Abstract
Objective: The effectiveness of treatment decisions and economic outcomes of using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (Gd-EOB-DTPA-MRI) were compared with extracellular contrast media-enhanced MRI (ECCM-MRI) and multi-detector computed tomography (MDCT) as initial procedures in patients with suspected hepatocellular carcinoma (HCC) in South Korea and Thailand. Methods: A decision-tree model simulated the clinical pathway for patients with suspected HCC from the first imaging procedure to a confirmed treatment decision. Input data (probabilities and resource consumptions) were estimated and validated by clinical experts. Costs for diagnostic alternatives and related treatment options were derived from published sources, taking into account both payer’s and hospital’s perspectives. Results: All experts from Korea and Thailand agreed that Gd-EOB-DTPA-MRI yields the highest diagnostic certainty and minimizes the need for additional confirmatory diagnostic procedures in HCC. In Korea, from the payer’s perspective, total cost was USD $3087/patient to reach a confirmed treatment decision using Gd-EOB-DTPA-MRI (vs $3205/patient for MDCT and $3403/patient for ECCM-MRI). From the hospital’s perspective, Gd-EOB-DTPA-MRI incurred the lowest cost ($2289/patient vs $2320/patient and $2528/patient, respectively). In Thailand, Gd-EOB-DTPA-MRI was the least costly alternative for the payer ($702/patient vs $931/patient for MDCT and $873/patient for ECCM-MRI). From the hospital’s perspective, costs were $1106/patient, $1178/patient, and $1087/patient for Gd-EOB-DTPA-MRI, MDCT, and ECCM-MRI, respectively. Conclusions: Gd-EOB-DTPA-MRI as an initial imaging procedure in patients with suspected HCC provides better diagnostic certainty and relevant statutory health insurance cost savings in Thailand and Korea, compared with ECCM-MRI and MDCT.
Background
In 2012 there were ∼782,000 new cases of liver cancer worldwide and most patients who develop liver cancer die within the first year after diagnosisCitation1. Hepatocellular carcinoma (HCC) represents ∼85% of all types of liver cancerCitation1. The incidence of liver cancer and, specifically, HCC, is higher in the Asia-Pacific region and Africa compared with Europe, Australia, New Zealand, and the AmericasCitation1–3.
The therapeutic options are determined by the extent of the tumor and presence of metastasesCitation4,Citation5. Only patients with early stage disease can be offered the potentially curative treatment options of surgery (resection) or liver transplantation. While the Barcelona Clinic Liver Cancer guidelines only recommend transcatheter arterial chemoembolization for intermediate-stage HCC and sorafenib for advanced-stage HCCCitation4, treatment guidelines from Asia have adopted several other non-curative therapeutic modalities for intermediate- and advanced-stage HCCCitation5–7.
Precise early detection, localization, and characterization of liver lesions are essential to ensure the choice of treatments with the highest positive impact on patients’ life expectancy and health-related quality-of-life. As surgery and transplantation are very complex and resource intensive, but also the only potentially curative treatment options, an important diagnostic goal is to identify reliably those patients with early stage HCC who could benefit from these approaches. High precision in diagnosing HCC can help reduce the necessity for additional diagnostic and staging procedures, as well as reduce the need for intra-surgical modificationsCitation8.
Gd-EOB-DTPA (gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid) is a contrast agent that is approved for use in magnetic resonance imaging (MRI) detection and characterization of liver lesions in patients with known or suspected focal liver disease. Gd-EOB-DTPA has the properties of both extracellular contrast material and liver-specific contrast material. In clinical trials, Gd-EOB-DTPA-MRI improved the detection and characterization of all lesion types compared with non-enhanced MRI and spiral computed tomography (CT)Citation8–10, and reduced the need for second imaging procedures and treatment modificationCitation8. However, there have been no studies that analyze the cost of using Gd-EOB-DTPA, in particular comparing it with the standard-of-care procedures, extracellular contrast media-enhanced MRI (ECCM-MRI), and three-phase multi-detector CT (MDCT).
The purpose of this study was to compare the economic consequences of using Gd-EOB-DTPA-MRI and the effectiveness of treatment decisions with those of ECCM-MRI and MDCT as initial imaging procedures in patients with suspected HCC in South Korea and Thailand. This is pertinent information for the decision-maker, specifically the payer, given the high prevalence of HCC in Asia.
Materials and methods
Ethical approval was not required for this analysis as no patients or patient data were used.
Literature search
The aim of this health economic model was to summarize a realistic clinical pathway for patients with suspected HCC, with key clinical decision points and related costs from the initial imaging procedure to the treatment decision. A literature search was conducted using the PubMed database, guidelines from international gastroenterological associations and societies, and relevant gastroenterology journals. Four guidelines for the diagnosis and treatment of HCC were identifiedCitation11–14.
Delphi panel for model validation
A preliminary model was constructed based on the published recommendations for the diagnosis and treatment of HCC. The structure was validated by clinical experts from China (×10), Korea (×4), and Thailand (×2) during three rounds of panels following a Delphi approach in order to achieve a transnational consensus on the model clinical pathway for HCC. Due to major differences in the patient pathways, however, China was excluded from further study at this point in the project.
Model structure
A six-step decision-tree model, which simulates the clinical pathway of patients with suspected HCC from the initial imaging procedure until the confirmed treatment decision, was used to compare the payer relevant costs of the three imaging procedures: Gd-EOB-DTPA-MRI, ECCM-MRI, and MDCT. Patients entered the model with suspected HCC due to lesions detected by ultrasound and/or elevated alpha fetoprotein (AFP) levels. Initially, there was a choice between the first diagnostic imaging procedures: MDCT, ECCM-MRI, or Gd-EOB-DTPA-MRI.
Subsequent therapeutic decisions depended on the number and size of the existing tumors. The diagnostic options ranged from one tumor sized <1 cm up to four or more tumors, with at least one tumor being larger than 3 cm. There was consensus among the clinical experts on the following assumptions: (1) patients with single tumors smaller than 1 cm would receive repeat ultrasound examinations at 3–4-month intervals for surveillance with no further cost impact within the model; (2) additional smaller lesions detected during the second imaging procedure with the same imaging features were considered to be HCC; and (3) if the second imaging procedure detected extra lesions with different features to the initially detected lesions, then a third diagnostic procedure could be used. The treatment decision followed biopsy or a maximum of three imaging examinations.
After three rounds of the Delphi panel the clinical experts reached consensus on the model structure (). The consequences of each initial choice of diagnostic procedure were calculated using probability data for epidemiological and diagnostic effectiveness, which had either been published or were estimated and challenged by the panel experts. The model concludes with the confirmed treatment option. Overall utilization and total costs were calculated for each first imaging procedure up to the confirmed treatment decision.
Figure 1. Structure of the decision tree from first diagnostic imaging to confirmed treatment decision. MDCT, three-phase multi-detector computed tomography; ECCM-MRI, extracellular contrast media-enhanced magnetic resonance imaging; Gd-EOB-DTPA-MRI, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid magnetic resonance imaging; HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
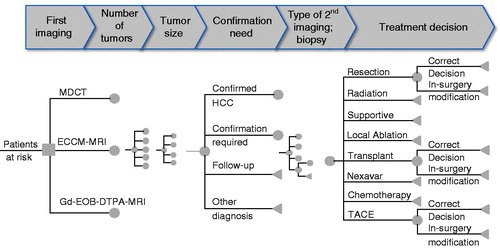
The results of the first imaging were either: confirmed HCC; further confirmation required (undetermined lesion); entering surveillance (only for a single tumor, <1 cm); or another diagnosis was made (no HCC).
After confirmation of HCC, staging was used to categorize patients and make a treatment decision according to the number and location of lesions, tumor size, vessel invasion, metastases, etc.Citation4,Citation5. The diagnostic procedure for staging could involve a second imaging procedure (CT, ECCM-MRI, or Gd-EOB-DTPA-MRI) or biopsy.
A second imaging process (CT, ECCM-MRI, or Gd-EOB-DTPA-MRI) could: (1) confirm HCC without extra lesions; (2) confirm HCC with extra lesions; or (3) exclude HCC. If HCC was excluded, the patient was disregarded in further model steps. Diagnosis could lead to eight treatment options for HCC depending on the staging results: (1) resection of the tumor(s); (2) liver transplantation; (3) supportive medication (e.g. morphine); (4) radiation therapy; (5) chemotherapy; (6) sorafenib-based chemotherapy; (7) local ablation; or (8) transcatheter arterial chemoembolization.
If, during surgery for resection, more liver lesions were discovered that had not been detected by imaging, this may have required intra-surgical modification of the surgery plan, increased surgery time, or rendered the surgery ineffective.
Patient characteristics
Patients with suspected HCC were included in the model if they had the following characteristics: (1) lesion(s) were detected using ultrasound and/or elevated AFP levels (≥200 ng/ml) or progressive increases in AFP levels (e.g. progressive increase in AFP levels by ≥10 ng/ml over initial levels for at least three consecutive examinations); (2) ≤ 65 years of age; (3) fully active and able to carry on all pre-disease activities without restriction (Eastern Cooperative Oncology Group performance status 0); (4) liver disease status of Child-Pugh Class A (well-compensated disease) or better; (5) no invasive tumor pattern (vascular invasion/extrahepatic spread) and no metastases (e.g. to the lung or bone); and (6) no extrahepatic co-morbidities.
Input probabilities and resource consumption
Input data (such as resource consumption and probabilities) were determined by face-to-face expert interviews and a rigorous process with pairs of physicians, consisting of one radiologist and one hepatologist: 10 and five pairs of physicians contributed their expertise in Korea and Thailand, respectively.
Estimates included the probabilities of the number and size of tumors after first imaging (); probability of HCC confirmation at first imaging; need for further confirmation, follow-up, or a different diagnosis (); and the need for further staging procedures such as a second imaging procedure or biopsy. Second, the experts estimated the probabilities for the treatment options relating to the diagnostic results, e.g. in how many patients out of 100 with a single tumor size <1 cm is a resection conducted. Finally, the resource consumption was estimated for each of the different imaging procedures and treatment options including labor costs, e.g. how long each professional is needed to conduct a resection, or which and how many laboratory tests were conducted related to the resection. All input data were challenged and validated in a Delphi panel process before they were applied in the country-specific models.
Table 1. Input probabilities for number and tumor size at first imaging.
Table 2. Input probabilities for confirmation of HCC at first imaging in Thailand and Korea.
Costs
The total cost from the hospital or the payer perspective in Korea or Thailand were calculated by accruing the relevant local cost of each diagnostic procedure or intervention for each of the initial diagnostic imaging options. Official local tariffs or published data were used to determine country-specific costs from the perspective of payers and hospitals. Where no published data were available, costs were estimated by interviewing experienced individuals (e.g. pharmacists and hospital controllers). The base year for all costs was 2010; all costs were inflated to the base year using current inflation rates. Where relevant, from the payer’s perspective, the calculations used the flat rates paid to the hospitals by the payer for different treatments or procedures (diagnosis-related group payment). Any cost items not included in the flat rates were added. From the hospital’s perspective the costs were evaluated through a micro-costing approach, where quantities (e.g. physician time for one resection) were first estimated in a Delphi panel and then multiplied with the unit costs from official tariffs (e.g. salary per minute). Relevant cost items from the hospital’s perspective were: personnel, medical procedures, laboratory tests, medical devices, hospitalization days, medication, and contrast media for each country.
Where Thailand and Korea follow different funding processes (e.g. for hospitals) this has been considered in determination of costs from the payer’s perspective. For Thailand, the flat rates paid by the payer () included the costs for contrast agents. For Korea, Resource-Based Relative Value (RBRV) scores were used to calculate the costs from a payer’s perspectiveCitation15. The total costs for imaging procedures and treatment options were calculated by adding the RBRVs to the respective relevant cost items from the payer’s perspective (). In the case of an incorrect treatment decision, the cost of modification was calculated in addition to the cost of the original treatment. A base case scenario was calculated using the best estimates of the underlying variables and model inputs, as derived from the interviews or other sources as indicated.
Table 3. Costs for diagnostic procedures and treatment options in Korea and Thailand from the hospital perspective.Table Footnotea
Sensitivity analyses
Sensitivity analyses were undertaken to assess the robustness of the results to changes in the input variables for both countries from payer and hospital perspectives. An initial analysis tested what maximum cost of Gd-EOB-DTPA (contrast only, not including other costs) in which the base case would be cost neutral compared with the other two imaging modalities (break-even cost). In addition, one-way sensitivity analyses were conducted for cost of diagnostic and therapeutic modalities including labor cost and key transition probabilities (confirmation required or more information needed, type of second imaging, and modification during surgery). The sensitivity analysis involved varying the input variables by ±20% or to the maximum or minimum possible values.
Results
Diagnostic certainty
There was a consensus among the experts that the need for further confirmatory diagnostic procedures to accurately diagnose HCC was reduced when using Gd-EOB-DTPA-MRI compared with MDCT or ECCM-MRI (). The need for further confirmation generally decreased with increasing size and number of tumors, and using Gd-EOB-DTPA-MRI remained advantageous in all cases.
Figure 2. Need for further confirmation of a single tumor in (a) Korea and (b) Thailand and four or more tumors in (c) Korea and (d) Thailand. ECCM-MRI, extracellular contrast media-enhanced magnetic resonance imaging; Gd-EOB-DTPA-MRI, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid magnetic resonance imaging; MDCT, three-phase multi-detector computed tomography.
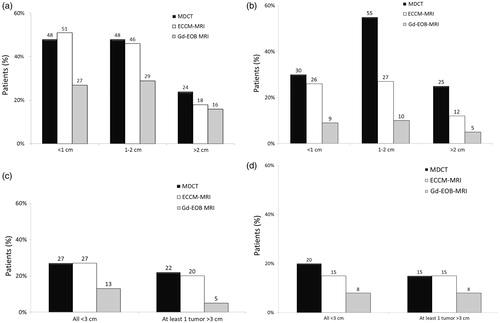
In Korea, the need for further diagnostic procedures was estimated to be reduced to 27–29% of cases for tumors sized <1 cm or 1–2 cm when using Gd-EOB-DPTA-MRI. In comparison, 46–51% of cases required confirmation when MDCT or ECCM-MRI was used initially (). In Thailand, further confirmation was needed in only 9% (tumor size <1 cm) or 10% (tumor size 1–2 cm) of cases when using Gd-EOB-DPTA-MRI, but in 26–55% when using one of the other two modalities for first diagnosis (). The need for confirmation was reduced by 16–46% in Thailand.
In Korea, confirmatory diagnostic procedures after using Gd-EOB-DPTA-MRI were needed for 13% of patients with four or more tumors that were all <3 cm, and only 5% of patients where at least one tumor was >3 cm (). When one of the other diagnostic modalities was used first, 20–27% of patients needed additional diagnostic confirmation. In Thailand, only 8% of patients with multiple tumors needed confirmation by another diagnostic procedure when Gd-EOB-DPTA-MRI was used first. With the other modalities, 15–20% of patients need further diagnostic confirmation ().
As can be seen in , input data vary between Thailand and Korea. While diagnosis and treatment follow the same pathway in the two countries, the actual number of patients in each category may depend on additional factors such as local clinical practice and the stage at which the patients are seen by the specialist hospitals.
The precision of the decision for resection is reported in . When using Gd-EOB-DPTA-MRI as the first diagnostic procedure in Korea, the need for intra-surgical modification was reduced by 61% and 26% compared with MDCT and ECCM-MRI, respectively. In Thailand, intra-surgical modifications were reduced by 80% when using Gd-EOB-DPTA-MRI as compared to a first diagnosis with MDCT, and were 69% lower than with ECCM-MRI.
Table 4. Reduction in intra-surgical treatment modification (mean across all tumor numbers and size categories).
Economic implications in the base case scenario
For Korea, the model indicates that relevant total payer costs per patient were USD 3087 when the first imaging procedure used was Gd-EOB-DTPA-MRI (). The total costs for using MDCT first were USD 3205 (i.e. cost difference of USD 117), whereas the costs when ECCM-MRI was used first were USD 3403 (i.e. USD 316 higher) compared with Gd-EOB-DTPA-MRI. Total costs included the patient pathway from the first imaging procedure up to and including the confirmed treatment decision, and cost difference for intra-surgical treatment modifications due to an incorrect first diagnosis. From the hospital’s perspective, Gd-EOB-DTPA-MRI was associated with the lowest cost (USD 2289), while the cost for MDCT was USD 2320 (USD 31 higher). The cost for using ECCM-MRI as a first diagnostic procedure was USD 2528, which was USD 239 higher than with Gd-EOB-DTPA-MRI.
Table 5. Total costs per patient from the payer’s and hospital’s perspective.
In Thailand, Gd-EOB-DTPA-MRI was the least costly alternative from the payer’s perspective (USD 702 vs USD 931 for MDCT and USD 873 for ECCM-MRI; ). From the hospital’s perspective, costs were USD 1106 for Gd-EOB-DTPA-MRI, USD 1178 for MDCT, and USD 1087 for ECCM-MRI ().
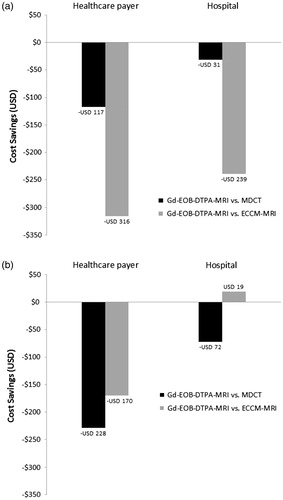
The total cost in Korea and Thailand of using Gd-EOB-DTPA-MRI as the first imaging procedure compared with MDCT or ECCM-MRI is illustrated in . Based on the difference between modalities being less than USD 100, Gd-EOB-DTPA-MRI as the first imaging procedure was deemed to be no more costly than MDCT in Korea and the least costly strategy in Thailand.
Figure 3. Total cost savings of using Gd-EOB-DTPA-MRI as the first imaging procedure compared with MDCT or ECCM-MRI in (a) Korea and (b) Thailand. ECCM-MRI, extracellular contrast media-enhanced magnetic resonance imaging; Gd-EOB-DTPA-MRI, gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetic acid magnetic resonance imaging; MDCT, three-phase multi-detector computed tomography.
Sensitivity analyses
Changes in cost of the initial imaging procedure had a moderate impact on the total per patient cost since the cost of the initial imaging was a relatively small percentage of the overall cost (mean =12%, range =4–28%). However, changing the cost of Gd-EOB-DTPA-MRI had a higher impact on the overall per patient cost than the two other imaging modalities because it was also predominantly used as the second imaging procedure after the other two imaging modalities: 99% and 64% of second imaging procedures after MDCT were Gd-EOB-DTPA-MRI in Korea and Thailand, respectively, and 88% and 97% after ECCM-MRI, respectively. The portion of patients that needed a second imaging procedure after MDCT in Korea and Thailand was 29% and 22%, respectively, 24% and 14% after ECCM-MRI, and 0% and 0.8% after Gd-EOB-DTPA-MRI.
The maximum cost of Gd-EOB-DTPA (contrast only, not including other costs) where it would break even with the other two imaging modalities is summarized in . From the Korean hospital perspective, the cost of Gd-EOB-DTPA-MRI only included cost of personnel but not Gd-EOB-DTPA, and in Thailand from the payer perspective the cost of Gd-EOB-DTPA was not separated from the overall cost of Gd-EOB-DTPA-MRI. Using Gd-EOB-DTPA remained cost neutral in Korea (payer perspective) at USD 410 and USD 620 when compared with MDCT and ECCM-MRI, respectively, and in Thailand (hospital perspective) at USD 280 and USD 150, respectively.
Table 6. Upper limit of cost of imaging with Gd-EOB-DTPA where it is cost neutral Gd-EOB-DTPA-MRI compared with the other two imaging modalities (USD).
The impact of the cost of treatment on overall per patient cost depended on the portion who underwent treatment. The most common procedure was transcatheter arterial chemoembolization (TACE; almost half the patients received TACE in Korea and ∼30% in Thailand) and it was the second most costly procedure after liver transplantation in Korea from both payer and hospital perspectives (USD 5808 and USD 5628, respectively). In Thailand, TACE was relatively low cost from a payer perspective (USD 469), but high from a hospital perspective (USD 2145). summarizes TACE cost sensitivity analyses. In Korea, TACE was the treatment procedure that contributed most to the total overall cost from both payer and hospital perspectives, and in Thailand from a hospital perspective. Since the number of patients receiving TACE in the three initial imaging groups was similar, changing the cost of TACE resulted in a modest change in the relative overall cost between the groups.
Table 7. Impact of TACE cost on overall per patient cost.
The cost of resection was USD 1259 and USD 811 from the Korean payer and hospital perspectives, respectively, and USD 266 and USD 628 in Thailand, respectively. Resection was undertaken in about a quarter of patients in Korea and ∼40% in Thailand, and these percentages were similar for the three imaging groups. Sensitivity analyses showed that the cost of resection had a limited impact on the overall cost in both countries. All other treatment modalities had limited impact on overall cost. Similarly, the transition probabilities for confirmation required, type imaging used for confirmation, and modification during surgery had limited impact on overall cost or relative cost differences between the three imaging groups.
summarizes the results of the personnel cost sensitivity analyses. Personnel cost only impacted on the hospital perspective. However, the impact of personnel cost on the total overall per patient cost was modest and the overall cost in Korea changed from the base case on average by ±2.8% and by ±1.3% in Thailand. The higher impact of personnel cost on total cost in Korea compared to Thailand is due to the nearly a 5-fold higher hospital personnel cost in Korea. Personnel cost for TACE, which was the only treatment modality with a sizable impact on overall cost, was only 7.2% of cost of TACE in Korea and 4% in Thailand. Neither of the two treatment modalities where personnel cost made up a large portion of the cost, namely resection (60%) and transplantation (56%), had much impact on overall cost. This can be explained by the lower number of patients undergoing those procedures and the relatively low total cost of the procedures compared to TACE.
Table 8. Impact of personnel cost impact on overall per patient cost, hospital perspective.
Discussion
Compared with the base case scenario, Gd-EOB-DTPA-MRI was the most cost-effective imaging procedure for diagnosing a patient with suspected HCC from the payer’s perspective in Thailand, and amongst the least costly alternatives in Korea. The lower total costs in Thailand and comparable costs to MDCT in Korea are mainly driven by a reduction of resource consumption with Gd-EOB-DTPA-MRI compared with the alternative imaging procedures. Expert opinion from the Delphi panel indicates that patients screened with Gd-EOB-DTPA-MRI have a higher probability of obtaining a confirmed diagnosis after the first imaging procedure compared with patients diagnosed using MDCT or ECCM-MRI. A recent study by Choi et al.Citation16 has confirmed the assumption derived from the expert opinion for this model. The comparative evaluation of the diagnostic performance of Gd-EOB-DTPA-MRI or dynamic CT and Gd-EOB-DTPA-MRI showed significantly higher sensitivity and accuracy of Barcelona Clinic Liver Cancer (BCLC) staging. When the diagnosis is confirmed at the first imaging step, there are no additional costs for further diagnostic procedures.
Higher diagnostic precision increases the care team’s confidence in terms of treatment choice. Therefore, in addition to cost savings, the time to treatment initiation is reduced. The latest evidence supports the hypothesis of improved health outcomes due to more precise diagnosis. Kim et al.Citation17 retrospectively compared recurrence-free survival rates of 323 patients who were diagnosed with Gd-EOB-DTPA-MRI to 377 patients who were diagnosed with a single-nodular HCC by dynamic 4-phase CT in Seoul, Korea. In an analysis of 285 matched pairs of patients, the group diagnosed by Gd-EOB-DTPA-MRI had significantly lower overall mortality (HR =0.66; 95% CI =0.44 − 0.99).
The expert panel in this study has already indicated that the higher diagnostic certainty also extends to the efficiency of resection surgery. The probability of detecting additional lesions during a resection, which results in treatment modification, is lower for patients first diagnosed with Gd-EOB-DTPA-MRI compared with the alternative options. Fewer intra-surgical treatment modifications are important from the hospital’s perspective as it means that the surgical team’s time is used more efficiently. In addition, this is also important for the patients because it reduces unnecessary surgical interventions and shortens surgery times. Kim et al.Citation17 showed in their retrospective analysis that additional HCC nodules were detected when using Gd-EOB-DTPA-MRI after initial CT, leading to changes in BCLC stages and modified treatment plans in 13.3% of patients. Thus, the payer and hospital can save the cost of treatment modifications by initially using Gd-EOB-DTPA-MRI in patients with suspected HCC. For the payer, this is only relevant if the costing is based on activity. With a diagnosis-related payment system, which has a flat rate payment per case, the additional time does not lead to additional costs. For the hospital, however, this may lead to considerable cost differences depending on the labor and equipment costs.
In general, labor costs have a major impact on the cost consequences of this model. In Thailand, labor costs are much lower than in Korea, which is a major reason for the high deviation in the results. In an environment with low labor costs, the relatively high price difference between the imaging agents has a much higher impact. In contrast, in Korea, the higher cost of Gd-EOB-DTPA-MRI becomes negligible in relation to the savings generated by the higher diagnostic certainty and the reduced need for intra-surgical modifications.
The modeling approach in this study has previously been used to compare different radiological procedures for the detection of liver metastases in European patients with colorectal cancerCitation18. This model showed similar results to those presented in our paper; in Sweden, Gd-EOB-DTPA-MRI was associated with reduced costs compared with ECCM-MRI and MDCT, while in Italy and Germany Gd-EOB-DTPA-MRI had lower costs than ECCM-MRI and similar costs to MDCT. Similar to our study, cost savings with Gd-EOB-DTPA-MRI arose as a result of improved pre-operative planning and reduced need for changes in the intra-operative planCitation18. The results of the European simulation were subsequently validated in a randomized trial in patients with suspected metastatic colorectal cancerCitation19. Use of Gd-EOB-DTPA-MRI as the first imaging strategy eliminated the need for further imaging, yielding benefits such as reduced workflow, lower costs, and improved accuracy of treatment planningCitation19. In addition, recent clinical studies have supported the estimated higher effectiveness of Gd-EOB-DTPA-MRI with even higher differences than estimated by the expert panel in this studyCitation20. A recent meta-analysis on small HCC <2 cm found that studies in which gadoxetate disodium was used had significantly higher sensitivities than those that used an extracellular agent (92% vs 67%, p ≤ 0.001)Citation21. However, specificity did not differ significantly between sub-groups (p ≥ 0.122). A consensus conference recommended Gd-EOB-DTPA-MRI as a method for characterizing HCC nodules detected in cirrhotic liver using ultrasonography, due to its high sensitivityCitation9.
It remains to be determined whether the results of this modeling study are transferable beyond Thailand and Korea. A recent comparison of current guidelines for the management of HCC revealed considerable differences in terms of diagnostic and treatment algorithmsCitation22. Three types of diagnostic algorithms were identified: (i) size-based; (ii) non-size-based, focusing on characteristic features of the nodules (i.e. a nodule is hypervascular in the arterial phase with washout in the portal venous or delayed phase); and (iii) diagnostic algorithms with no criteria, which only provide diagnostic tools without any detail. Obviously, the model cannot directly be transferred to the latter two diagnostic environmentsCitation22. In order to do this, local cost data would have to be used and the model would have to be adapted according to local guidelines and utilization patterns.
Sensitivity analysis
In the absence of real-life clinical data, many input variables were estimated based on expert opinion and, therefore, it was important to test the robustness of the model and results by varying these input data. Sensitivity and break-even analyses confirmed that the results were fairly robust across a range of assumptions; varying the key parameters associated with cost estimation (i.e. Gd-EOB-DTPA, personnel and TACE costs, and transition probabilities [confirmation required, type imaging used for confirmation and modification during surgery]) had limited impact on the relative overall per patient cost between the three imaging groups.
Limitations of this model
This study has a number of limitations. Basically, a model can only approximate the clinical reality by considering all key clinical pathways and outcomes. Clinical studies should be conducted to validate the model results in a similar way, as has been done for a previous model on the use of Gd-EOB-DTPA-MRI for the diagnosis of liver metastases of colorectal cancerCitation23. Furthermore, due to a lack of data, all input parameters (e.g. distribution of tumor size after diagnosis and resource consumption), except costs, were estimated by clinical expert panels. However, a large degree of care was taken to ensure that the experts achieved a consensus during a rigorous process. Nevertheless, in order to minimize future reliance on expert opinion we have recently performed a systematic review on diagnostic accuracyCitation24. Important assumptions of this study are that physicians are able to confirm HCC after only one imaging procedure, and that they can also decide on the subsequent treatment. Although a model conducted via Delphi panel input, and not a clinical study, the Zech et al.Citation18 model and the subsequent VALUE study both showed that the need for additional diagnostic procedures was reduced when initial screening was conducted with Gd-EOB-DTPA. This gives credence to the methodology of health economic models conducted from Delphi panels. Additionally, it is assumed that the patient pathway does not differ between Korea and Thailand. Due to differences in diagnostic and therapeutic procedures, China had to be excluded from this study. This model also only estimates the cost consequences from the payer’s or hospital’s perspective. Societal costs, cost savings, or quality-of-life aspects are not considered.
In addition, since this study used radiologist–hepatologist pair input, it could be argued that the sample was not comprehensive. However, each pair comprised experts in the field of HCC diagnosis for Korea and Thailand and, thus, reflect best knowledge of clinical practice and outcomes from these two countries.
Safety was also not considered in this study. Each additional procedure induces further risks for the patient. For example, radiation exposure from MDCT is associated with additional cancer risk for the patients who are already, by definition, “at risk”Citation25. Likewise, each biopsy exposes patients to additional risks that can be avoided or reduced with a high degree of certainty of the first diagnosis, as is seen with Gd-EOB-DTPA-MRICitation26.
Conclusion
Gd-EOB-DTPA-MRI as the first imaging procedure was deemed to be the least costly strategy in Thailand and no more costly than MDCT in Korea for patients with suspected HCC. The reduction in costs is mainly driven by the fact that Gd-EOB-DTPA-MRI is associated with better diagnostic certainty, meaning that patients require fewer imaging procedures than those screened with either MDCT or ECCM-MRI. Therefore, even though the cost of Gd-EOB-DTPA-MRI is higher than for the alternative procedures, it is offset by the reduced need for other diagnostic procedures.
Transparency
Declaration of funding
This study was funded by Bayer Healthcare, Berlin, Germany.
Declaration of interest/other relationships
JML has received fees for participation in review activities and has received writing assistance and administrative support from Springer Healthcare. JML has also received board membership from Bayer Healthcare, grants from Bayer Healthcare and Guerbet, and payment for lectures from Bayer Healthcare and Siemens Healthcare. SP has received consulting fees/honoraria for this study, grants for this study and travel/accommodations/meeting expenses unrelated to this study. AS has received consulting fees/honoraria for this study. APH and HR have received consulting fees/honoraria from Bayer Healthcare for the analytical components in this study and for writing or reviewing of the manuscript. APH and RH’s institution has received consultancy fees for other projects with Bayer Healthcare and other pharmaceutical and diagnostic companies. KB is an employee of Bayer Healthcare. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
| Abbreviations | ||
| AFP | = | alpha fetoprotein |
| CT | = | computed tomography |
| ECCM-MRI | = | extracellular contrast media-enhanced MRI |
| Gd-EOB-DTPA-MRI | = | gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging |
| HCC | = | hepatocellular carcinoma |
| MDCT | = | multidetector computed tomography |
| MRI | = | magnetic resonance imaging |
| RBRV | = | Resource-Based Relative Value |
Acknowledgments
We would like to thank IMS and specifically, I. Utzmann and D. Gasche, for the diligent work in the modeling and conduction of the Delphi panels as well as for providing some of the country-specific retail cost data. The authors thank Vicky Hinstridge of inScience Communications, Springer Healthcare, for medical writing support, funded by Bayer Healthcare.
References
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed April 19, 2016
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27
- Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am 2010;24:899-919
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74
- Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011;29:339-64
- Han KH, Kudo M, Ye SL, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology 2011;81(1 Suppl):158-64
- Kim SE, Lee HC, Kim KM, et al. Applicability of the BCLC staging system to patients with hepatocellular carcinoma in Korea: analysis at a single center with a liver transplant center. Korean J Hepatol 2011;17:113-19
- Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol 2012;57:421-9
- Lee JM, Zech CJ, Bolondi L, et al. Consensus report of the 4th International Forum for gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging. Korean J Radiol 2011;12:403-15
- Fowler KJ, Brown JJ, Narra VR. Magnetic resonance imaging of focal liver lesions: approach to imaging diagnosis. Hepatology 2011;54:2227-37
- Kudo M, Okanoue T, Hepatology JSo. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology 2007;72(1 Suppl):2-15
- Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36
- Park JW, Korean Liver Cancer Study G, National Cancer C. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol 2004;10:88-98
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711
- Ministry of Health and Welfare. http://www.mohw.go.kr/eng/index.jsp. Accessed August 1, 2012
- Choi SH, Byun JH, Kwon HJ, et al. The usefulness of gadoxetic acid-enhanced dynamic magnetic resonance imaging in hepatocellular carcinoma: toward improved staging. Ann Surg Oncol 2015;22:819-25
- Kim HD, Lim YS, Han S, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology 2015;48:1371-82
- Zech CJ, Grazioli L, Jonas E, et al. Health-economic evaluation of three imaging strategies in patients with suspected colorectal liver metastases: Gd-EOB-DTPA-enhanced MRI vs. extracellular contrast media-enhanced MRI and 3-phase MDCT in Germany, Italy and Sweden. Eur Radiol 2009;19(3 Suppl):S753-S63
- Zech CJ, Korpraphong P, Huppertz A, et al. Randomised multicentre trial on gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg 2014;101:613-21
- Park Y, Kim SH, Jeon YH, et al. Gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI versus gadobenate dimeglumine (Gd-BOPTA)-enhanced MRI for preoperatively detecting hepatocellular carcinoma: an initial experience. Korean J Radiol 2010;11:433-40
- Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR Imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology 2016;278:82-94
- Song P, Tobe RG, Inagaki Y, et al. The management of hepatocellular carcinoma around the world: a comparison of guidelines from 2001 to 2011. Liver Int 2012;32:1053-63
- Zech CJ, Justo N, Lang A, et al. Cost evaluation of gadoxetic acid-enhanced magnetic resonance imaging in the diagnosis of colorectal-cancer metastasis in the liver: results from the VALUE Trial. Eur Radiol 2016; [Epub ahead of print]
- Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR Imaging-A systematic review and meta-analysis. Radiology 2015;275:97-109
- Chang ML, Hou JK. Cancer risk related to gastrointestinal diagnostic radiation exposure. Curr Gastroenterol Rep 2011;13:449-57
- Mullhaupt B, Durand F, Roskams T, et al. Is tumor biopsy necessary? Liver Transpl 2011;17(2 Suppl):S14-S25
