suppl):S1-S99
- Lassmann-Vague V, Clavel S, Guerci B, et al; Société francophone du diabète (ex ALFEDIAM). When to treat a diabetic patient using an external insulin pump. Expert consensus. Société francophone du diabète (ex ALFEDIAM) 2009. Diabetes Metab 2010;36:79-85
- German Diabetes Association, Matthaei S, Bierwirth R, et al. Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes 2009;117:522-57
- Grunberger G, Abelseth JM, Bailey TS, et al. Consensus statement by the American Association of Clinical Endocrinologists/American College of Endocrinology Insulin Pump Management Task Force. Endocr Pract 2014;20:463-89
- Guzmán JR, Lyra R, Aguilar-Salinas CA, et al; ALAD Consensus Group. Treatment of type 2 diabetes in Latin America: a consensus statement by the medical associations of 17 Latin American countries. Latin American Diabetes Association. Rev Panam Salud Publica 2010;28:463-71
- David G, Shafiroff J, Saulnier A, et al. Multiple daily injection therapy (MDI) versus durable insulin pump therapy in type II diabetics: a breakeven analysis. Value Health 2012;15:A65
- David G, Gill M, Gunnarsson C, et al. Switching from multiple daily injections to CSII pump therapy: insulin expenditures in type 2 diabetes. Am J Manag Care 2014;20:e490-7
