Abstract
Objective: To calculate costs per median overall survival (OS) month in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone acetate plus prednisone (AA + P) or enzalutamide. Methods: Median treatment duration and median OS data from published Phase 3 clinical trials and prescribing information were used to calculate costs per median OS month based on wholesale acquisition costs (WACs) for patients with mCRPC treated with AA + P or enzalutamide. Sensitivity analyses were performed to understand how variations in treatment duration and treatment-related monitoring recommendations influenced cost per median OS month. Cost-effectiveness estimates of other Phase 3 trial outcomes were also explored: cost per month of chemotherapy avoided and per median radiographic progression-free survival (rPFS) month. Results: The results demonstrated that AA + P has a lower cost per median OS month than enzalutamide ($3231 vs 4512; 28% reduction), based on the following assumptions: median treatment duration of 14 months for AA + P and 18 months for enzalutamide, median OS of 34.7 months for AA + P and 35.3 months for enzalutamide, and WAC per 30-day supply of $8007.17 for AA + P vs $8847.98 for enzalutamide. Sensitivity analyses showed that accounting for recommended treatment-related monitoring costs or assuming identical treatment durations for AA + P and enzalutamide (18 months) resulted in costs per median OS month 8–27% lower for AA + P than for enzalutamide. Costs per month of chemotherapy avoided were $4448 for AA + P and $5688 for enzalutamide, while costs per month to achieve median rPFS were $6794 for AA + P and $7963 for enzalutamide. Conclusions: This cost-effectiveness analysis demonstrated that costs per median OS month, along with costs of other Phase 3 trial outcomes, were lower for AA + P than for enzalutamide. The findings were robust to sensitivity analyses. These results have important implications for population health decision-makers evaluating the relative value of therapies for mCRPC patients.
Introduction
Prostate cancer is the most commonly diagnosed non-skin cancer among men. While important advances have recently been made in the treatment of prostate cancer, in 2014 nearly 30 000 prostate-cancer patient deaths were recorded in the USCitation1. Prostate cancer is the third most costly cancer in the US, with estimated expenditures of $13.4 billion in 2014Citation2. Historically, few treatment options have been available for patients who progress to metastatic castration-resistant prostate cancer (mCRPC). Documented survival with mCRPC has been short, even with docetaxel plus prednisone (every 3 weeks) that demonstrated an overall survival benefit of 2.4 months (median overall survival: 18.9 vs 16.5) compared to mitoxantrone plus prednisone every 3 weeksCitation3.
More recently, several novel therapies, including oral agents, have become available to fill this unmet need for effective mCRPC treatment. In recent studies, Sipuleucel-T, an immunotherapy, showed a survival advantage compared to placebo, but did not delay disease progressionCitation4. Cabazitaxel, a chemotherapeutic agent, improved overall survival compared to mitoxantrone in patients with mCRPC previously treated with docetaxelCitation5. Abiraterone acetate, an androgen biosynthesis inhibitor inhibiting the activity of the enzyme CYP17, was initially approved by the FDA in April 2011 for mCRPC patients who had received prior chemotherapy with docetaxel and received an expanded indication in December 2012 that included chemotherapy-naïve mCRPC patients. Abiraterone acetate plus prednisone demonstrated a survival benefit in both post-chemotherapy and chemotherapy-naïve patients with mCRPC, when compared to prednisone aloneCitation6,Citation7. Similarly, enzalutamide, an androgen receptor inhibitor, was initially approved by the FDA in August 2012 for mCRPC patients who previously received docetaxel and was granted an expanded indication that included chemotherapy-naïve patients in September 2013. In phase 3 studies of post-docetaxel and docetaxel-naïve mCRPC patients, enzalutamide prolonged survival compared to placeboCitation8,Citation9.
Despite the availability of these newer non-cytotoxic treatment options, their optimal usage, sequencing, or combinations have not been established, as the magnitude of their survival benefit or their cost-effectiveness has not been directly compared in head-to-head phase 3 studies of either chemotherapy-experienced or chemotherapy-naïve populations. In the absence of direct head-to-head trials of any of these agents, several indirect comparisons of clinical effectiveness and cost-effectiveness of abiraterone acetate and enzalutamide have been reported based on clinical trial data in patients with mCRPC who had received previous chemotherapy. Such studies have reported that, while the two agents were similar in terms of survival benefit, abiraterone acetate was less costly than enzalutamide in this patient populationCitation10–12. However, to the best of our knowledge, no study evaluating both cost and efficacy considerations has been conducted to date in patients who have not undergone docetaxel-based chemotherapy.
To fulfill this gap in the literature and to better understand the cost-effectiveness of the two latest non-cytotoxic agents in chemotherapy-naïve mCRPC from the perspective of population health decision-makers, we calculated the cost per median overall survival (OS) month for treatment with abiraterone acetate plus prednisone or enzalutamide in asymptomatic/mildly symptomatic patients with mCRPC who have not received prior chemotherapy.
Methods
Data sources
Clinical data for this study design, in particular, median treatment duration and median overall survival, were obtained from final analyses of randomized, controlled Phase 3 trials of abiraterone acetate plus prednisone vs prednisone (the COU-AA-302 study) and enzalutamide vs placebo (the PREVAIL study), as reported in published literatureCitation7,Citation9,Citation13,Citation14 or, if unavailable, in the product’s prescribing informationCitation15,Citation16. The study designs for these two trials were generally similar with respect to their inclusion and exclusion criteria, as well as disease progression criteria, as summarized in . The baseline demographic and clinical characteristics of patients included in these trials were also generally similar (). In both studies, the co-primary end-points were overall survival and radiographic progression-free survival. Overall survival was used as the basis of the cost-effectiveness comparison for this analysis. Overall survival was defined identically in both trials as the time from randomization to death due to any cause.
Table 1. Study-design criteria for studies COU-AA-302 and PREVAIL.
Table 2. Baseline characteristics of COU-AA-302 and PREVAIL populations.
This study utilizes data from the final analysis results obtained in the COU-AA-302 (abiraterone acetate plus prednizone vs placebo plus prednisone) or PREVAIL (enzalutamide vs placebo) and from a previously published study as inputs for the calculations on cost per median OS month and sensitivity analysesCitation14,Citation16,Citation17.
The current prescribing information for abiraterone acetate plus prednisone (May 2015) reports Phase 3 trial median overall survival data based upon the final analysis of study COU-AA-302, which captured a median of 49 months of patient follow-upCitation14,Citation15. The current prescribing information for enzalutamide (October 2015) reports Phase 3 trial median overall survival data based upon the final analysis of the PREVAIL study collected over a median of 31 months of patient follow-upCitation16.
Drug treatment costs were based on wholesale acquisition costs (WAC), which are manufacturers’ published list prices, and were obtained from Analysource/First Databank. WAC pricing does not reflect any discounts, price concessions, or chargebacks extended to wholesalers or other end users and are not intended to represent an actual sales price to customersCitation18. The WACs obtained were for a 30-day supply and were effective on 6 May 2015 for abiraterone acetate, on 2 April 2015 for enzalutamide, and on 12 July 2013 for prednisone.
Statistical analyses
Both the COU-AA-302 and PREVAIL Phase 3 trials demonstrated an overall reduction in the risk of death as compared to the comparator based upon hazard ratios generated with Cox proportional hazards models. Hazard ratios indicate that the risk of achieving a pre-defined endpoint is different in a treatment as compared to a control group, but may not provide meaningful information about the magnitude of the effect (i.e. the amount of time required for equivalent proportions of patients to achieve the endpoint)Citation19. Comparison of hazard ratios between these studies is not possible because different comparators were utilized in the two trials. Instead, as mentioned by Spruance et al.Citation20, time-to-event curves provide time-based parameters that can be used for indirect comparison of the active arms in the two trials. This analysis, therefore, uses median overall survival, that is, the time at which 50% of the treated patients remain alive, as a common time-based parameter in studies COU-AA-302 and PREVAIL.
The cost per median OS month was calculated by dividing the total treatment cost by the number of months to achieve median OS for each treatment:
Drug treatment costs were calculated as the median treatment duration (in months) multiplied by the WAC for a 30-day supply:
A sensitivity analysis was performed to evaluate the impact on cost per median overall survival month if the treatment duration for abiraterone acetate plus prednisone was adjusted to be equivalent to that reported for enzalutamide, without assuming changes to median overall survival time.
Another sensitivity analysis evaluated the cost per median overall survival month when treatment-related monitoring costs were included in addition to drug WAC. Treatment-related monitoring costs were based on recommendations in the prescribing information for abiraterone acetate, specifically: monitoring patients for hypertension, hypokalemia, and fluid retention at the start of treatment and at least monthly during treatment; monitoring for signs and symptoms of adrenocortical insufficiency; and measuring serum transaminases (ALT and AST) and bilirubin at the start of treatment, every 2 weeks for the first 3 months after starting treatment, and monthly thereafterCitation15. The prescribing information for enzalutamide recommends to monitor the International Normalized Ratio in patients who may be treated concomitantly with warfarin, but it was not considered in this analysis, because the information on the proportion of patients with concomitant use of warfarin and enzalutamide was not publicly available (although not yet published work from the authors, using retrospective healthcare claims from September 2012–July 2014, showed that 12% of patients treated with enzalutamide were treated with warfarin during the 6 months prior to enzalutamide initiation, which may lead to potential drug interaction upon enzalutamide initiation)Citation16,Citation21. Treatment-related monitoring costs were calculated for the median treatment duration using average paid costs for monitoring services observed in 2013 in a large healthcare claims data set (Truven Commercial Claims and Encounters and Medicare Supplemental Dataset identified through the Truven Treatment Pathways tool). In this sensitivity analysis, the cost per median overall survival month was calculated as follows:
The treatment cost per median radiographic progression-free survival (rPFS) month was also estimated as a further sensitivity analysis. The rPFS was the co-primary end-point in COU-AA-302 and PREVAIL and both studies defined rPFS as absence of death by any cause or absence of radiographic progression according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria for soft tissue imaging and to the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria for bone scans.Citation16,Citation7 The cost per month of treatment to median rPFS was evaluated as follows:
Finally, a sensitivity analysis was performed to estimate the cost per month of chemotherapy avoided, calculated as follows using the median time to chemotherapy:
The model inputs for the calculation of cost per median overall survival month, cost per median rPFS month and cost per month of chemotherapy avoided are summarized in ; assumptions for the calculation of recommended treatment-related monitoring costs are summarized in .
Table 3. Inputs for drug treatment cost calculation.
Table 4. Treatment-related monitoring cost assumptions.Table Footnotea
Results
At the final analysis of the COU-AA-302 trial, 741 deaths had occurred. Median overall survival for abiraterone acetate plus prednisone was 34.7 months vs 30.3 months for prednisone, a net survival improvement of 4.4 months (hazard ratio [95% confidence interval] = 0.81 [0.70–0.93]; p = 0.0033)Citation14. Median treatment duration for the abiraterone acetate plus prednisone arm was 13.8 months (rounded up to 14 months to align with a payer perspective) and the overall median follow-up time was 49.2 monthsCitation14.
At the final analysis for enzalutamide (PREVAIL), 784 deaths had occurred. Median overall survival for enzalutamide was 35.3 months vs 31.3 months for placebo, a net survival improvement of 4.0 months (hazard ratio [95% confidence interval] = 0.77 [0.67–0.88]; p = not specified)Citation16. Median treatment duration for the enzalutamide arm was 17.5 months (rounded up to 18 months), and the overall median follow-up time was 31 monthsCitation16.
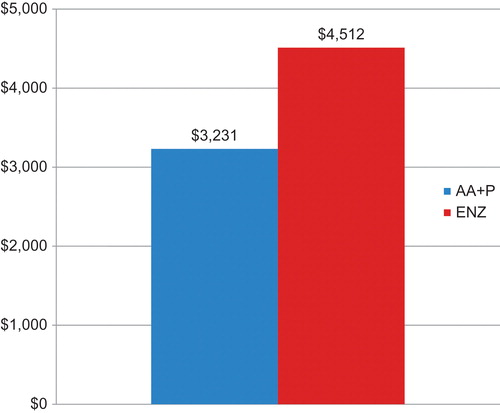
Based on the model inputs from the final analyses of both trials, the estimated total treatment cost was $112,100 for abiraterone acetate plus prednisone and $159,264 for enzalutamide. The cost per median overall survival month at the final analysis was $3231 for abiraterone acetate plus prednisone and $4512 for enzalutamide (). These results suggest that the cost per median overall survival month is ∼28% lower for abiraterone acetate plus prednisone than for enzalutamide.
Figure 1. Estimated cost per median overall survival month in chemotherapy-naïve mCRPC patients with mCRPC treated with abiraterone acetate plus prednisone or enzalutamide. AA + P, Abiraterone acetate plus prednisone; ENZ, enzalutamide.
The results of the sensitivity analyses using the overall survival end-point are summarized in .
Figure 2. Sensitivity analyses of estimated cost per median overall survival month in chemotherapy-naïve patients with mCRPC treated with abiraterone acetate plus prednisone or enzalutamide. AA + P, Abiraterone acetate plus prednisone; ENZ, enzalutamide.
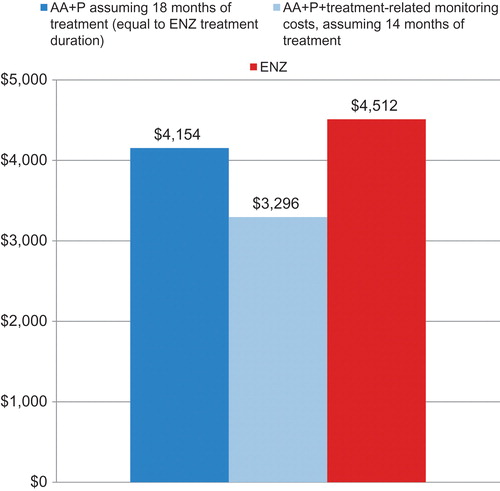
The first sensitivity analysis assessed the impact of median treatment duration on cost per median OS month. When median treatment duration for abiraterone acetate plus prednisone was assumed to be equivalent to that observed for enzalutamide (18 months), the total cost for abiraterone acetate plus prednisone increased to $144,129. The resulting cost per median overall survival month was estimated as $4154, an increase of $923 per median overall survival month compared to the base model, yet still lower by 8% for abiraterone acetate plus prednisone than for enzalutamide ($4512 per month of overall survival for patients treated with enzalutamide).
The second sensitivity analysis evaluated the addition of treatment-related monitoring costs on the total cost per OS month. Treatment-related monitoring costs for abiraterone acetate plus prednisone over the 14 month treatment duration totaled $2274, bringing total drug plus monitoring costs to $114,375 and cost per median overall survival month to $3296, or an increase of $66 per median overall survival month compared to the base model, and still lower by 27% for abiraterone acetate plus prednisone than for enzalutamide.
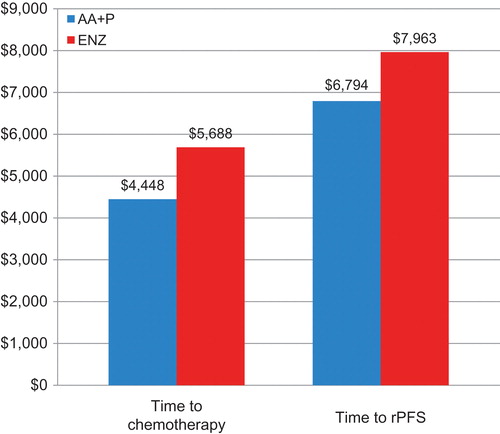
displays the results of the sensitivity analyses performed chemotherapy avoidance and rPFS. The cost per median month of chemotherapy avoided was $4448 for abiraterone acetate plus prednisone, and $5688 for enzalutamide, or a $1240 (22%) reduction per month of chemotherapy avoided for abiraterone acetate patients as compared to enzalutamide patients. The cost per median rPFS month was $6794 for treatment with abiraterone acetate plus prednisone and $7963 for enzalutamide. This translates into a $1169 (15%) lower cost per month of rPFS for abiraterone acetate patients as compared to enzalutamide patients.
Figure 3. Sensitivity analyses of estimated cost per median month of chemotherapy avoided and per median radiographic progression-free survival month in chemotherapy-naïve patients with mCRPC treated with abiraterone acetate plus prednisone or enzalutamide. AA + P, Abiraterone acetate plus prednisone; ENZ, enzalutamide; rPFS, radiographic progression-free survival.
Discussion
To the best of our knowledge, this is one of the first studies to estimate the cost of expected clinical benefit for patients with mCRPC treated with abiraterone acetate plus prednisone or enzalutamide who had not received prior chemotherapy. This economic evaluation of cost per median OS month was based upon inputs of treatment duration and OS time as found in publications of Phase 3 trials and prescribing information of the products. Treatment with abiraterone acetate plus prednisone was observed to result in lower cost per median overall survival month than treatment with enzalutamide. This result was robust to the assumption of equivalent treatment durations for abiraterone acetate plus prednisone and enzalutamide and to the inclusion of estimated treatment-related monitoring costs recommended by the prescribing information for abiraterone acetate plus prednisone. Furthermore, the study also demonstrated higher cost of treatment with enzalutamide compared to abiraterone acetate plus prednisone per month of chemotherapy avoided and per month of median rPFS.
The conclusions derived from this analysis are consistent with previously reported results of cost comparisons of abiraterone acetate and enzalutamide in populations of patients with mCRPC previously treated with chemotherapy. In particular, an indirect cost-effectiveness analysis found that, in this population, treatment with abiraterone acetate provided substantial cost savings compared to treatment with enzalutamide, primarily due to lower drug acquisition costsCitation11. Similarly, a cost-effectiveness analysis of the pivotal studies involving abiraterone acetate plus prednisone, enzalutamide, and cabazitaxel in docetaxel-treated patients with mCRPC demonstrated that treatment with abiraterone acetate plus prednisone was the most cost-effective when compared to the other two active treatments, with estimated costs of abiraterone acetate treatment estimated at $123.4K/quality-adjusted life year compared to placebo, while enzalutamide and cabazitaxel treatments were, respectively, estimated at $154.3K/quality-adjusted life year and $163.2K/quality-adjusted life year compared to placeboCitation22. Furthermore, a model of expected pharmacy costs, in which treatment duration, adherence, and the number of treated patients were held constant, estimated that treatment with abiraterone acetate plus prednisone was less costly than treatment with enzalutamide in patients with mCRPC previously treated with docetaxelCitation12.
Recent studies also reported results of cost comparisons of abiraterone acetate plus prednisone and enzalutamide in populations of patients with mCRPC including patients previously treated with chemotherapy and patients not previously treated with chemotherapy. Using a payor perspective, a large retrospective study of two databases demonstrated that a majority of patients were treated with abiraterone acetate plus prednisone and that they had significantly lower monthly pharmacy costs than patients treated with enzalutamideCitation23. Moreover, a budgetary impact analysis of abiraterone acetate plus prednisone vs enzalutamide for the treatment of patients with mCRPC reported that pharmacy spending increased when first-line enzalutamide replaced first-line abiraterone acetate plus prednisoneCitation24.
Finally, a recent study from Bui et al.Citation25 that compared, from a payor perspective, the cost of treatment with enzalutamide to the cost of other mCRPC treatments in chemotherapy-naïve mCRPC patients also found a higher total and monthly cost of treatment with enzalutamide as compared to treatment with abiraterone acetate. Yet, Bui et al.Citation25 included in their study adverse event costs that were estimated to be slightly higher for abiraterone acetate patients ($198.68/month) than for enzalutamide patients ($162.09/month) without providing sufficient details about how the inputs and assumptions drive the adverse event cost estimations. Of note, addition of the adverse event costs noted by Bui et al.Citation25 would not change the conclusions of the present analysis. When monitoring cost assumptions used in the present study and the adverse event costs estimated by Bui et al.Citation25 in their study are considered, the cost per OS month for abiraterone plus prednisone is $1218 (27%) lower than for patients treated with enzalutamide.
This study has a number of limitations. First, because no prospective, randomized, controlled, head-to-head trial data comparing abiraterone acetate plus prednisone and enzalutamide exist, this evaluation was based on accepted methodology for indirect comparisonCitation26 using the median overall survival observed in the active treatment arms of the COU-AA-302 and PREVAIL Phase 3 trials, at the final analysis.
Furthermore, the model assumptions were derived from data in the prescribing information of the two products and publications based on Phase 3 trial data final analyses. While the Phase 3 trial designs were generally similar with respect to their inclusion and exclusion criteria, and the included patient populations had generally similar baseline characteristics, no attempt was made in this study to adjust the model assumptions for any differences in these components, or for follow-up time, events occurring after discontinuation of the active treatment (except radiographic progression and chemotherapy treatment that were used in sensitivity analyses), or magnitude of the placebo response. This may be a particularly important limitation, since overall survival of patients could have been influenced by treatments received after discontinuation of the study drug. Most notably, since the PREVAIL trial was performed later than the COU-AA-302 trial, PREVAIL patients had more available options than COU-AA-302 patients after discontinuation. Hence, in PREVAIL, at the interim analysis, 21% of enzalutamide-treated patients received abiraterone as a subsequent anti-neoplastic therapyCitation13,Citation14; whereas none of the abiraterone acetate plus prednisone-treated patients in COU-AA-302 received enzalutamide as a subsequent anti-neoplastic agent. No adjustment has been made to these study results to account for this difference in subsequent therapy. However, the sensitivity analyses that were conducted to estimate the treatment cost per median month of chemotherapy avoided and per median month of rPFS found higher costs for enzalutamide than for abiraterone acetate plus prednisone in both cases, whereas these two end-points may have been less impacted by subsequent events and treatments.
In addition, the base model reflected drug acquisition costs (i.e. WAC) only. Cost considerations were limited to the costs of the active comparator based on each clinical trial. Nevertheless, a sensitivity analysis accounting for the costs of recommended treatment-related monitoring in patients treated with abiraterone acetate was conducted and yielded similar conclusions. Costs associated with ancillary pharmaceuticals, clinic visits, hospitalizations, physician visits, laboratory evaluation, or subsequent therapies were not included. The model also did not consider the impact of other pricing conventions (e.g. average sales price, average wholesale price), nor did it account for contracting or pricing discounts or for different contract-based pricing conventions that may be used by commercial or Medicare or other government payors.
Finally, this analysis did not account for real-world differences that may exist in patient characteristics, treatment durations, or utilization of these products (e.g. longer or shorter treatment durations), as compared to observations in Phase 3 trials.
Conclusions
Based upon Phase 3 clinical trial results in asymptomatic or mildly symptomatic chemotherapy-naïve patients with mCRPC, this cost-effectiveness analysis demonstrated that the cost per median overall survival month was lower for treatment with abiraterone acetate plus prednisone than for treatment with enzalutamide. Consistent results were obtained even when equivalent duration of treatment was assumed and when estimated recommended treatment-related monitoring costs for abiraterone acetate plus prednisone were included. The costs per median month of chemotherapy avoided and per median radiographic progression-free survival month were also found to be lower for treatment with abiraterone acetate plus prednisone than for treatment with enzalutamide. These results have important implications for population health decision-makers assessing the relative value of new therapies for this patient population.
Transparency
Declaration of funding
The research was funded by Janssen Scientific Affairs, LLC, in Horsham, PA.
Declaration of financial/other relationship
Two of the authors (Pilon and Lefebvre) are employees of Analysis Group, Inc., a consulting company that has received research grants from Janssen Scientific Affairs. Two of the authors (Ellis and Queener) are employees of Janssen Scientific Affairs.
Acknowledgment
Writing assistance was provided by Lucia Antràs, PhD, of Analysis Group, Inc.
References
- American Cancer Society. Cancer Facts & Figures 2014. Georgia, US: American Cancer Society, 2014 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/index. Accessed April 5, 2016
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the U.S.: 2010-2020. J Natl Cancer Inst 2011;103:117-28
- Tannock IF, de Wit R, Berry WR, et al; for the TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet 2010;376:1147-54
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;365:1995-2005
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368:138-48
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33
- Chopra A, Haaland B, Lopes G. Comparative effective analysis between enzalutamide and abiraterone in the treatment of metastatic castration-resistant prostate cancer [abstract]. J Clin Oncol 2013;31(6 Suppl):abstr 217
- Li T, Thompson M, Todd MB, et al. An indirect treatment comparison (ITC) and cost-effectiveness analysis of abiraterone acetate and enzalutamide for the treatment of metastatic castration-resistant prostate cancer (mCRPC) post-chemotherapy [abstract]. J Clin Oncol 2014;32(4 Suppl):abstr 270
- Ellis LA, McKenzie RS, Senbetta M. Cost considerations for new oral therapies for patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with docetaxel. Poster presented at the 24th International Prostate Cancer Update; February 19-22, 2014; Vail, CO
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66:815-25
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152-60
- ZYTIGA® [package insert]. Hornsham, PA: Janssen Biotech, Inc., 2015
- XTANDI® [package insert]. Northbrook, IL: Astellas Pharma US, Inc., 2015
- Beer TM, Sternberg CN, Higano CS, et al. Enzalutamide (ENZA) in men with chemotherapy-Naïve metastatic castration-resistant prostate cancer (mCRPC): final analysis of the phase 3 PREVAIL study. Poster presented at the 2015 ASCO Annual Meeting, Chicago, IL
- First DataBank. Drug Pricing Policy. California, US: First Databank, 2015 http://www.fdbhealth.com/policies/drug-pricing-policy/. Accessed February 13, 2015
- Uno H, Wittes J, Fu H, et al. Alternatives to hazard ratios for comparing the efficacy or safety of therapies in noninferiority studies. Ann Intern Med 2015;163:127-34
- Spruance SL, Reid JE, Grace M, et al. Hazard ratio in clinical trials. Antimicrob Agents Chemother 2004;48:2787-92
- Pilon D, Ellis L, Xiao Y, et al. Consideration of concomitant medication use in selection of FDA-approved drugs indicated for prostate cancer, Under revision for publication
- Wilson L, Tang J, Zhong L, et al. New therapeutic options in metastatic castration-resistant prostate cancer: Can cost-effectiveness analysis help in treatment decisions? J Oncol Pharm Practice 2014;20:417-25
- Ellis LA, Lafeuille M-H, Gozalo L, et al. Treatment sequences and pharmacy costs observed for abiraterone and enzalutmide: considerations for U.S. Payers. Poster presented at the 2015 AMCP’s 27th Annual Meeting & Expo, San Diego, CA
- Ellis LA, Pilon D, Lefebvre P. (Budgetary impact analysis of abiraterone acetate plus prednisone (AA + P) versus enzalutamide (ENZ) for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). Poster presented at the 2015 AVBCC 5th Annual Conference, Washington, DC
- Bui C, O’Day K, Flanders S, et al. Budget impact analysis of enzalutamide for the treatment of metastatic castration-resistant prostate cancer from a US payer perspective. Poster presented at the 2015 ISPOR 20th Annual International Meeting, Philadelphia, PA
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
