Abstract
Objective: To quantify and compare hospital length of stay (LOS) and costs between hospitalized non-valvular atrial fibrillation (NVAF) patients treated with either apixaban or warfarin via a large claims database.
Methods: Adult patients hospitalized with AF were selected from the Premier Perspective Claims Database (01JAN2013-31MARCH2014). Patients with evidence of valvular heart disease, valve replacement procedures, or pregnancy during the index hospitalization were excluded. Patients treated with apixaban or warfarin during hospitalization were identified. Propensity score matching (PSM) was performed to control for baseline imbalances between patients treated with apixaban or warfarin. Primary outcomes were hospital LOS (days), post-medication administration LOS, and index hospitalization costs, and were compared using paired t-tests in the matched sample.
Results: Before PSM, 2894 apixaban and 124,174 warfarin patients were identified. Patients treated with warfarin were older and sicker compared to those treated with apixaban. After applying PSM, a total of 2886 patients were included in each cohort, and baseline characteristics were balanced. The mean (standard deviation [SD] and median) hospital LOS was significantly (p = 0.002) shorter for patients treated with apixaban for 5.1 days (5.7 and 3) compared to warfarin for 5.5 days (4.8 and 4). The trend appeared consistent in the hospital LOS from point of apixaban or warfarin administration to discharge (4.5 vs 4.7 days, p = 0.051). Patients administered apixaban incurred significantly lower hospitalization costs compared to those administered warfarin ($11,262 vs $12,883; p < 0.001).
Conclusions: Among NVAF patients, apixaban treatment was associated with significantly shorter hospital LOS and lower costs when compared to warfarin treatment.
Introduction
Atrial fibrillation (AF) is the most common heart dysrthythmia diagnosed in the USCitation1. AF prevalence has increased with an increasingly aging population in the US, and is expected to continue this trend substantially in the coming decadesCitation2,Citation3. In 2010 there were an estimated 5.2 million cases of AF and the number of AF cases is projected to increase to 12.1 million cases in 2030Citation3. Furthermore, AF carries a significant financial burden, costing the US healthcare system ∼$26 billion annually, with hospitalizations accounting for a majority of the costs (52%)Citation4. Non-valvular AF (NVAF) refers to AF that is not associated with rheumatic mitral stenosis, mechanical or bioprosthetic heart valves, or mitral valve repairCitation5. NVAF affects ∼85–95% of the overall population with AFCitation6,Citation7. NVAF has long been identified as a significant risk factor for disabling or fatal ischemic stroke and systemic embolismCitation8. Specifically, NVAF is associated with a 5-fold increase in stroke risk compared to those without NVAF, and AF-related strokes tend to be more severe than non-AF-related strokesCitation9,Citation10.
Until recently, vitamin K antagonists (VKAs), like warfarin, were the main drugs recommended for the reduction of risk of stroke in NVAF patients with a CHADS2 score ≥2. However, due to warfarin’s narrow therapeutic index and multiple drug–drug and drug–food interactions, requiring regular INR monitoring with dose adjustments, only about half of eligible AF patients in the US receive warfarin therapyCitation9,Citation11. Non-VKA oral anticoagulants (NOACs), including dabigatran, apixaban, and rivaroxaban, have demonstrated similar or superior stroke risk reduction without the need for routine monitoring and dose adjustments, compared to warfarin, and are alternative treatments for NVAF patientsCitation12. Apixaban has been shown to be superior to dose-adjusted warfarin in terms of reduction in the rates of stroke and systemic embolism, major bleeding, and all-cause mortalityCitation13. Previous studies have reported that NVAF patients treated with NOACs had significantly shorter hospital length of stay (LOS) compared to those treated with warfarinCitation14–16. Moreover, NOACs are becoming increasingly popular, particularly among patients with lower CHADS2 and HAS-BLED scoresCitation17. By June 2013, more than 60% of patients newly prescribed oral anticoagulants were prescribed dabigatran, rivaroxaban, or apixabanCitation17. Apixaban was approved in December 2012; therefore, insufficient evidence exists regarding clinical and economic outcomes among NVAF patients in real-world settings. The goal of this study was to estimate and compare index hospitalization costs and hospital LOS for real-world, hospitalized NVAF patients treated with either warfarin or apixaban.
Methods
This study used data from the Premier Perspective Claims Database (PCD) from January 2012 through March 2014 and identified patients for the study population from January 2013 through March 2014. Premier is a large US hospital-based clinical and economic database developed for quality and utilization. This database contains detailed billing and coding information for more than 2.5 million daily service records for an average of more than 5 million de-identified hospital discharges per year from 2000 to the present. The hospitals included are nationally representative based on geographic location, bed size, and teaching hospital status. Data elements include hospital characteristics, and patient demographics, diagnoses, and procedures during the inpatient admission. Patients’ medical information is derived from records utilized for billing purposes at the hospital level. Additionally, the Premier PCD cost variables reflect billable amounts, including drugs, and hospital costs, which include all supplies, labour, and depreciation of equipment. It is the sum of the fixed (overhead) and variable (direct) costs contained in the dataset. Premier data are de-identified and fully compliant with all Health Insurance Portability and Accountability Act privacy and security requirements.
Study sample
The study period ranged from January 2012–March 2014. Adult patients (≥18 years) were selected from the Premier PCD during the identification period (January 2013–March 2014) if they were diagnosed with AF (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code: 427.31) during an inpatient admission. The first AF hospitalization during the identification period was defined as the index hospitalization.
In order to evaluate NVAF patients, those with evidence of mitral valvular heart disease (ICD-9-CM codes: 093.21, 394.xx, 369.xx, 424.0, 746.5, 746.6), valve replacement procedures (ICD-9 procedure codes: 35.05-.09, 35.20–35.28, 35.97), or pregnancy (ICD-9-CM diagnosis codes: 630-679, V22–V24, V27, V28, V61.6, V61.7, 792.3, 796.5 or ICD-9 procedure codes: 72–75.99) were excluded. Patients with a discharge date after February 28, 2014 were excluded. Patients were categorized into cohorts based on anticoagulant treatment received during the index hospitalization. If patients were administered apixaban during the index hospitalization then they were placed in the Apixaban Cohort and if they were administered warfarin they were placed in the Warfarin Cohort. If patients were administered both apixaban and warfarin or other NOACs (dabigatran or rivaroxaban) during the index hospitalization they were excluded. Patients were followed until re-admission or end of the study period.
Covariates
Demographic and clinical characteristics were collected during hospitalizations 12 months prior to and during the NVAF index hospitalization. Covariates included gender, age, region, race, pre-index Charlson Comorbidity Index (CCI) score, CHADS2 score (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism), CHA2DS2-VASc score (CHADS2 plus vascular disease, age 65–74 years, sex category; Supplementary Material, Table 1), HAS-BLED score (hypertension, abnormal renal and liver function, stroke, bleeding, labile International Normalized Ratios [INRs], elderly age [≥65 years], drug therapy and alcohol intake; Supplementary Material, Table 2), and pre-index comorbid conditionsCitation18–21. The CCI score predicts the 10-year mortality for a patient who may have a range of 22 co-morbid conditionsCitation18. The CHADS2 and CHA2DS2-VASc scores predict stroke riskCitation20. The HAS-BLED score can range from 0–9, with a score ≥3 associated with a high bleeding riskCitation21,Citation22. Co-morbid conditions included diabetes, congestive heart failure, hypertension, stroke, vascular disease, renal insufficiency, venous thromboembolism, acute coronary syndrome, drug and alcohol use, chronic kidney disease, chronic obstructive pulmonary disease, and anemia.
Table 1. Baseline characteristics of hospitalized NVAF patients treated with apixaban and warfarin.
Table 2. Baseline characteristics of hospitalized NVAF patients treated with apixaban and warfarin after 1:1 PSM.
Outcome variables
The outcome variables included hospital LOS, index hospitalization costs, and re-admission rates. Hospital LOS was defined as the number of days from hospital admission to hospital discharge. Post-medication administration LOS was conducted in which hospital LOS was measured from first apixaban or warfarin administration to hospital discharge. Index hospitalization costs were calculated for each patient during the index hospitalization and included all billable costs (e.g. procedures, medications, room and board, and hospital costs) incurred during the hospitalization. Hospitalization costs were adjusted to 2014 US dollars using the Consumer Price Index (CPI) for Medical Care Services. Hospital re-admissions within 30 days of the index hospitalization were flagged. Re-admissions were only captured if the patient was re-admitted to the same hospital in which the index hospitalization took place, as re-admissions to other hospitals are not captured in the database.
Statistical analysis
The cohorts were compared for baseline and outcome variables. Percentages and standard deviations (SDs) were calculated for dichotomous and polychotomous variables and p-values were calculated using the Chi-square test. Means, medians, and SDs were provided for continuous variables, and p-values were calculated using the student t-test. The level of significance was set to α = 0.05.
Propensity score matching (PSM) was performed to balance baseline characteristics between the two cohortsCitation23,Citation24. An unconditional logistic regression model was fitted to determine the baseline variables associated with the treatment cohorts, designated as 1 for apixaban and 0 for warfarin. Using this model, a propensity score was developed for each patient and represented the probability of being a member of the Apixaban Cohort. Covariates in the propensity model included age, gender, race, insurance coverage, patient type (inpatient, other), admission type, hospital characteristics (region, population served [urban, rural], hospital setting, hospital bed size), CCI score, CHA2DS2-VASc score, HAS-BLED score, co-morbid conditions, VTE, hemodialysis or thrombocytopenia during index hospitalization, and any hospitalization during 12 months prior to index hospitalization. Each patient in the Apixaban Cohort was matched to a patient in the Warfarin Cohort with the closest propensity score, using the nearest neighbor technique with a caliper of ±0.01Citation25. The balance of measured covariates between the matched cohorts was assessed using absolute standardized differences, which are not influenced by sample size. An absolute standardized difference of ≤0.1 indicates a negligible difference in measured variables between groupsCitation26. After matching, index hospitalization costs, hospital LOS, and 30-day re-admission were compared between the two matched cohorts using paired t-tests and McNemar tests. A sensitivity analysis was conducted by comparing post-medication administration LOS. Additionally, costs were stratified by LOS and CHADS2 score. All statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Descriptive results
During their first AF hospitalization, 130,241 patients were treated with apixaban or warfarin. Of those, 219 patients were excluded because they were administered both apixaban and warfarin during the hospitalization, and 2954 patients were excluded because they were treated with other NOACs. After all of the criteria were applied, 127,068 patients in the study sample were identified, 2894 apixaban patients and 124,174 warfarin patients. Patients treated with warfarin were older (age ≥75 years: 58.2% vs 50.6%, p < 0.001), less likely to be female (47.1% vs 49.5%, p = 0.012), white (77.4% vs 83.7%, p < 0.001), and to have managed care insurance (10.5% vs 17.7%, p < 0.001) compared to those treated with apixaban. Of the apixaban and warfarin patients, 12.8% and 24.8% were hospitalized within 12 months prior to their index hospitalization, respectively. Patients treated with apixaban had significantly lower CHADS2, CHA2DS2-VASc, and HAS-BLED scores (). Among patients treated with apixaban and warfarin, 1.5% and 2.0% died during their hospitalization, respectively. AF was the most common discharge diagnosis for patients in both cohorts. Other common discharge diagnoses included septicemia, systolic heart failure, and cerebral embolism.
Before matching, patients treated with apixaban had a shorter hospital LOS (5.0 vs 6.4 days, p < 0.001) and lower index hospitalization costs ($11,239 vs $15,094, p < 0.001). Patients in the apixaban cohort were less likely to be re-admitted within 30 days compared to patients in the warfarin cohort (10.2% vs 12.2%, p = 0.002).
Multivariate results
After 1:1 PSM, the demographic and clinical characteristics between the two cohorts were well balanced, and 2886 patients were matched in each cohort. The mean CHA2DS2-VASc score was 4.0 for both cohorts, indicating a high stroke risk. There were no significant differences in baseline characteristics between the two cohorts ().
After matching, there was a significant difference in hospital LOS between the two cohorts. Patients in the Apixaban Cohort had a significantly shorter average hospital LOS compared to those in the Warfarin Cohort (5.1 vs 5.5 days, p = 0.002; ). There was a 1-day difference in the median hospital LOS (3 vs 4 days). Furthermore, significantly more patients treated with warfarin had a hospital LOS from 3–14 days compared to those treated with apixaban (). The mean index hospital LOS from point of apixaban or warfarin administration followed the same trend (4.5 vs 4.7 days, p = 0.051); however, it was not statistically significant.
Figure 1. Average hospital LOS for NVAF patients. LOS, length of stay; NVAF, non-valvular atrial fibrillation.
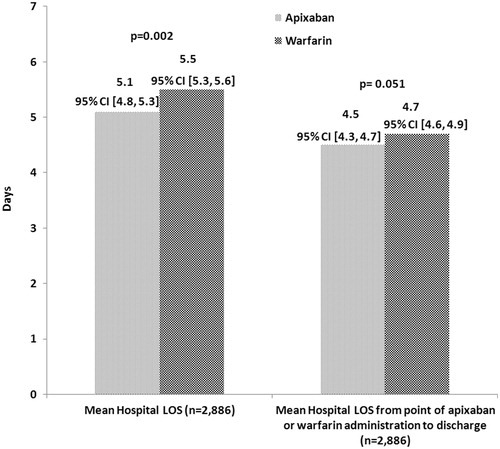
Table 3. Outcomes of hospitalized NVAF patients treated with apixaban and warfarin after 1:1 PSM.
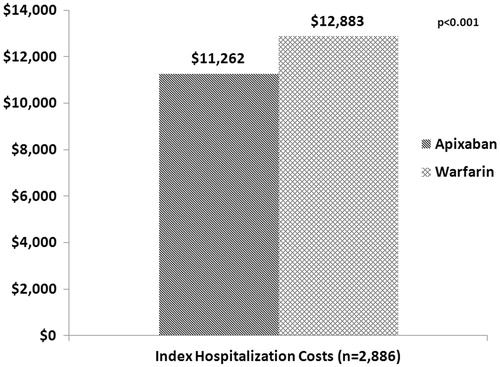
In turn, patients treated with apixaban had significantly lower hospitalization costs compared to those treated with warfarin ($11,262 vs $12,883, p < 0.001; ). This trend remained apparent when hospitalization costs were stratified by LOS and CHADS2 score. Patients in both the low-to-moderate and high stroke risk groups incurred significantly lower hospitalization costs when treated with apixaban compared to warfarin. Additionally, patients treated with apixaban who had a hospital LOS of 0–2 ($5033 vs $5797, p = 0.040), 3–7 ($9554 vs $10,179, p < 0.001), and 8–14 days ($21,008 vs $22,386, p < 0.001) incurred significantly lower hospitalization costs compared to those treated with warfarin. The trend remained consistent for longer hospital stays (>14 days), but this was not statistically significant. After matching the two cohorts, there was no significant difference in the 30-day re-admission rate (10.3% [n = 291] vs 10.8% [n = 306]).
Discussion
In this retrospective claims study, apixaban treatment resulted in significantly lower hospital LOS and hospitalization costs compared to warfarin treatment among NVAF patients. This study provides real-world evidence to demonstrate the economic benefits of apixaban that may be a result of more favorable clinical outcomes, as observed in clinical trials; however, this hypothesis still needs to be validated. Due to apixaban’s recent market entry, further research needs to be completed with a larger sample size.
Patients treated with apixaban had significantly shorter hospital LOS, which resulted in significantly lower hospitalization costs. Before matching, apixaban patients were younger and healthier than warfarin patients; however, after PSM, the baseline clinical and demographic characteristics were balanced between the two cohorts. PSM controls confounders, and because the cohorts are balanced on all observable covariates, it is assumed that they are also closely balanced for unobservable characteristics. After matching, patients treated with apixaban had an estimated 1 day shorter median LOS. This shorter LOS for apixaban patients resulted in ∼$1600 per patient lower estimated index hospitalization costs, including pharmacy costs, compared to warfarin patients.
Additionally, the trend remained apparent when hospitalization costs were stratified by LOS and stroke risk. About 80% of the study population had a LOS between 0–7 days. Among patients with a LOS between 0–2 and 3–7 days, those treated with apixaban incurred, on average, $764 and $624, respectively, less during the index hospitalization compared to those treated with warfarin. Hospitalization costs increased significantly with each additional day of hospitalization. Furthermore, among patients with a CHADS2 score ≥2, those treated with apixaban incurred more than $1100 lower hospitalization costs compared to those treated with warfarin. In the study population, there was no significant difference in the 30-day re-admission rate; however, this was an early view study due to apixaban’s market entry in January 2013. After apixaban has been on the market for a longer period of time, further research needs to be conducted to compare all-cause and major bleeding-related re-admission rates.
Few studies have evaluated hospital LOS and costs among patients treated with NOACs compared to warfarin using real-world data. A similar analysis of hospitalized NVAF patients treated with apixaban or warfarin was conducted by Farr et al.Citation27 using the MarketScan Hospital Discharge Database. They found that the mean (SD) and median hospital LOS were significantly shorter in apixaban patients (4.5 [4.2] and 3 days) compared to warfarin patients (5.4 [5.0] and 4 days; p < 0.001). Previous studies have also shown that patients treated with other NOACs incur significantly lower hospitalization costs and have shorter hospital stays. In a retrospective study using the Premier database, rivaroxaban users had significantly shorter hospital LOS (0.8 days) and lower hospitalization costs, estimated to be $1284, compared to warfarin usersCitation15,Citation28. However, a sub-group analysis showed that, when patients were stratified by whether or not they were administered parenteral anticoagulants, hospital LOS was significantly shorter only among patients administered parental anticoagulants in addition to rivaroxabanCitation29. Further analysis in the Humana database showed that patients administered rivaroxaban were significantly less likely to have an AF-related hospitalization (0.40 vs 0.57, p = 0.022), lower AF-related hospitalization costs ($2872 vs $4147, p = 0.020), and shorter hospital LOS (2.1 vs 3.0, p = 0.014) compared to matched warfarin patientsCitation30,Citation31. In another retrospective claims study examining treatment-naïve, newly-diagnosed AF patients, those treated with dabigatran had an estimated 0.7 days shorter stay compared to warfarin and $2000 lower estimated hospitalization costsCitation14. The results from previous retrospective studies are similar to the hospital LOS and cost differences reported in this study.
Due to apixaban’s recent market entry, there is limited information regarding how apixaban is used in real-world clinical settings. Numerous cost-effectiveness Markov models have been developed to compare treatment costs between NOACs and warfarin. Using data from clinical trials, the models determined that NOACs produced greater quality-adjusted life expectancy than warfarin. Furthermore, apixaban provided the greatest monetary and quality-adjusted life year value compared to dabigatran, rivaroxaban, and warfarinCitation32,Citation33. Medical costs for clinical events among patients who were prescribed any NOACs were estimated to be lower compared to warfarin. Apixaban use showed the largest difference, with a $485 cost reductionCitation34. Additionally, it was determined that apixaban was the most cost-effective treatment for stroke risk reductionCitation35–37. When retrospective claims data were used to predict medical costs, patients treated with apixaban had medical cost reductionsCitation38. Most of the Markov models are based on clinical trials; therefore, results pertaining to the comparison of real-world economic outcomes in a clinical setting need further assessment.
Like any retrospective claims study, our study has several limitations. Propensity score methods do not address confounding in unobserved characteristics such as unmeasured health status and socioeconomic status. Other measures, such as AF duration, pre-index oral anticoagulation use, over-the-counter medication use, and patient health behavior could not be measured; therefore, these estimates may be biased due to residual confounding. Baseline characteristics were captured for 12 months prior to and during the index hospitalization through inpatient settings; therefore, the comorbidity indices and frequency of comorbid conditions may be under-estimated. In addition, as is the case for claims-based studies, there may be coding errors or diagnoses entered for administrative processing rather than clinical completeness.
The Premier PCD contains information from a large number of hospitals across the US; however, the results may not be generalizable to the entire US population of NVAF patients. The Premier PCD represents only hospital costs and does not represent medical care received outside a hospital facility. In addition, re-admissions recorded in the database are only those occurring at the same hospital in which the index hospitalization took place; therefore, re-admission rates may be under-estimated. Furthermore, the identification period in this study started in January 2013 at the same time as apixaban entered the market, which resulted in a smaller sample size and shorter follow-up period for apixaban patients. As an early-view analysis, the results may evolve over time.
Conclusion
Results of this early view study indicate that NVAF patients treated with apixaban had significantly shorter hospital LOS and lower index hospitalization costs compared to those treated with warfarin. These findings demonstrate that apixaban treatment can lower costs compared to the standard treatment, and may have important clinical and economic implications for hospitals, payers, and patients.
Transparency
Declaration of funding
Study funding provided by Pfizer Inc. and Bristol-Myers Squibb Company.
Declaration of financial/other relationships
JM, WT, and JT are employees of Pfizer Inc. KP was an employee of Pfizer Inc. at the time of the study. LV and PS are employees of Bristol-Myers Squibb Company. AB and JK were employees of Bristol-Myers Squibb Company at the time of the study. LX, AK, and OB are employees of STATinMED Research, which received funding from Pfizer Inc. and Bristol-Myers Squibb Company to conduct this study and develop this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Material.docx
Download MS Word (13.8 KB)Acknowledgments
Statistical programming for this study was provided by Juan Du, MS, of STATinMED Research and funded by Pfizer Inc. and Bristol-Myers Squibb Company.
References
- Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):429S-56S
- Marinigh R, Lip GY, Fiotti N, et al. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol 2010;56:827-37
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-7
- Limone BL, Baker WL, Kluger J, et al. Novel anticoagulants for stroke prevention in atrial fibrillation: a systematic review of cost-effectiveness models. PLoS One 2013;8:e62183
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. JACC 2014;64:e1-76
- Levy S, Maarek M, Coumel P, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. Circulation 1999;99:3028-35
- HalperinJL, HuismanM, DienerHC, et al. Patterns of newly detected atrial fibrillation and antithrombotic treatment in North America (GLORIA™-AF Phase II). American College of Cardiology 64th Annual Scientific Session in San Diego, CA, March 16, 2015
- Harburger JM, Aronow WS. Newer anticoagulants for non-valvular atrial fibrillation. Pharmaceuticals (Basel) 2012;5:469-80
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449-57. doi:10.1001/archinte.1994.00420130036007.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5
- De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;110:1087-107
- Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625-51
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Fonseca E, Walker DR, Hess GP. Abstract 258: Dabigatran etexilate is associated with shorter hospital length of stay and lower hospital costs compared to warfarin in treatment-naive, newly-diagnosed non-valvular atrial fibrillation patients. Circ Cardiovasc Qual Outcomes 2013;6:A258
- Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay: is rivaroxaban associated with shorter inpatient stay compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:645-53
- Farr A, Jung Y, Johnston S. Comparison of hospital length of stay between non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Chicago, IL: American Heart Association, 2014. pp 15-19
- Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med 2014;127:1075-82
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-429
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983a;70:41-55
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-8
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-matched samples. Stat Med 2009;28:3083-107
- Farr AM, Jing Y, Johnston S, et al. Comparison of hospital length of stay between hospitalized non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Hosp Pract 2015;43:172-9
- Laliberté F, Pilon D, Raut MK, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:1521-8
- Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay of nonvalvular atrial fibrillation patients who were administered rivaroxaban versus warfarin with and without pretreatment parenteral anticoagulant therapies. Hosp Pract 2014;42:17-25
- Laliberté F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on hospitalization days and other health care resource utilization in patients with non-valvular atrial fibrillation patients: an observational study from a cohort of matched users. Clin Ther 2015;37:554-62
- Laliberté F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among non-valvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther 2015;32:216-27
- Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health 2013;16:498-506
- Canestaro WJ, Patrick AR, Avorn J, et al. Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2013;6:724-31
- Deitelzweig S, Amin A, Jing Y, et al. Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ 2012;15:776-85
- Harrington AR, Armstrong EP, Nolan PE, Jr., et al. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676-81
- Lip GY, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210
- Wisløff T, Hagen G, Klemp M. Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation. Pharmacoeconomics 2014;32:601-12
- Amin A, Stokes M, Wu N, et al. Estimated medical cost reductions associated with apixaban in real-world patients with non-valvular atrial fibrillation. J Med Econ 2013;16:1193-202