ABSTRACT
The first reports that it is possible to emit dioxins from the heat and power generation sector are from the beginning of the 1980s. Detailed research proved that the emission of dioxins might occur during combustion of hard coal, brown coal, and furnace oil as well as coke-oven gas. The emission of dioxins occurs in wood incineration; wood that is clean and understood as biomass; or, in particular, wood waste (polluted). This paper thoroughly discusses the mechanism of dioxin formation in thermal processes, first and foremost in combustion processes. The parameters influencing the quantity of dioxins formed and the dependence of their quantity on the conditions of combustion are highlighted. Furthermore, the methods of reducing dioxin emissions from combustion processes (primary and secondary) are discussed. The most efficacious methods that may find application in the heat and power generation sector are proposed; this is relevant from the point of view of the implementation of the Stockholm Convention resolutions in Poland with regard to persistent organic pollutants.
In this study, the problem of emission of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from the heat and power generation sector is discussed. It is proven that practically all combustion processes may be a source of emission of dioxins into the atmosphere. The implementation of the Stockholm Convention with reference to persistent organic pollutants will require that the measures leading to the reduction of dioxin emissions will also be taken in this sector. A critical analysis of the actually available emission reduction methods was performed and such methods that might find a practical application are highlighted.
INTRODUCTION
Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), referred to as dioxins and furans for short, are widely considered to be one of the most dangerous poisons in the environment. However, the literature data indicate that although dioxins display neither acute toxicity nor carcinogenicity in reference to human organisms, as was widely held in the 1970s, dioxins are not neutral for all living organisms, including humans. They are allocated to the group of the so-called “endocrine disrupters”—substances disturbing endocrine hormone secretion—and because of this, their emissions should be reduced.
The Stockholm Convention signed on May 23, 2001 during the international conference of government plenipotentiaries regarding persistent organic pollutants introduced the relevant reduction in the production, utilization, export, and import of persistent organic pollutants and strict requirements concerning the evidence and monitoring. One of the most important resolutions resulting from the Stockholm Convention is an obligation to the continuous minimization and the final elimination of the release of PCDDs, PCDFs, hexachlorobenzene (HCB), and polychlorinated biphenyls (PCBs). Fulfilling the commitments that result from the Stockholm Convention requires a detailed identification of the emission of sources of dioxins to the environment be performed and a detailed analysis of the existing possibilities of reduction of emissions from the main sources. In Poland, the inventory control of dioxin emissions has been carried out in a detailed way since the mid-1990s, but the reduction of emissions of PCDD/Fs requires accurate knowledge and understanding of the mechanisms of their formation and the knowledge of the existing methods and the emission reduction technologies.
Within the background of the known results of the more than 20-yr-long investigation, it is reported that dioxins (PCDDs) and furans (PCDFs) are generated as an undesirable side product in practically all combustion processes (including the processes occurring during municipal, industrial, medicinal, or sewage sludge incineration as well as during mineral fuel combustion, i.e., hard coal, brown coal, and biomass combustion) certain production industrial processes (e.g., the production of pesticides, paper, and cellulose), and in the iron and steel industry and nonferrous metallurgy. In the 1970s and 1980s, the main source of PCDD/F emissions in most countries in the world was the municipal waste incineration plants. At present, as a result of tightening of the emission standards, development of new combustion technologies, and the application of efficient systems of flue gas cleaning, the situation has changed and the contemporary incineration plants emit flue gases containing dioxins and furans at a concentration similar to that which usually occurs in the polluted air of cities. Still another important PCDD/F emission source is small medical (hospital) waste incinerators. These caused emissions of approximately 200–400 g of toxicity equivalents (TEQ) per year (∼10% of total) in Europe in 2001,Citation1 but after implementation of the Waste Incineration DirectiveCitation2 in European Union (EU) countries, the dioxin emissions from those plants rapidly dropped. The meaningful source today in Poland and many European countries is the energy and metallurgic sector, encompassing the iron and steel industry, nonferrous metallurgy, and secondary scrap processing. However, a very important element is the uncontrolled household waste incineration in household furnaces and the combustion of solid fuels of low quality (including hard and brown coal) in the small, outdated, and overexploited local boiler houses, in which the conditions of incineration and combustion are very negative from the point of view of thermodynamics and chemistry of combustion and incineration. At this moment, these are the main sources of dioxin emissions into the air in most European countries, including Poland.Citation3
SOURCES OF DIOXIN EMISSIONS
The first information about dioxins originating from the process of combustion appeared in 1977–1978. OlieCitation4 found dioxins in flue gases coming from Dutch waste incineration plants. BuserCitation5 also found dioxins in volatile ashes from Swiss incineration plants. The theory then appeared that PCDDs and PCDFs had their sources in combustion processes—it was referred to as “trace chemistry of fire” from the title of an article that was published in 1980 in the respected and prestigious magazine, Science.Citation6 In accordance with this theory, the basic sources of dioxin emissions to the environment, especially to the atmosphere, may be as follows: waste incineration plants, power plants heated with mineral fuels, car engines (petrol and diesel), fireplaces, grills, and cigarettes.
Summarizing in 1992 the first decade of intensive research on dioxin formation, RappeCitation7 concluded that the environmental pollution by dioxins may have resulted from emissions from the following sources:
| • | Chemical reactions: This is the result of side reactions occurring in chemical synthesis processes; for instance, the production of chlorinated pesticides, chlorophenoxy herbicides, chlorophenols, and PCBs. Another important source is the production and industrial utilization of chlorine (e.g., polyvinyl chloride [PVC]); in particular, electrolysis combined with the production of chlorine, whitening of cellulose pulp with chlorine, etc. | ||||
| • | Thermal processes: This concerns the processes of municipal, medicinal, and chemical waste incineration and the production of iron, steel, magnesium, and nickel. Exhaust gases are also sources of emissions of dioxins to the environment. | ||||
| • | Photochemical reactions: These may include the photochemical dechlorination of higher chlorinated dioxins, the formation of lower chlorinated, more toxic congeners, photochemical cyclization of o-phenoxy-phenols, or the photochemical dimerization of chlorophenols. | ||||
| • | Enzymatic reactions: This is a process of generation of PCDD/Fs from chlorophenols under the influence of peroxidases, which may occur in the natural conditions of sewer system, in sewage sludge, or in lake and river bottoms. | ||||
In 2000, BakerCitation8 asked himself a fundamental question whether combustion is a main source of dioxin emissions to the environment. He formulated a thesis that dioxin emissions are an immanent feature of economical growth and the quantity of dioxin emissions in a given country depends mainly on the gross domestic product and is proportional to the emissions of carbon dioxide (CO2). He illustrated his considerations with data concerning the quantity of the gross domestic product and CO2 and dioxin emissions from several European countries and the United States (of a various degree of the economical growth), obtaining a satisfactory correlation between the aforementioned quantities.
Today, in light of the available rich subject literature, one may recognize that the main sources of dioxin emissions to the environment are first and foremost thermal processes, including combustion and other (e.g., metal-lurgic) processes.
MECHANISM OF DIOXIN FORMATION IN THERMAL PROCESSES
Understanding of the mechanism of dioxin generation in thermal processes requires an understanding of the chemistry of combustion and the mechanisms pollutant generation in combustion processes. The combustion of gaseous fuels is the simplest case of combustion in which a simple oxidation reaction of hydrocarbons occurs in agreement with the simplified scheme of the reaction, which is as follows:
The basic products of the oxidation reaction are CO2 and water. In the case of insufficient aeration of the combustion zone, carbon monoxide (CO) is generated as a product of the reaction. Flammable gas usually mixes well with the air, which contributes to the fact that combustion is of a kinetic nature (i.e., it is controlled by the rate of the chemical reaction of oxidation). In the occasional situations in which the flammable gas is not mixed with the air, the process may be controlled by the phenomenon of oxygen diffusion into the reaction zone. Although the reaction zone is not sufficiently aerated, CO and elemental carbon (EC; soot) become the products of the combustion process because the rate of the water generation reaction is higher than the rate of carbon oxidation. The combustion of liquid fuels is a more complex process because if the reaction of oxidation (combustion) is to occur, it is then necessary to evaporate the liquid because its vapors are prone to combustion. Liquid spraying contributes to evaporation; however, it is very difficult to obtain a homogenous mixture of liquid vapors and the air. Hence, the combustion of liquid fuels rarely has a kinetic character because this is a diffusive combustion. Organic liquids usually have a more complex chemical structure than gases; that is why one deals more frequently with the generation of not fully combusted products, among which there are also other organic compounds,
In an extreme case, a situation may occur in which a full decomposition of organic compounds contained in fuel may not take place because of deficiency of the air originating from the combustion process, and the whole carbon will not be subjected to oxidation. In consequence, apart from gaseous products, a solid product (i.e., an EC, or soot) will form as a result of combustion:
The process of solid fuel combustion occurs in a more complex way. This is a multidirectional and multistage process—the combination of the processes of combustion (oxidation), gasification, and thermal decomposition (including pyrolysis—an anaerobic process). Depending on the temperature, one may distinguish several stages in the process of solid fuel combustionCitation9:
| • | 100–200 °C—The process of thermal drying and water separation (a physical process). | ||||
| • | 250 °C—The processes of reductive deoxidation, reduction, decomposition of esters of sulfuric acid, separation of bound moisture and CO2, depolymerization, and the onset of hydrogen sulfide release. | ||||
| • | 340 °C—Generation of aliphatic compounds (including unsaturated ones) and the onset of the release of methane and other aliphatic compounds. | ||||
| • | 380 °C—Carbonification in the process of low-temperature carbonization. | ||||
| • | 400 °C—The onset of production of carbon compounds with oxygen and nitrogen. | ||||
| • | 400–600 °C—The change of bithumic substances into low-temperature carbonization oil or low-temperature carbonization tar. | ||||
| • | 600 °C—Cracking of bithumic substances in the direction of thermally durable substances (gases, hydrocarbons of short-chain structure) and generation of new substances (benzene derivatives as a result of cyclization of unsaturated aliphatic compounds). | ||||
| • | >600 °C—A further course of the hypothetical dimerization reaction of butylene synthesis, dehydration to butadiene, formation of cyclohexane, and thermal aromatization to benzene and other higher aromatic compounds. | ||||
The process of combustion of such a nonhomogenous material (as waste undoubtedly is and irrespective of whether this is municipal, industrial, or medicinal waste or sewage sludge) is the source of emission of many chemical compounds, among which there are often toxic, carcinogenic substances. The main part of waste is organic matter; therefore, the emission of CO2 and water vapor (as well as CO in the case of incomplete combustion) is obvious. The presence of substances containing in a molecule other elements (such as sulfur, nitrogen, chlorine, or fluoride [apart from carbon and hydrogen]) in waste will result in dust emissions. On the other hand, the presence of noncombustible substances (so-called “ash-forming matter”) in the combusted material will result in the emission of dust. The mechanism of sulfur dioxide or nitrogen oxide formation is very well known and has been described many times in publications concerning the energetic combustion of mineral fuels. Analogously, the mechanism of hydrochloride or hydrogen fluoride has been thoroughly studied.
Nonetheless, one must be aware that the real process of combustion is far from ideal (i.e., complete and total combustion). The combustion process (thermal decomposition and oxidation) of many organic compounds (particularly those contained in waste) does not occur in an ideal way; that is, with the generation of only CO2, CO, and water. Many intermediate products of decomposition and oxidation not prone to further decomposition usually form in this process. Seemingly, in the drastic conditions of combustion at the temperature of approximately 1000 °C, all organic substances must be prone to combustion. Unfortunately, this is not true. Many chemical compounds (frequently flammable ones) are not prone to an utter destruction in the course of waste combustion. Several organic compounds form as a result of many secondary reactions occurring in the combustion zone and behind this zone. The simplified scheme of pollutant generation in the combustion processes is shown in
Figure 1. A simplified scheme of pollutant generation in the process of flammable substance combustion or incineration (fuel, waste, etc.).
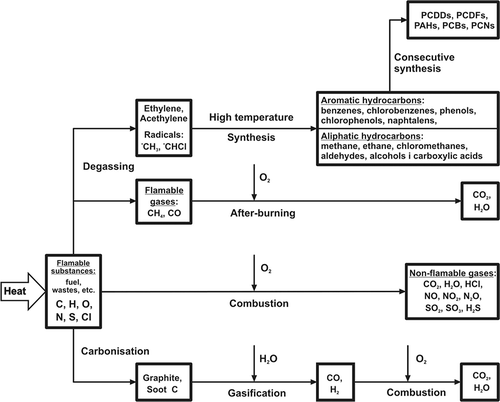
The effect of this phenomenon is the presence of the following pollutants in flue gases from waste incineration plantsCitation10:
| • | Hydrocarbons C1 and C2 | ||||
| • | Acrylonitrile, acetonitrile | ||||
| • | Benzene, toluene, ethyl benzene, xylene | ||||
| • | 1,2-Dichlorobenzene, 1,4-dichlorobenzene, 1,2,4-trichlorobenzene, HCB | ||||
| • | Phenol, 2,4-dinitrophenol, 2,4-dichlorophenol, 2,4,5-trichlorophenol, pentachlorophenol | ||||
| • | Chloromethane, chloroform, methylene chloride, carbon tetrachloride (CCl4) | ||||
| • | 1,1-Dichloroethane, 1,2-dichloroethane, 1,1,1-trichloroethane, 1,1,2-trichloroethane, 1,1,2,2-tetrachloroethane | ||||
| • | 1,1-Dichloroethylene, trichloroethylene, tetrachloroethylene | ||||
| • | Formaldehyde, acetic aldehyde, acetone, methyl ethyl ketone | ||||
| • | Vinyl chloride, diethyl phthalate | ||||
| • | Formic acid, acetic acid, and many others. | ||||
Further research carried out by other scientistsCitation11–14 proved the presence of more than 350 chemical (organic) compounds of various types in flue gases from waste incineration plants in concentrations greater than 5 μg/m3.
Additionally, the presence of unsaturated organic compounds (i.e., compounds containing double or triple carbon-carbon bonds [CAC or C'C] in a molecule) in waste contributes to the fact that at the temperature of approximately 500–800 °C, the reaction of multiring aromatic hydrocarbons occurs.Citation15,Citation16 Multiring aromatic hydrocarbons belong to a category that includes some of the most dangerous substances in the environment. Many of them (e.g., benzo[a]pyrene, benzo[a]anthracene, benzo[k] fluoranthene, dibenzo[a,h]anthracene, and indeno[1,2,3-c,d]pyrene) are categorized by the International Agency of Cancer Research (IARC) as substances of proven carcinogenic activity. They are emitted to the atmosphere from practically all combustion or incineration processes and not just from waste incineration plants.Citation17
The presence in waste of many elements containing metals brings about their emission to the atmosphere from combustion processes. Those metals are contained mainly in dust and gravel. Their particular distribution in gravel, volatile ashes, dust released in the dedusting systems of waste incineration plants, and dust emitted into the atmosphere was the subject of detailed investigations.Citation18,Citation19 Certain metals such as mercury, arsenic, selenium, and partially cadmium are emitted in the form of vapors; others such as chromium, cobalt, nickel, manganese, copper, thallium, and vanadium are bound mainly in gravel or dust.Citation20
In accordance with the scheme for the synthesis of dioxins demonstrated in , of crucial importance is the stage of generation of hydrocarbon radicals, including halogenated ones (first of all chlorinated), and simple unsaturated hydrocarbons (e.g., ethylene and acetylene).
Those reactions occur at elevated temperatures, and at a subsequent stage their products are subjected to further reactions, including cyclization (i.e., formation of aromatic compounds, and frequently chlorinated ones, including chlorobenzene and chlorophenols).Citation21
PCDDs and PCDFs are generated in practically each combustion processCitation6,Citation22 of solid and liquid fuels and of gaseous fuels (however, rarely) if chlorine, oxygen, and organic matter appear in the zone of the appropriate temperatures with or without a catalyst such as copper, alumina, iron, etc. This thesis is not only valid for the combustion processes but also for most thermal processes occurring at the temperatures of 200–700 °C. The general course of the reaction is as follows:
On the basis of the critical analysis of numerous literature reports, the following methods of dioxin generation in the stream of flue gases from the combustion process may be assumed:
| • | Dioxins introduced into the system that did not participate in any chemical reactions or were subjected to the reactions of partial chlorination and/or dechlorination of a molecule.Citation23 | ||||
| • | Dioxins generated as a result of generation of aromatic rings and rearrangement of substituents with the participation of chlorinated precursors such as PVC, PCBs, chlorinated benzenes, and chlorinated phenols that occur behind the combustion zone when the reaction proceeds in a gaseous phase at 500–700 °C.Citation24 | ||||
| • | Dioxins are generated behind the combustion zone as a result of nonchlorinated reactions of organic compounds (e.g., polyaromatic hydrocarbons (PAHs), lignite, brown coal, etc.) and subsequent chlorination. Dioxins are also generated from the chlorinated organic precursors of various types (e.g., chlorophenols, chlorobenzenes, polychlorinated diphenylethers, PCBs, etc.) as well as other products of partial combustion that may react with themselves and on the surface of metals included in the composition of volatile ash particles,Citation25 which may be subjected to the reactions of chlorination and dechlorination. | ||||
| • | The synthesis of dioxins generated behind the zone of combustion from EC (the carbonificated residues of the combustion process, nonburnt carbon in the particles of dust, i.e., soot) and organic and inorganic chlorine in the presence of volatile ash at 200–400 °C is most frequently referred to as de novo synthesis.Citation26 | ||||
The basic difference in those mechanisms is the source of carbon and the temperature range at which the synthesis proceeds.Citation27 In light of the research results, it seems that the crucial mechanism from which the most dioxins are generated is the third mechanism, then the second one, whereas the fewest dioxins are generated from the first mechanism.Citation28
Analyzing the before-mentioned ways of dioxin formation in a waste gas stream from thermal processes, including the process of solid fuel combustion and, first and foremost, waste, it must be stated that the first case is relatively improbable. PCDDs and PCDFs are not chemical substances of high thermal stability. In conditions of combustion (the presence of oxygen, mixing, flow) almost all organic compounds, including dioxins, are prone to decomposition at 850 °C. According to Gullet and Seeker,Citation29 dioxins are prone to 99.9% destruction just at 700 °C, whereas the temperature of decomposition of other dioxin synthesis precursor compounds may be approximately 800 °C. On the other hand, HCB may reach approximately 930 °C. In cases of oxygen deficiency, the boundary of organic compound decomposition may increase.Citation30 Nevertheless, investigations carried out by HunsingerCitation31 proved that temperatures greater than 900 °C and oxygen deficiency bring about a complete decomposition of dioxins, which means that the dioxin synthesis may progress after the last supply of air to the combustion process and at temperatures below 900 °C. Thus, it seems unlikely that dioxins contained in the material subjected to combustion at temperatures reaching even 1000 °C could not be prone to destruction; however, in cases of bad construction of a combusting device or generation of cold zones in a combustion chamber, this possibility cannot be excluded.
As has been mentioned, at temperatures greater than 900 °C, hydrocarbon radicals, simple hydrocarbons (frequently of unsaturated character), and flammable gases subsequently participate in many complex synthesis reactions (including halogenation, oxyhalogenation, oxidation, recombination, and disproportionation) and are released from incinerated waste and other solid and liquid fuels.Citation31–35 As a result of those reactions, the products of so-called “partial combustion” are generated, in which one may include first and foremost the following substances: phenols, chlorophenols, chlorobenzenes, alkylobenzenes, simple aliphatic hydrocarbons, aldehydes, ke-tones, alcohols, and simple carboxylic acids.Citation10,Citation11,Citation13,Citation14
The key point here is the reaction described for the first time by Aubrey and van Wazer,Citation36 in which aromatic compounds are formed from aliphatic compounds at elevated temperature,
A particular role here is played by acetylene. This compound always occurs in flue gases from combustion processes and it is the precursor of the generation of many chloroaromatic compounds.Citation37 At the first stage, acetylene is subjected to chlorination to dichloroacetylene as a result of exchanging the ligands; next, it is subjected to cyclization to HCB or condensation to hexachlorobutadiene.Citation38 Further researchCitation39,Citation40 proved that chlorobenzenes, chlorophenols, and chloronaphtalenes can form from acetylene. A relevant role of acetylene in the formation of dioxin precursors in combustion processes is underlined by Cieplik.Citation41
Nonetheless, a key factor in generating dioxins from synthesis in a gaseous phase is the combustion process conditions. The investigationsCitation42 proved a very close dependence of the formation of chlorinated and nonchlorinated aromatic compounds in a combustion and afterburning chamber (at 650–900 °C) on the combustion parameters. Nonoptimal combustion conditions cause an increase in concentrations of mono-, di-, and trichloro-dibenzo-pdioxins; dibenzofurans; and PAHs, whereas the concentrations of chlorinated benzenes, PCBs, and higher chlorinated PCDD/Fs increase only slightly. In 1997, GulletCitation29 noticed that the side products of bad combustion conditions of gaseous fuel (i.e., a natural gas) may constitute a sufficient source of carbon for the synthesis of dioxins. Similar observations were also made by Froese with regard to combustion of acetylene, ethane, and ethylene.Citation43,Citation44 The mechanisms of dioxin formation in a gaseous phase were the subject of numerous examinations widely described in the literature. One of the most interesting observations is the finding that one of the paths of dioxin synthesis in a gaseous phase may run through the formation of PCBs.Citation45 Today, the most important precursors of dioxin synthesis in a gaseous phase include benzene; naphthalene; phenanthrene; acetone; trichloroethane; benzaldehyde; dibenzofuran; phenol; mono-, di-, and trichlorophenols; chlorobenzenes; tetrachloroethylene; polychlorinated naphthalenes; and polychlorinated diphenyl ethers.Citation46,Citation47 The rate of dioxin formation from different precursors varies; for instance, the rate of formation of chlorophenols is visibly greater than the rate of formation of those compounds from chlorobenzenes.Citation48 Dioxin formation precursors can also include PVC and dioxazine, phthalocyanine, and chloroanil dyes.Citation5,Citation7,Citation49,Citation50 Those precursors may interact with one another at 500–700 °C in a gaseous phaseCitation24 and on the surface of volatile ash at 200–500 °C.Citation51
Behind the combustion zone at temperatures below 500 °C, dioxins are formed as a result of a series of catalytic reactions proceeding on the surface of dust that contains metals. It is most frequently assumed that dioxins are formed there according to two mechanisms: (1) the catalytic synthesis from precursors such as chlorobenzene, chlorophenols, polychlorinated naphthalenes, and PCBs (analogously, as is the case in a gaseous phase); and (2) the catalytic synthesis from EC (soot) contained in particles of dust, molecules of multiring aromatic hydro-carbons, and gaseous chlorine and oxygen.
The first of those two mechanisms was described for the first time in 1987 by Karasek.Citation26 Numerous investigations carried out later proved that this reaction essentially proceeds as a reaction of chlorobenzene or chlorophenol condensation or eventually as a reaction of the selective oxychlorination of polychlorinated naphthalenes or PCBs. This reaction may be catalyzed by many metals, including copper, titanium, manganese, cobalt, and zinc, where copper displays the greatest catalytic abilities.Citation52 In recent years, the most complex description of the catalytic synthesis of dioxins from precursors (mainly chlorophenols) proceeding on the surface of dust was presented by Milligan,Citation53,Citation54 Evans,Citation55,Citation56 Khachatryan,Citation57 and Ryu.Citation58–60 On the basis of their investigations, it may be concluded that the systems of congeners of PCDDs and PCDFs directly depend on the type of precursors taking part in the condensation reaction.
Analyzing the aforementioned publications, it is clearly seen that in the mechanism of synthesis from precursors (progressing in a gaseous phase and proceeding on the surface of dust particles catalyzed by certain metals), lower chlorinated dioxins containing from one to four chlorine atoms are formed.Citation61 Considering the formation of dioxins from precursors in a gaseous phase and on the surface of volatile ash (the catalytic reaction), one may notice that the problem that from where these higher chlorinated dioxins (containing from four to eight chlorine atoms in a molecule) appear in waste gases is not determined. The answer to this question is given by the third mechanism of dioxin generation—de novo synthesis.
The third mechanism of dioxin formation and the second one in the zone behind the combustion chamber, in the area of flue gas cooling, is the mechanism of de novo synthesis, which was discovered in the 1980s by Stieglitz et al. De novo synthesis is a catalytic, heterophase reaction in which dioxins form without producing intermediate gaseous products. This reaction proceeds at 200– 500 °C from carbon molecules contained in volatile ash that react with oxygen and chlorine on the surface of a catalyst.Citation25,Citation62,Citation63 On the basis of the investigations carried out using Citation13C isotopes, it was found that de novo synthesis from carbon progresses via formation and closing of benzene rings.Citation62 The source of carbon in de novo synthesis is frequently the EC contained in the volatile ash particles as a remaining product after the processes of thermal decomposition and oxidation in combustion. In practice, the total burnout of carbon contained in fuel and small quantities of carbon that have not been burnt out occur in waste gases in the form of soot. Seemingly, multiring aromatic hydrocarbons are contained in soot (i.e., PAHs),Citation64 but at this time they are seldom treated as the most important carbon source for de novo synthesis.Citation65,Citation66 Nevertheless, there are many publications showing that multiring aromatic hydrocarbons are emitted in flue gases from incineration power plantsCitation67,Citation68 in relatively high concentrations (even by 3 times more than dioxins and furans) and may constitute a relevant element in the chain of de novo synthesis.Citation69 The emission of dioxins from the process of clean wood combustion suggests that large, complex molecules of organic compounds such as lignite or brown coal may also be the source of carbon.Citation70 In the end, dioxins may also be generated without the presence of organic precursors during metallurgic processes; for instance, during chlorination and calcination of silicate black iron ore or steelmaking and smelting of magnesium,Citation71,Citation72 or from carbon molecules on the surface of volatile ashes behind the combustion chamber in boilers or incineration plants.Citation73,Citation74 StieglitzCitation74 observed a very strong correlation between carbon content in dust particles and the quantity of dioxins generated in de novo synthesis. A similar phenomenon was observed by Wunsch,Citation75 Milligan,Citation76,Citation77 and recently by Kakuta.Citation78
Chlorinating factors in de novo synthesis may be free elemental chlorine, chlorinated organic compounds, and volatile inorganic chlorine salts; for example, sodium chloride or iron(III) chloride formed in thermal processes at approximately 440 °C.Citation79 Nevertheless, a basic role in dioxin generation is played by the free chlorine radical concentration in the zone of the combustion reaction and in the cooling zone. Depending on the form in which chlorine occurs (free chlorine, hydrochloride, chlorine bound in gravel, chlorine bound in volatile ashes), it exerts a varied influence on dioxin formation.Citation80 Recent examinationsCitation81 proved that organic chlorine has a greater potential than inorganic chlorine for dioxin formation. However, it has been shown that the role of the free chlorine fraction, which may take part in PCDD/F synthesis, is a small one that is very dependent on temperature.Citation82
Despite an obvious role of chlorine in de novo synthesis, the data from more than 1900 investigations on an industrial scaleCitation83 show that there is no clear relationship between the quantity of chlorine introduced in fuel to the combustion process and the quantity of dioxins emitted in the process.Citation84 On the other hand, there is a relationship between the quantity of dioxin emitted and the concentration of chlorine or hydrochloride at the outlet of the combustion chamber.
Volatile ash plays a very important role as a heterogenic catalyst in the formation and decomposition of dioxins.Citation85,Citation86 The key to chlorination of particles in de novo synthesis is the Deacon reactionCitation87 of catalytic oxidation of hydrochloride generated during combustion to elemental chlorine,Citation88 which is in turn capable of running the chlorination reaction catalyzed by the copper compounds copper(I) chloride, copper(II) chloride, copper(II) oxide, copper(I) oxide, and copper(II) sulfate.Citation89
The Deacon reactions apart from copper compounds may also catalyze manganese, nickel, cobalt, vanadium, molybdenum, iron, aluminum, sodium, potassium, zinc, and magnesium.Citation90 Other metals (e.g., nickel) do not display the catalytic activity or decrease the quantity of dioxins formed (e.g., chromium).Citation91,Citation92
The rate of de novo reaction is limited by the rate of decomposition of the carbon matrix directly to dioxins or their precursors. In light of the known research results, de novo synthesis is a slow reaction, even a very slow one, and the reaction of dioxin formation (e.g., from chlorophenols in a gaseous phase) is approximately 10Citation2 to 10Citation5 quicker.Citation93 De novo synthesis proceeds in the combustion installation behind the combustion zone at 200–400 °C on the sedimented volatile dust particles. The temperature of 350 °CCitation63,Citation94 is considered to be an optimal temperature for de novo synthesis. The oxygen concentration influences de novo synthesis. This is obvious if one considers that oxygen is present in the molecules of dibenzo-pdioxin and of dibenzofuran. The investigations confirmed that the increase of oxygen concentration in the zone behind the combustion zone in the relevant way increases the quantity of dioxins formed; analogously, the increase of hydrochloride gives the identical effect.Citation95
Summarizing the aforementioned review of literature concerning the methods of dioxin formation in combustion processes, one must mention a considerable influence of the combustion conditions on the quantity of dioxins and furans that form. Under the conditions of combustion, one should understand first and foremost the temperature; the time of residence of gases generated as a result of combustion in the elevated temperature zone; the oxygen concentration in the combustion zone; and the concentration of carbon oxide in flue gases, which is connected with the oxygen concentration. Bad combustion conditions (i.e., too low temperature and too high concentration of carbon oxide) trigger the generation of partial combustion products such as chlorobenzenes and chlorophenols, which are precursors of dioxins that may be prone to the condensation reaction at elevated temperatures and in the low-temperature zones in the presence of metals catalyzing the condensation reactions. Bad combustion conditions bring about the formation of multiring aromatic hydrocarbons and the fact that carbon that has not been burnt out in dust is lifted from the process. This constitutes a perfect raw material for de novo synthesis, which in turn means that there is a direct impact of the combustion conditions on the quantity of dioxins formed in all three known and aforementioned mechanisms.
DIOXIN EMISSIONS FROM POWER AND HEAT GENERATION SECTOR
The first reports on the subject of the possibility of dioxin emissions from the power generation sector were released in the early 1980s.Citation96,Citation97 Detailed investigations proved that dioxin emissions may occur during hard coal,Citation98–109 brown coal,Citation110–112 furnace oil,Citation113,Citation114 and coke-oven gas combustion.Citation115 In all cases the dioxin concentrations in flue gases do not exceed 0.1 ng TEQ/m3, although in the case of modern coal power plants heated with hard coalCitation106 they attain values below 0.001 ng TEQ/m3. According to Meij,Citation116 dioxin concentrations in Dutch power plants are 0.0015–0.0032 ng TEQ/m3. In the case of brown coalCitation112 and coke-oven gas,Citation115 dioxin concentrations are slightly higher—at slightly below 0.1 ng TEQ/m3. As is easily seen, the dioxin concentrations in emitters of large objects of mineral fuel energetic combustion are mostly 1 order lower than the concentrations in the emitters of waste incineration plants. Taking into consideration the huge streams of flue gases that one deals with in power plants, the emissions of dioxins even at very low dioxin concentrations in flue gases are significant. On the other hand, co-incineration of waste in coal power plants may be the source of the dioxin emission increases up to the known level from waste incineration plant emissions.Citation117–120
A perfect example of emissions from energetic boilers of various types in Poland are the data that have been demonstrated recently by Grochowalski and KonieczyńskiCitation121, which are presented in .
Table 1. Typical concentrations PCDD/Fs in flue gases and the emission indicators for energetic boilers of medium-capacity municipal heat and power plants.118
A much greater problem is wood combustion as an energetic fuel. Wood as a fuel has been applied since time immemorial. Because of its low sulfur content, it is commonly regarded as an ecological fuel. Because it is also a renewable fuel, clean wood as biomass has become a desired product and is commonly regarded as a fuel that is environmentally friendly. The real research results of bio-mass emission properties show that because of this fact, it is not as environmentally friendly as it would seem to be. Generally, the sulfur dioxide emissions are considerably lower than the emissions during coal combustion, but the simultaneous emissions of nitrogen oxides and CO2 are slightly higher, whereas the emissions of organic micropollutants (the products of partial combustion) are significantly higher. The emissions of dioxins in wood combustion processes (understood as biomass or in particular wood waste) also occur. On one hand this is an effect of the presence of chlorine in wood material; on the other hand, it is an effect of the slight sulfur content and the simultaneous lack of an inhibitory effect of sulfur on the formation of dioxins. The first results of investigations of emissions of dioxins from the wood combustion process come from the first half of the 1980s.Citation122,Citation123 Later on the problem was the subject of research of many more authors.Citation124–141 According to those investigations, the concentrations of dioxins in flue gases are 0.1–2 ng TEQ/m3. The emission of dioxins also occurs during the combustion of plywoodCitation142 or medium-density fiberboard plates,Citation143 and the concentrations may even reach a few tens of nanograms TEQ per cubic meter. The intensity of dioxin emissions significantly increases if wooden waste is subjected to combustion particularly after prior impregnationCitation144,Citation145; for example, by means of pentachlorophenol146–148 or using chromium (chromated copper arsenate [CCA]).Citation149–154 In extreme cases the dioxin concentrations in flue gases from the combustion of wood impregnated by CCA may attain a few thousand nanograms TEQ per cubic meter. The elevated dioxin concentration may also occur at the stage of lightening the hearth heated with wood.Citation155 The source of chlorine for PCDD/F formation is the small amount of this element contained in wood.
METHODS FOR REDUCTION OF DIOXIN EMISSIONS
The methods for limiting the emission of dioxins from technological processes may be divided into two groups: primary and secondary methods.
Primary methods may be defined as the interference into a technological process and creation of such conditions in its course that the amount of dioxins is possibly the smallest.Citation156 Among the primary methods, the problem of crucial importance is to avoid the presence of chlorine in thermal processes. The processes of dioxins formation are nonequilibrium processes, and even a trace amount of chlorine and organic material in the elevated temperature zone (200–700 °C) will result in the production of dioxins. Unfortunately, the presence of chlorine is frequently unavoidable.
The next factor of importance is temperature. Dioxins are not as persistent of chemical compounds as they are widely considered to be, and they are decomposed at a temperature of 700 °C.Citation157 In Europe, according to the Waste Incineration Directive,Citation2 all incineration plants should be designed, equipped, built, and operated in such a way that the gas resulting from the process is heated (after the last injection of combustion air, in a controlled and homogeneous fashion, and even under the most unfavorable conditions) to a temperature of 850 °C for 2 sec. If hazardous wastes with a content of more than 1% halogenated organic substances (expressed as chlorine) are incinerated, then the temperature must be raised to 1100 °C. McKayCitation158 recommended achieving a temperature above 1000 °C for at least 2 sec in high-turbulence (Reynolds number >50,000) conditions with approximately 3–6% excess of oxygen. These conditions are well known as the 3-T (temperature, time, and turbulence) conditions.
Unfortunately, most dioxins in thermal processes are produced as a result of de novo synthesis, and the optimal range of the temperatures used in de novo synthesis is 300–350 °C. Analogously, one may observe production of dioxins during the process of gas cooling from 700 to 200 °C. The amount of dioxins produced as a consequence of de novo synthesis is inversely proportional to the rate of gas cooling (taking away the heat); thus, it is essential that one aims at the building of very efficient systems of heat recovery (heat exchangers) and the eventual cooling of gases by quenching.Citation159 Furthermore, the presence of CO and soot as well as the dust containing metals (e.g., copper, iron, nickel, aluminum, or zinc) favors de novo synthesis.Citation160 That is why one may include in the primary limitation methods flue gas afterburning (so as to minimize the presence of CO and soot) and hot gas scrubbing or such adjustment of the flow rate by heat recovery systems that the gas contained in those systems does not deposit on them. The formation of zones in the system of heat recovery, in which one may observe the process of dust and soot deposition, brings about an increase in the amount of dioxins as a result of de novo synthesis.
In combustion processes, the issue of utmost importance is the appropriate control of combustion so that it is as similar as possible to the process of full and complete combustion.Citation161 The research of combustion and formation of organic micropollutants (e.g., the products of incomplete combustion) in this process led to the conclusion that one of the most important parameters determining their emissions, including dioxins, is the high concentration of CO and unburned organic carbon and low concentration of oxygen in flue gases (i.e., the quality of combustion).Citation161 The effect of chlorine and hydrogen chloride (HCl) in flue gases is negligible.Citation162 Those observations were confirmed by Seeker.Citation162 According to those results, the concentration of CO (i.e., the conditions of combustion) considerably influences the amount and emission of dioxins generated in this process.
One may include the presence of sulfur dioxide in flue gases among the primary methods of dioxin emission restriction because of its inhibition of dioxin synthesis. During the extensive research of waste combustion processes, it was concluded that the presence of sulfurdioxide in the combustion zone to a large extent decreases the amount of dioxins. Indeed, in the gas phase one may observe the reaction between chlorine and sulfur dioxideCitation163,Citation164:
The reaction has become an argument in the debates concerning the possibility of waste co-combustion in power stations and thermal-electric power stations. The results of the research carried out by the U.S. Environmental Protection Agency (EPA) and the confirmation of dioxin emissions from combustion processes have made it possible to perceive the problem in a different way. It is essential to remember that at elevated temperatures one may notice the competitive Deacon reaction—the catalytic decomposition of HCl:
Hence, it is obvious that no chance of complete elimination of dioxins in the combustion process exists. Further research concerning an effect of sulfur dioxide on the emission of dioxins indicates that the inhibitive influence of sulfur dioxide is not so high and is strongly dependent on its being in excess. On the other hand, the Deacon reaction possesses its own optimum that is dependent on the temperature and concentration of HCl.
It must be underlined that good results in the scope of the limitation of dioxin production are obtained using inhibitors. Some of the most frequently encountered inhibitors are nitrogen and sulfur compounds, which bring about the blocking of the metallic active centers in the particles of volatile dust, on the surface of which one may notice de novo synthesis.Citation165–168
Many investigations were carried out all over the world. The strong inhibition effect was proven for gaseous and liquid ammonia, inorganic salts such as ammonium sulfate and ammonium thiosulfate, and organic compounds such as urea, ethanolamine, methyl mercaptan, etc.Citation167,Citation169–173
To summarize, the aforementioned primary methods of the restriction of dioxin emissions have a chance to substantially decrease the amount of dioxins, but it is not possible to completely eliminate their production. Moreover, in practice, one may observe that it is not possible to reduce the concentration of dioxins in flue gases below the required level 0.1 ng TEQ/m3 using only primary methods.Citation158
With regards to the secondary methods of the reduction of dioxin emissions, the methods of utmost importance are the following:
| • | Adsorption on the activated carbon (on solid medium or the stream method) | ||||
| • | Catalytic decomposition of dioxins in the vanadium catalyst | ||||
| • | The filtrating-catalytic method “REMEDIA” | ||||
| • | The absorbing-adsorbing method “ADIOX” | ||||
| • | The radiation method (using an electron beam) | ||||
| • | The corona discharge method | ||||
| • | Adsorption on carbon nanotubes | ||||
| • | Nanocatalysis | ||||
Similar to most chemical substances, dioxins are well adsorbed on active carbon.Citation174 Two variants of the adsorption process are known: the adsorption on the solid bedCitation175–177 and the so-called “stream adsorption.” In the first case, great adsorbers filled with active carbon are the most frequently used. Active carbon should be replaced at regular time intervals. The variations of those devices are the adsorbers with a moving bed of active carbon, which operate in a flowing manner.Citation178–180 The carbon used is most frequently directed to combustion taking place in a waste incineration plant. Another variation may be the application of adsorbers with a fluidized bedCitation181 or with a monolithic honeycomb bed.Citation182 The stream absorption is connected with the introduction of certain amounts of powdered active carbon (sometimes in the mixture with powdery calcium oxide known as Sorbalit, Sorbacal, Spongiacal, etc.) to the stream of flowing gases and, then, gas scrubbing on a fabric filter.Citation183 The method of adsorption on a solid or moving bed of active carbon has found its application in municipal waste incineration plants. Such a solution was used in the modern (commissioned in 1998) waste incineration plant in Cologne, a new municipal solid waste incineration plant (Pfaffenau) in Vienna, in the hazardous waste and sewage sludge incineration plant of EBS Simmering in Vienna, and in the only Polish waste incineration plant in Warsaw. In medicinal waste incineration plants, there is a widely used method of stream adsorption combined with dry desulfurization using Sorbalit as a sorbent. It must be added that the stream adsorption method of dioxin elimination has recently been used with success in one of the Belgian steel plants. The efficacy of dioxin elimination is usually 95%. The adsorption methods also present some disadvantages, such as the problem of used adsorbent management, which comprises considerable amounts of dioxins as well as heavy metals (mercury in particular). The method with the application of active carbon (most of all, adsorption on the solid bed) has one important flaw; namely, the possibility of spontaneous carbon ignition at elevated temperatures (>200 °C) and burning the plant. Therefore, the application of the method of the reduction of dioxin emissions is gradually diminished.
It must be highlighted that the catalytic method is deprived of this disadvantage. During the exploitation of the catalytic systems of nitrogen oxide removal from flue gases, it was noticed that dioxins in waste gas are subject to efficacious decomposition. The research done by Hagen-meierCitation184 indicates that the vanadium-tungsten catalyst on the titanium oxide carrier effectively decomposes PCDD/Fs with the release of CO2, water, and HCl. Such a catalyst is widely used in the catalytic reduction of nitrogen oxide (with the addition of ammonia) in the process of selective catalytic reduction (SCR). Further researchCitation185–194 indicated that it is feasible to effectively reduce the emission of nitrogen oxides and PCDD/Fs on the appropriately prepared catalyst of vanadium(V) oxide (V2O5)-tungsten oxide (WO3)/titanium dioxide (TiO2) in the presence of ammonia. Today it is known that on the tungsten or platinum catalyst one may observe dechlorination and oxidation of dioxins (the partition of those processes— dechlorination and oxidation—are dependent on the composition of the catalyst).Citation195
The products of those reactions are CO2, water, and HCl. The temperature of the process is in the range of 250–300 °C. The efficacy of dioxins elimination using the catalytic methods is 95–99%. The numerous industrial applications confirm the high efficacy of the method.Citation196 It must be added that much research was conducted using the other catalysts, such as the platinum or perovskite catalysts; however, those trials with platinum catalyst were completed with no success because of the secondary synthesis of dioxins on the catalyst.Citation197–199 Disadvantages of the catalytic method include its high price, which is almost double that of the systems with sorbent injection and a fabric filter and slightly higher than the systems with a moving bed of active carbon.
Quite recently, a new method of dioxin elimination from waste gases appeared. The method was developed at the GORE Associates Company under the name “REMEDIA”Citation200–203 and it includes such methods as adsorption, dedusting, and catalytic decomposition. The main part constitutes membranes produced from Teflon and glass fibers that are specially modified with a layer of vanadium compounds. The membranes operate at 180–250 °C. The preliminary adsorption of dioxins occurs (those which are in the solid form) on the layer of dust separated on the filtrating membranes. On the other hand, those dioxins that were not absorbed are subject to dechlorination and oxidation using vanadium salts deposited on the membrane. The efficacy of dioxin elimination is 95%. According to the scientists who developed the method, it is slightly cheaper than the method with injection of the sorbent and fabric filter and is 60% cheaper than catalytic systems. The experiments using the filtrating-catalytic method in the IVRO municipal waste incineration plant (the capacity of two lines at 47,000 Mg/yr) in Roeselare, Belgium, are positive.Citation204,Citation205 As a result of the observations lasting 42 months, it was confirmed that 99.95% of the restriction of dioxin emissions and the systematic maintenance of the dioxin concentration in waste gases is in the range of 0.008–0.037 ng TEQ/m3. It must be added that a similar installation was recently used in the municipal waste incineration plant in Liberec in the Czech Republic.Citation206
The absorbing-adsorbing method (ADIOX) was recently developed by the Swedish company Go˝taverken Miljo˝. The method applies the so-called “memory effect”Citation207 that is observed in waste incineration plants. The effect is based on the absorption of dioxins in the elements of flue gas cleaning systems made of plastics (i.e., polypropylene) and their subsequent desorption from packing elements to the stream of flue gases in the case of the relevant decrease of their concentration in the flue gas stream as a result of, for instance, the application of primary methods emissions reduction.
The solution of ADIOX encompasses the introduction of activated carbon into polypropylene applied to the process of the elements of flue gas wet purification (mainly packing elements). The molecules of dioxins, which are absorbed in polypropylene, are additionally adsorbed on the surface of activated carbon and efficaciously eliminated from the flue gas stream in a persistent manner. The used elements, saturated with dioxins, are burnt in the waste incineration plant after their replacement. Because the process of dioxin production is a nonequilibrium process, it does not influence the increase in PCDD/F emissions from the emitter of the incineration plant. The method was applied in several municipal and hazardous waste incineration plants (i.e., Kloding, Thisted, Fasan, Holstebro, and Glostrup in Denmark; Umeå and Malmö in Sweden; Tredi Salaise in France; and Trondheim in Norway) with good results.Citation208–214
The search for new methods in the reduction of dioxin emissions continues. The promising effects include the application of a radiation method (with a beam of electrons)Citation215–221 that was previously used for the simulta neous elimination of sulfur dioxide and nitrogen oxides in power plants. Furthermore, it is reported that the application of corona discharge permits one to reduce the emission of dioxins.Citation222,Citation223 Use of the 21st-century tech nique of nanotechnology to remove dioxins has also been proposed. One of the variations includes using the unique adsorbing abilities of carbon nanotubesCitation224–228; another is using the nanoparticles of transition metals (e.g., titanium and vanadium) introduced to flue gases, the presence of which causes the catalytic decomposition of dioxins.Citation229–232 Nonetheless, this research is being carried out on a laboratory scale similar to the first examinations using ionic liquids in the selective absorption of dioxins and furans.Citation233
In conclusion, among the secondary methods of dioxin emission reduction, one may recommend the catalytic method (SCR) as the most important and most efficacious one, allowing for simultaneous and considerable reductions in the emissions of PCDD/Fs and reduced emissions of nitrogen oxides. Very good results are also obtained with the application of a filtrating-catalytic method. Numerous installations provide a chance to disseminate the technology in industry. The obstacle may be a high price of filtrating sacks applied in this method. However, it seems that the utilization of adsorption technologies using active carbon has only a slight chance of being implemented. The utilization of a radiation method (with an electron beam) may also be promising because it is an effective method for the reduction of sulfur dioxide and nitrogen oxide emissions on a commercial scale of production. The application of adsorption using carbon nanotubes seems very intriguing; however, the actual state of the technique does not give a chance to implement the method on a large scale.
CONCLUSIONS
The intensity and structure of PCDD/F emissions to the atmosphere in Poland for recent years are shown in .Citation234 For comparison, shows the fraction of particular economy sectors in the state dioxin emissions in select countries of the EU and Poland.Citation3
Table 2. Changes in emissions of PCDD/Fs from particular economy sectors in Poland in the years 1996 –2005 (g I-TEQ/yr)
Table 3. Fraction (as % of total) of particular economy sectors in the total emission of PCDD/Fs in chosen EU countries and in Poland in 2002 (according to the European Dioxin Inventory1)
Analyzing the data summarized in the above tables, one may infer that in Poland and other EU countries, the most important source of dioxin emissions is fuel combustion in municipal economies. This is a sector in which the chances for dioxin emissions are relatively small. In most EU countries, the fraction of emissions from the power generation sector is relatively low, in the range of 0.5–3.2%, whereas the fraction of other combustion processes in industry (mainly boiler houses in plants) is greater, and in certain cases it even reaches 20% (Belgium). This is an area of potential emissions reduction activities in which the chance for successful emissions reduction is relatively great.
It seems that the basic method of reduction of dioxin emissions in the energy sector should be the modernization of existing boilers combined with introduction of low-emission combustion technologies. At the relatively high sulfur content in combusted coals there exists a great chance for a considerable reduction of PCDD/F emissions. The successively restricted emission standards, especially for dust emission, should additionally allow for reduction of dioxin concentrations in flue gases, whereas the probable introduction of catalytic reduction of nitrogen oxides in certain energetic objects should also significantly decrease the emission of PCDD/Fs. Very good results are obtained by application of the filtration-catalytic method. Numerous operating installations provide a chance to distribute this technology in the energy sector as well. However, an obstacle here may be a high price of filtration bags applied in this method. On the other hand, the utilization of adsorption techniques using active coal has a slight chance to be implemented. The application of adsorption in carbon nanotubes appears to be very interesting. Nevertheless, the actual state of technology does not provide a chance to implement this method on a large scale. It may also be promising to use the radiative method (with an electron beam) because it is an effective method of sulfur dioxide or nitrogen oxide emissions reduction that operates on a technical scale.
In most European countries the emissions of PCDDs and PCDFs have systematically decreased for years. This is the result widely undertaken actions leading to emissions reduction from industrial sources but also from waste incineration plants and the metallurgic sector. The greatest progress in this scope has been attained by the most economically developed and richest countries of the EU— France, Germany, Belgium, The Netherlands, Austria, Great Britain, Denmark, Luxemburg, Switzerland, and Norway. In particular, a considerable decrease is observed in terms of emissions from waste incineration plants. This is a result of the implementation of the strict legal regulations reducing the admissible concentrations of PCDD/Fs in flue gases (Directive 2000/76/EC2). Actions aimed at the emissions reduction from the metallurgic sector are also observed in many countries. The basic and most important source of dioxin emissions slowly becomes the combustion of fuels in the heating sector, particularly in small heating units heated with coal or wood.
In Poland a systematic slight decrease of dioxin emissions to the atmosphere may be observed. This is first and foremost connected with the liquidation of many old waste incineration plants (mainly medicinal ones) that do not practically possess any systems of flue gas cleaning. Similar to many EU countries, the most important source of dioxin emissions is fuel combustion in the municipal economy.
Poland is the side of the international convention signed in 2002 in Stockholm with regard to persistent organic pollutants.Citation235 Its ratification brings about the necessity of a considerable decrease in total dioxin emissions. This is mainly connected with the necessity of emissions reduction in the metallurgic and energetic sectors; however, in all probability it will not bring too great of effects. The main emission source is and will be in the nearest future fuel combustion in individual heating systems, and the only solution for the future appears to be the large-scale introduction of central heating within cities.
REFERENCES
- Mininni , G. , Sbrilli , A. , Braguglia , C.M. , Guerriero , E. , Marani , D. and Dioxins , Rotatori, M. 2007 . Furans and Polycyclic Aromatic Hydrocarbons Emissions from a Hospital and Cemetery Waste Incinerator . Atmos. Environ. , 41 : 8527 – 8536 .
- Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the Incineration of Waste; OJ L 332, 28.12.2000; European Commission: Brussels, Belgium, 2000; pp 91–111.
- The European Dioxin Emission Inventory. Stage II; DG ENV; European Commission: Brussels, Belgium, 2000.
- Olie , K. , Vermeulen , P.L. and Hutzinger , O. 1977 . Chlorodibenzo-p-Dioxins and Chlorodibenzo-Furans Are Trace Components of Fly Ash and Flue Gas of Some Municipal Waste Incinerators in the Netherlands . Chemosphere , 6 : 455 – 459 .
- Buser , H.R. , Bosshardt , H.P. and Rappe , Ch. 1978 . Identification of Polychlorinated Dibenzo-p-Dioxin Isomers Found in Fly Ash . Chemosphere , 7 : 165 – 72 .
- Bumb , R.R. , Crummett , W.B. , Artie , S.S. , Gledhill , J.R. , Hummel , R.H. , Kagel , R.O. , Lamparski , L.L. , Luoma , E.V. , Miller , D.L. , Nestrick , T. J. , Shadroff , L.A. , Stehl , R.H. and Woods , J.S. 1980 . Trace Chemistries of Fire: a Source of Chlorinated Dioxins . Science , 210 : 385 – 390 .
- Rappe , C. 1992 . Sources of PCDDs and PCDFs. Introduction, Reactions, Levels, Patterns, Profiles and Trends . Chemosphere , 25 : 41 – 44 .
- Baker , J.I. and Hites , R.A. 2000 . Is Combustion the Major Source of Polychlorinated Dibenzo-p-Dioxins and Dibenmzofurans to the Environment? A Mass Balance Investigation . Environ. Sci. Technol. , 34 : 2879 – 2886 .
- Thome-Kozmiensky , K.J. 1994 . Thermal Waste Treatment , Berlin , , Germany : EF-Verlag für Energie- und Umwelttechnik GmbH .
- Eduljee , G.H. 1994 . Waste Incineration and the Environment , Edited by: Hester , R.E. and Harrison , R.M. London : Royal Society of Chemistry .
- Jay , K. and Stieglitz , L. 1995 . Identification and Quantification of Volatile Organic Components in Emission of Waste Incineration Plants . Chemo-sphere , 30 : 1249 – 1260 .
- Mascolo , G. , Spinosa , L. , Lotito , V. and Mininni , G. 1997 . Bagnuolo, G. Lab-Scale Evaluations on Formation of Products of Incomplete Combustion in Hazardous Waste Incineration: Influence of Process Variables . Water Sci. Technol. , 36 : 219 – 226 .
- Trenholm , A. 1998 . Identification of PICs in Hazardous Waste Combustion Emissions . Waste Manage. , 18 : 485 – 492 .
- Wienecke , J. , Kruse , H. , Huckfeldt , U. , Eickhoff , W. and Wassermann , O. 1995 . Organic Compounds in the Flue Gas of a Hazardous Incinerator . Chemosphere , 30 : 907 – 913 .
- Liow , M.Ch. , Lee , W.J. , Chen , S.J. , Ch , Wang, L. , Chung , Ch.H and Chen , J.H. 1997 . Emission Polycyclic Aromatic Hydrocarbons from Medical Waste Incinerators . J. Aerosol Sci. , 28 : 549 – 550 .
- Liu , K. , Xie , W. , Zhao , Z.B. , Pan , W.P. and Riley , T.T. 2000 . Investigation of Polycyclic Aromatic Hydrocarbons in Fly Ash Form Fluidized Bed Combustion Systems . Environ. Sci. Technol. , 34 : 2273 – 2279 .
- Mastral , A.M. , Callen , M.S. and Garcia , T. 2000 . Toxic Organic Emissions from Coal Combustion . Fuel Process. Technol. , 67 : 1 – 10 .
- Belevi , H. and Langmeier , M. 2000 . Factors Determining the Element Behavior in Municipal Solid Waste Incinerators . Laboratory Experiments; Environ. Sci. Technol. , 34 : 2507 – 2512 .
- Belevi , H. and Moench , H. 2000 . Factors Determining the Element Behavior in Municipal Solid Waste Incinerators. Field Studies . Environ. Sci. Technol. , 34 : 2501 – 2506 .
- Sukrut , S. , Thipse , E. and Dreizin , L. 2002 . Metal Partitioning in Products of Incineration of Municipal Solid Waste . Chemosphere , 46 : 837 – 849 .
- McEnally , C.S. , Pfefferle , L.D. and Atakan , B. 2006 . Kohse-Höinghaus. Studies of Aromatic Hydrocarbon Formation Mechanisms in Flames: Progress towards Closing the Fuel Gap . Prog. Energy Combust. Sci. , 32 : 247 – 294 .
- Crummett , W.B. and Townsend , D.J. 1984 . The Trace Chemistries of Fire Hypothesis: Review and Update . Chemosphere , 13 : 777 – 798 .
- Tosine , H.M. , Clement , E.R. , Ozvacic , V. and Wong , G. 1985 . Levels of PCDD/ PCDF and Other Chlorinated Organics in Municipal Refuse . Chemo-sphere , 14 : 821 – 827 .
- Ballschmiter , K. , Zoller , W. , Buchert , H. and Clas , Th. 1985 . Correlation between Substitution Pattern and Reaction Pathway in the Formation of Polychlorodibenzofurans . Fresenius J. Anal. Chem. , 322 : 587 – 594 .
- Vogg , H. and Stieglitz , L. 1986 . Thermal Behavior of PCDD/PCDF in Fly Ash from Municipal Incinerators . Chemosphere , 15 : 1373 – 1378 .
- Karasek , F.W. and Dickson , L.C. 1987 . Model Studies of Polychlorinated Dibenzo-p-Dioxin Formation during Municipal Refuse Incineration . Science , 237 : 754 – 756 .
- Tuppurainen , K. , Asikainen , A. , Ruokojärvi , P. and Ruuskanen , J. 2003 . Perspectives on the Formation of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans during Municipal Solid Waste (MSW) Incineration and Other Combustion Processes . Acc. Chem. Res. , 36 : 652 – 658 .
- Huang , H. and Buekens , A. 1995 . On the Mechanisms of Dioxin Formation in Combustion Processes . Chemosphere , 31 : 4099 – 4117 .
- Gullett , B. and Seeker , R. 1997 . “ Chlorinated Dioxin and Furan Control and Monitoring ” . In Paper Presented at the ICCR Meeting Research Triangle Park , NC
- Gullett , B.K. , Bruce , K.R. and Beach , L.O. 1990 . The Effect of Metal Catalysts on the Formation of Polychlorinated Dibenzo-p-Dioxin and Polychlorinated Dibenzofuran Precursors . Chemosphere , 20 : 1945 – 1952 .
- Hunsinger , H. , Jay , K. and Vehlow , J. 2000 . Formation and Destruction of PCDD/F inside a Grate Furnace . Organohalogen Compd. , 46 : 86 – 89 .
- Ballschmiter , K. and Swerev , M. 1987 . Reaction Pathways for the Formation of Polychlorodibenzodioxins (PCDD) and -Furans (PCDF) in Combustion Processes. I . Fresenius Z. Anal. Chem. , 328 : 125 – 127 .
- Ballschmiter , K. , Braunmiller , I. , Niemczyk , R. and Swerev , M. 1988 . Reaction Pathways for the Formation of Polychlorodibenzodioxins (PCDD) and -Furans (PCDF) in Combustion Processes. II. Chlorobenzenes and Chlorophenols and Precursors in the Formation of Polychlorodibenzodioxins and -Dibenzofurans in Flame Chemistry . Chemosphere , 17 : 995 – 1005 .
- Rubey , W.A. , Dellinger , B. , Hall , D.L. and Mazer , S.L. 1985 . High-Temperature Gas-Phase Formation and Destruction of Polychlorinated Dibenzofurans . Chemosphere , 14 : 1483 – 1494 .
- Young , C.M. and Voorhees , K.J. 1991 . PIC Formation Mechanisms in the Thermal Decomposition of 1,2-Dichlorobenzene . Chemosphere , 23 : 1265 – 1277 .
- Aubrey , N.E. and van Wazer , J.J. 1964 . Equilibrium Rearrangement of Perchlorocarbon Compounds . J. Am. Chem. Soc. , 86 : 4380 – 4383 .
- Lenoir , D. , Wahrmeier , A. , Sidhu , S.S. and Taylor , P.H. 2001 . Formation and Inhibition of Chloroaromatic Micropollutants Formed in Incineration Processes . Chemosphere , 43 : 107 – 114 .
- Lenoir , D. , Wahrmeier , A. , Schramm , K.W. , Kaune , A. , Zimmermann , R. , Taylor , P.H. and Sidhu , S.S. 1998 . Thermal Formation of Polychlorinated Dibenzodioxins and -Furans: Investigation of Relevant Pathways . Environ. Eng. Sci. , 15 : 37 – 47 .
- Taylor , P.H. , Wehrmeier , A. , Sidhu , S.S. , Lenoir , D. and Schramm , K.W. 2000 . Copper Catalyzed Chlorination and Condensation of Acetylene and Dichloroacetylene . Chemosphere , 40 : 1297 – 1303 .
- Wehrmeier , A. , Lenoir , D. , Sidhu , S.S. , Taylor , P.H. , Rubey , W.A. , Kettrup , A. and Dellinger , B. 1998 . The Role of Copper Species in Chlorination and Condensation Reactions of Acetylene . Environ. Sci. Technol. , 32 : 2741 – 2748 .
- Cieplik , M.K. , Oviedo , M.C. and Louw , R. 2000 . On the Possible Role of Acetylene in Gas Phase Dioxin Formation . Chemosphere , 40 : 195 – 199 .
- Blumenstock , M. , Zimmermann , R. and Schramm , K.W. 2000 . Kettrup, A. Influence of Combustion Conditions on the PCDD/F, PCB, PCBz, and PAH Concentrations in the Post Combustion Chamber of a Waste Incineration Pilot Plant . Chemosphere , 40 : 987 – 993 .
- Froese , K.L. and Hutzinger , O. 1996 . Polychlorinated Benzene, Phenol, Dibenzo-p-Dioxin and Dibenzofuran in Heterogeneous Combustion Reactions of Acetylene . Environ. Sci. Technol. , 30 : 998 – 1008 .
- Froese , K.L. and Hutzinger , O. 1996 . Polychlorinated Benzene and Polychlorinated Phenol in Heterogeneous Combustion Reactions of Ethylene and Ethane . Environ. Sci. Technol. , 30 : 1009 – 1013 .
- Pandelova , M. , Lenoir , D. and Schramm , K.W. 2005 . Proving of a Hypothesis of Thermal PCDF Formation in Laboratory-Scale Furnaces . Organohalogen Compd. , 67 : 2230 – 2231 .
- Stanmore , B.R. 2004 . The Formation of Dioxins in Combustion Systems; Combust . Flame , 136 : 398 – 427 .
- Cieplik , M.K. , de Jong , V. , Bozovic , J. , Liljelind , P. , Marklund , S. and Louw , R. 2006 . Formation of Dioxins from Combustion Micropollutants over MSWI Fly Ash . Environ. Sci. Technol. , 40 : 1263 – 1269 .
- Ghorishi , S.B. and Altwicker , E.R. 1996 . Rapid Formation of Polychlorinated Dioxins/Furans during the Heterogeneous Combustion of 1,2-Dichlorobenzene and 2,4-Dichlorophenol . Chemosphere , 32 : 133 – 144 .
- Heindl , H. and Hutzinger , O. 1989 . Search for Industrial Sources of PCDD/ PCDFs: IV . Phthalocyanine Dyes; Chemosphere , 18 : 1207 – 1211 .
- Williams , D.T. , LeBel , G.L. and Benoit , F.M. 1992 . Polychlorodibenzodioxins and Polychlorodibenzofurans in Dioxazine Dyes and Pigments . Chemosphere , 24 : 169 – 180 .
- Dickson , L.C. , Lenoir , D. and Hutzinger , O. 1989 . Surface Catalyzed Formation of Chlorinated Dibenzo-Dioxins and Dibenzo-Furans during Incineration . Chemosphere , 19 : 277 – 282 .
- Qian , Y. , Zheng , M. , Liu , W. , Ma , X. and Zhang , B. 2005 . Influence of Metal Oxides on PCDD/Fs Formation from Pentachlorophenol . Chemo-sphere , 60 : 951 – 958 .
- Milligan , M.S. and Altwicker , E.R. 1996 . Chlorophenol Reactions on Fly Ash. 1. Adsorption/Desorption Equilibria and Conversion to Polychlorinated Dibenzo-p-Dioxin . Environ. Sci. Technol. , 30 : 225 – 229 .
- Milligan , M.S. and Altwicker , E.R. 1996 . Chlorophenol Reactions on Fly Ash. 2. Equilibrium Surface Coverage and Global Kinetics . Environ. Sci. Technol. , 30 : 230 – 236 .
- Evans , C.S. and Dellinger , B. 2000 . Surface-Mediated Formation of Polybrominated Dibenzo-p-Dioxins and Dibenzofurans from the High-Temperature Pyrolysis of 2-Bromophenol on a CuO/Silica Surface . Environ. Sci. Technol. , 39 : 4857 – 4863 .
- Evans, C.S. Ph.D. thesis, Louisiana State University, Baton Rouge, LA, 2004.
- Khachatryan , L. , Lomnicki , S. and Dellinger , B. 2007 . An Expanded Reaction Kinetic Model of the CuO Surface-Mediated Formation of PCDD/F from Pyrolysis of 2-Chlrophenol . Chemosphere , 68 : 1741 – 1750 .
- Ryu , J.Y. and Mulholland , J.A. 2005 . Metal-Mediated Chlorinated Dibenzo-p-Dioxin (CDD) and Dibenzofuran (CDF) Formation from Phenols . Chemosphere , 58 : 977 – 988 .
- Ryu , J.Y. 2008 . Formation of Chlorinated Phenols, Dibenzo-p-Dioxins, Dibenzofurans, Benzenes, Benzoquinones and Perchloroethylenes from Phenols in Oxidative and Copper (II) Chloride Catalyzed Thermal Process . Chemosphere , 71 : 1100 – 1109 .
- Ryu , J.Y. , Takeuchi , M. and Mulholland , J.A. 2006 . Is Phenol Condensation One of the Major Pathways in the Formation of Polychlorinated Dibenzofurans in Municipal Waste Incinerators? Model Prediction vs. Field Observation . Sci. Total Environ. , 357 : 237 – 246 .
- Wikström , E. , Ryan , S. , Touati , A. and Gullett , B.K. 2004 . In Situ Formed Soot Deposit as a Carbon Source for Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans . Environ. Sci. Technol. , 38 : 2097 – 2101 .
- Stieglitz , L. and Vogg , H. 1987 . On Formation Conditions of PCDD/PCDF in Fly Ash from Municipal Waste Incinerators . Chemosphere , 16 : 1917 – 1922 .
- Stiegliz , L. , Zwick , G. , Beck , J. , Roth , W. and Vogg , H. 1989 . On the De Novo Synthesis of PCDD/PCDF on the Fly Ash of Municipal Waste Incinerators . Chemosphere , 18 : 1219 – 1226 .
- Calcote , H.F. 1981 . Mechanism of Soot Nucleation in Flames—a Critical Review . Combust. Flame , 42 : 215 – 242 .
- Jay , K. and Stieglitz , L. 1991 . On the Mechanism of Formation of Polychlorinated Aromatic Compounds with Copper (II) Chloride . Chemosphere , 22 : 987 – 996 .
- Schoonenboom , M.H. and Olie , K. 1995 . Formation of PCDDs and PCDFs from Anthracene and Chloroanthracene in a Model Fly Ash System . Environ. Sci. Technol. , 29 : 2005 – 2009 .
- Eiceman , G.A. , Clement , R.E. and Karasek , F.W. 1979 . Analysis of Fly Ash from Municipal Incinerators for Trace Organic Compounds . Analyt. Chem. , 51 : 2343 – 2350 .
- Akimoto , Y. , Aoki , T. , Nito , S. and Innouye , Y. 1997 . Oxygenated Polycyclic Aromatic Hydrocarbons from MSW Incinerator Fly Ash . Chemosphere , 34 : 263 – 273 .
- Iino , F. , Imagawa , T. , Takeuchi , M. and Sadakata , M. 1999 . De Novo Synthesis Mechanism of Polychlorinated Dibenzofurans from Polycyclic Aromatic Hydrocarbons and Characteristic Isomers of Polychlorinated Naphthalenes . Environ. Sci. Technol. , 33 : 1038 – 1043 .
- Nestrick , T.J. , Lamparski , L.L. and Crummett , W.B. 1987 . Thermolytic Surface-Reaction of Benzene and Iron (III) Chloride to Form Chlorinated Dibenzo-p-Dioxins and Dibenzofurans . Chemosphere , 16 : 777 – 790 .
- Oehme , M. , Larssen , S. and Brevik , E.M. 1989 . Formation of Polychlorinated Dibenzofurans and Dibenzo-p-Dioxins by Production Processes for Magnesium and Refined Nickel . Chemosphere , 18 : 1379 – 1389 .
- Lahl , U. 1993 . Sintering Plants of Steel Industry—the Most Important Thermical PCDD/F Source in Industrialized Regions . Organohalogen Compd. , 11 : 311 – 314 .
- Addink , R. , van Bavel , B. , Visser , R. , Wever , H. , Slot , P. and Olie , K. 1990 . Surface Catalyzed Formation of Polychlorinated Dibenzo-p-Dioxins/ Dibenzofurans during Municipal Waste Incineration . Chemosphere , 20 : 1929 – 1934 .
- Stieglitz , L. , Eichberger , M. , Schleihauf , J. , Beck , J. , Zwick , G. and Will , R. 1993 . The Oxidative Degradation of Carbon and Its Role in the De Novo Synthesis of Organohalogen Compd . in Fly Ash; Chemosphere , 27 : 343 – 350 .
- Wunsch , P. , Leichsenring , S. , Schramm , K.W. and Kettrup , A. 1994 . Temperature Dependence of PCDD/F Formation in Boiler Ash . Chemosphere , 29 : 1235 – 1243 .
- Milligan , M.S. and Altwicker , E.R. 1993 . The Relationship between the De Novo Synthesis of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans and Low Temperature Carbon Gasification in Fly Ash . Environ. Sci. Technol. , 27 : 1595 – 1601 .
- Milligan , M.S. and Altwicker , E.R. 1995 . Mechanistic Aspects of the De Novo Synthesis of Polychlorinated Dibenzo-p-Dioxins and Furans in Fly-Ash from Experiments Using Isotopically Labeled Reagents . Environ. Sci. Technol. , 29 : 1353 – 1358 .
- Kakuta , Y. , Matsuto , T. , Tojo , Y. and Tomikawa , H. 2007 . Characterization of Residual Carbon Influencing the De Novo Synthesis of PCDD/Fs in MSWI Fly Ash . Chemosphere , 68 : 880 – 886 .
- Fängmark , I. , Strömberg , B. , Berge , N. and Rappe , C. 1994 . Influence of Post-combustion Temperature Profiles on the Formation of PCDDs, PCDFs, PCBzs and PCBs in a Pilot Incinerator . Environ. Sci. Technol. , 28 : 624 – 629 .
- Wikström , E. , Ryan , S. , Touati , A. and Gullett , B.K. 2003 . Key Parameters for De Novo Formation of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans . Environ. Sci. Technol. , 37 : 1962 – 1970 .
- Wey , M.Y. , Liu , K.Y. , Yu , W.J. , Lin , C.L. and Chang , F.Y. 2008 . Influences of Chlorine Content on Emission of HCl and Organic Compounds in Waste Incineration Using Fluidized Beds . Waste Manage. , 28 : 406 – 415 .
- Liu , K. , Pan , W.-P. and Riley , J.T. 2000 . A Study of Chlorine Behavior in a Simulated Fluidized Bed Combustion System . Fuel , 79 : 1115 – 1124 .
- Rigo , H.G. and Handler , A.J. 1998 . Is There a Strong Dioxin-Chlorine Link in Commercial Scale Systems? . Chemosphere , 37 : 2031 – 2046 .
- Schaub , W.M. 1995 . Chlorine in Equals Dioxin Out? … It's Not That Simple . Resources Conserv. Recycling , 14 : 157 – 170 .
- Hagenmaier , H. , Kraft , M. , Brunner , H. and Haag , R. 1987 . Catalytic Effect of Fly Ash from Waste Incineration Facilities on the Formation and Decomposition of Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans . Environ. Sci. Technol. , 21 : 1080 – 1084 .
- Hagenmaier , H. , Brunner , H. , Haag , R. and Kraft , M. 1987 . Copper Catalyzed Dechlorination/Hydrogenation of Polychlorinated Dibenzo-p-Dioxins, Polychlorinated Dibenzofurans and Other Chlorinated Aromatic Compounds . Environ. Sci. Technol. , 21 : 1085 – 1088 .
- Griffin , R.D. 1986 . A New Theory of Dioxin Formation in Municipal Solid Waste Combustion . Chemosphere , 15 : 1987 – 1990 .
- Huang , H. and Buekens , A. 1996 . De Novo Synthesis of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans: Proposal of a Mechanistic Scheme . Sci. Total Environ. , 193 : 121 – 141 .
- Gullet , B.K. , Bruce , K.R. , Beach , L.O. and Drago , A.M. 1992 . Mechanistic Steps in the Production of PCDD and PCDF during Waste Combustion . Chemosphere , 25 : 1387 – 1392 .
- Hisham , M.W.M. and Benson , S.W. 1995 . Thermochemistry of the Deacon Process . J. Phys. Chem. , 99 : 6194 – 6198 .
- Öberg , T. , Öhström , T. and Bergström , J.G.T. 2007 . Metal Catalyzed Formation of Chlorinated Aromatic Compounds: a Study of the Correlation Pattern in Incinerator Fly Ash . Chemosphere , 67 : S185 – S190 .
- Öberg , T. , Bergbäck , B. and Filipsson , M. 2008 . Catalytic Effect by Metal Oxides on the Formation and Degradation of Chlorinated Aromatic Compounds in Fly Ash . Chemosphere , 71 : 1135 – 1143 .
- Dickson , L.C. , Lenoir , D. and Hutzinger , O. 1992 . Quantitative Comparison of De Novo and Precursor Formation of Polychlorinated Dibenzo-p-Dioxins under Simulated Municipal Solid Waste Incinerator Postcombustion Conditions . Environ. Sci. Technol. , 26 : 1822 – 1826 .
- Fiedler , H. 1993 . Formation and Sources of PCDD/PCDF . Organohalogen Compd. , 11 : 221 – 228 .
- Gulett , B. and Touati , A. 2002 . Sensitivity of PCDD/F Formation to Hazardous Waste Firing Rate and Combustion Quality . Organohalogen Compd. , 26 : 155 – 158 .
- Mahle , N.H. and Whiting , L.F. 1980 . The Formation of Chlorodibenzo-p-Dioxin by Air Oxidation and Chlorination of Bituminous Coal . Chemo-sphere , 9 : 693 – 699 .
- Chiu , C. , Thomas , R.S. , Lockwood , J. , Li , K. , Halman , R. and Lao , R.C.C. 1983 . Polychlorinated Hydrocarbons from Power Plants, Wood Burning and Municipal Incinerators . Chemosphere , 12 : 607 – 616 .
- Garcia , J.P. , Beyne-Masclet , S. and Mouvier , G. 1992 . Emissions of Volatile Organic Compounds by Coal-Fired Power Stations . Atmos. Environ. , 25 : 1589 – 1597 .
- Wienecke , J. , Kruse , H. and Wassermann , O. 1992 . Organic Compounds in the Flue Gas from a Power Station with Circulating Fluid Bed Combustion of Coal . Chemosphere , 25 : 1889 – 1895 .
- Luijk , R. , Dorland , C. , Kapteijn , F. and Govers , H.A.J. 1993 . The Formation of PCDDs and PCDFs in the Catalyzed Combustion of Carbon: Implications for Coal Combustion . Fuel , 72 : 343 – 347 .
- Riggs , K.B. , Brown , T.D. and Schrock , M.E. 1995 . PCDD/PCDF Emissions from Coal-Fired Power Plants . Organohalogen Compd. , 24 : 51 – 56 .
- Chagger , H.K. and Jones , J.M. 1999 . Pourkashanian, M. Emission of Volatile Organic Compounds from Coal Combustion . Fuel , 78 : 1527 – 1538 .
- Mastral , A.M. and Callen , M.S. 2000 . A Review on Polycyclic Aromatic Hydro-carbon (PAH) Emissions from Energy Generation . Environ. Sci. Technol. , 34 : 3051 – 3057 .
- Fernandez-Martinez , G. , Lopez-Mahia , P. , Muniategui-Lorenzo , S. , Prada-Rodriguez , D. and Fernandez-Fernandez , E. 2001 . Distribution of Volatile Organic Compounds during the Combustion Process in Coal-Fired Power Stations . Atmos. Environ. , 35 : 5823 – 5831 .
- Fernandez-Martinez , G. , Lopez-Vilarino , J.M. , Lopez-Mahia , P. , Muniategui-Lorenzo , S. , Prada-Rodriguez , D. , Abad , E. and Rivera , J. 2002 . Dioxin Assessment in Coal-Fired Power Stations from Spain . Organohalogen Compd. , 59 : 203 – 206 .
- Fernandez-Martinez , G. , Lopez-Vilarino , J.M. , Lopez-Mahia , P. , Muniategui-Lorenzo , S. , Prada-Rodriguez , D. , Abad , E. and Rivera , J. 2004 . First Assessment of Dioxin Emissions from Coal-Fired Power Stations in Spain . Chemosphere , 57 : 67 – 71 .
- Chen , C.M. 2004 . The Emission Inventory of PCDD/PCDF in Taiwan . Chemosphere , 54 : 1413 – 1420 .
- Martinez , M.A. , Sanz , P. , Fabrellas , B. , Rivera , J. and Abad , E. 2006 . PCDD/F Emission in Power Generation Plants: Evaluation in the Frame of Spanish Dioxin Inventory . Organohalogen Compd. , 68 : 2276 – 2279 .
- Lin , L.F. , Lee , W.J. , Li , H.W. , Wang , M.S. and Chang-Chen , G.P. 2007 . Characterization and Inventory of PCDD/F Emissions from Coal-Fired Power Plants and Other Sources in Taiwan . Chemosphere , 68 : 1642 – 1649 .
- Herrmann , D. and Herzschuh , R. 1999 . Formation of PCDD/Fs during Thermal Treatment of Lignite . J. Anal. Appl. Pyrolysis , 49 : 211 – 220 .
- Thusz , U. , Popp , P. , Ehrlich , C. and Kalkoff , W.-D. 1995 . Domestic Lignite Combustion of Polychlorodibenzodioxins and Furans (PCDD/F) . Chemosphere , 31 : 2591 – 2602 .
- Thusz , U. , Herzchub , R. , Popp , R. , Ehrlich , C. and Kalkoff , W.D. 1997 . PCDD/F in Flue Gas and in Bottom Ash of Lignite Domestic Combustion and the Role of the Salt Content of the Burned Briquettes . Chemosphere , 34 : 1091 – 1103 .
- Schleicher , O. , Jensen , A. , Herrmann , T. , Roots , O. and Tordik , A. 2004 . Dioxin Emission from Two Oil Shale Fired Power Plants in Estonia . Organo-halogen Compd. , 66 : 1635 – 1641 .
- Wang , Y.F. , Chao , H.R. , Wu , C.H. , Wang , L.C. , Chang-Chein , G.P. , Yang , H.H. , Lin , D.Y. and Tsou , T. 2009 . C Emissions of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans from a Heavy Oil-Fueled Power Plant in Northern Taiwan . J. Hazard. Mater. , 163 : 266 – 272 .
- Kim , S.C. , Na , J.G. , Choe , S.H. , Lee , J.H. , Kim , Y.H. , Hwanh , S.R. , Cheon , Y.J. , Choi , J.H. , Lee , C.J. , Choi , K.S. and Hwang , Y.Y. 2003 . PCDDs/ PCDFs Emission from Power Station Using Coke-Oven Gas as Fuel . Organohalogen Compd. , 63 : 81 – 85 .
- Meij , R. and te Winkel , H. 2007 . The Emissions of Heavy Metals and Persistent Organic Pollutants from Modern Coal-Fired Power Stations . Atmos. Environ. , 41 : 9262 – 9272 .
- Frankenhäuser , M. , Hiltunen , M. , Manninen , H. , Palonen , J. , Ruuskanen , J. and Vartiainen , T. 1994 . Emissions from Co-Combustion of Used Packaging with Peat and Coal . Chemosphere , 29 : 2057 – 2066 .
- Frankenhäuser , M. , Manninen , H. , Koja , I. , Ruuskanen , J. , Virtanen , T. , Vesterinen , T.R. and Virkki , J. 1993 . Organic Emissions from Co-Combustion of Mixed Plastics with Coal in a Bubbling Fluidized Bed Boiler . Chemo-sphere , 27 : 309 – 316 .
- Ruuskanen , J. , Virtiainen , T. , Koja , I. , Manninen , H. , Oksanen , J. and Frankenhäuser , M. 1994 . Formation of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Co-Combustion of Mixed Plastics with Coal. Exploratory Principal Component Analysis . Chemosphere , 28 : 1989 – 1999 .
- Gulyurutlu , I. , Crujeira , A.T. , Abelha , P. and Cabrita , I. 2007 . Measurements of Dioxin Emissions during Co-Firing in a Fluidized Bed . Fuel , 86 : 2090 – 2100 .
- Grochowalski , A. and Konieczyński , J. 2008 . PCDDs/PCDFs, dl-PCBs and HCB in Flue Gas from Coal Fired CFB Boilers . Chemosphere , 73 : 97 – 103 .
- Nestrick , T.J. and Lamparski , L.L. 1983 . Assessment of Chlorinated Dibenzo-p-Dioxin Formation and Potential Emission to Environment from Wood Combustion . Chemosphere , 12 : 617 – 626 .
- Clement , R.E. , Tosine , H.M. and Ali , B. 1985 . Levels of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Wood Burning Stoves and Fireplaces . Chemosphere , 14 : 815 – 819 .
- Bacher , R. , Swerev , M. and Ballschmiter , K. 1992 . Profile and Pattern of Monochlorodibenzodioxins through Octachlorodibenzodioxins and Octachlorodibenzofurans in Chimney Deposits from Wood Burning . Environ. Sci. Technol. , 26 : 1649 – 1655 .
- Koleda , J. , Gass , H. , Wilken , N. , Jager , J. and Zeschmar-Lahl , B. 1993 . Determination and Reduction of PCDD/F Emissions from Wood Burning Facilities . Organohalogen Compd. , 11 : 401 – 404 .
- Koleda , J. , Gass , H. , Wilken , N. , Jager , J. and Zeschmar-Lahl , B. 1994 . Determination and Reduction of PCDD/F Emissions from Wood Burning Facilities . Chemosphere , 29 : 1927 – 1938 .
- Schatowitz , B. , Brandt , G. , Gafner , F. , Schlumpf , E. , Bühler , R. , Hasler , P. and Nussbaumer , T. 1994 . Dioxin Emission from Wood Combustion . Chemosphere , 29 : 2005 – 2013 .
- Schmoeckel , G. and Streit , A. 1994 . Emissions of Organic Substances in Wood Burning , Augsburg , , Germany : Bavarian State Office for the Environment .
- Marklund , S. , Wikström , E. , Löfvenius , G. , Fängmark , I. and Rappe , C. 1994 . Emissions of Polychlorinated Compounds in Combustion of Biofuel . Chemosphere , 28 : 1895 – 1904 .
- Pohlandt , K. and Marutzku , R. 1994 . Concentration and Distribution of Polychlorinated Dibenzo-p-Dioxins (PCDD) and Polychlorinated Dibenzofurans (PCDF) in Wood Ash . Chemosphere , 28 : 1311 – 1314 .
- Oehme , M. and Müller , M.D. 1995 . Levels and Congener Patterns of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Solid Residues from Wood Fired Boilers. Influence of Combustion Conditions and Fuel Type . Chemosphere , 30 : 1527 – 1539 .
- Pagger , E. and Theuermann , J. 1996 . Analysis of Organic Compounds (PCDD/Fs in Flue Gas: PCDD/Fs, PAHs, PCBs in Different Ashes) of Wood Waste Fired Boilers . Organohalogen Compd. , 27 : 171 – 176 .
- Andresson , P. and Marklund , S. 1998 . Emissions of Organic Compounds from Biofuel Combustion and Influence of Different Control Parameters Using Laboratory Scale Incinerator . Chemosphere , 36 : 1429 – 1443 .
- Chagger , H.K. , Kendall , A. , McDonald , A. , Pourkashanian , M. and Williams , A. 1998 . Formation of Dioxins and Other Semi-Volatile Organic Compounds in Biomass Combustion . Appl. Energy , 60 : 101 – 114 .
- Nussbaumer , T. and Hasler , P. 1999 . Formation and Properties of Aerosols from Wood Combustion (in German) . Eur. J. Wood Wood Prod. , 57 : 13 – 22 .
- Wunderli , S. , Zanegg , M. , Dolezal , I.S. , Grujer , E. , Moser , U. and Wolfenberger , M. 2000 . Determination of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Solid Residues from Wood Combustion by HRGC/ HRMS . Chemosphere , 40 : 641 – 649 .
- Strecker , M. , Marutzky , R. , Pieper , A. and Bahadir , M. 2001 . Reducing Emissions of Nitrogen Oxides (NOx) and Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans (PCDD/PCDF) in the Energy Recovery of Waste Wood in Furnace with the Bottom Supply Using Construction and Operational Methods , Osnabrück , , Germany : German Federal Environmental Foundation .
- Schleicher , O. , Jensen , A. , Blinksbjerg , P. , Thomsem , E. and Schilling , B. 2002 . Dioxin Emissions from Biomass Fired Energy Plants and Other Sources in Denmark . Organohalogen Compd. , 56 : 147 – 150 .
- Lavric , E.D. , Konnov , A.A. and de Ruyck , J. 2004 . Dioxin Levels in Wood Combustion—a Review . Biomass & Bioenergy , 26 : 115 – 145 .
- Lavric , E.D. , Konnov , A.A. and de Ruyck , J. 2005 . Modeling the Formation of Precursors of Dioxins during Combustion of Woody Fuel Volatiles . Fuel , 84 : 323 – 334 .
- Neuer-Etscheidt, K. Ph.D. thesis, Technical University of Munich, MunichGermany, 2006.
- Muto , H. and Sugawara , T. 2001 . Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Plywood Combustion Gas . Chemosphere , 45 : 145 – 150 .
- Samaras , P. , Skodras , G. , Sakellaropoulos , G.P. , Blumenstock , M. , Schramm , K.W. and Kettrup , A. 2001 . Toxic Emissions during Co-Combustion of Biomass-Waste Wood-Lignite Blends in an Industrial Boiler . Chemosphere , 43 : 751 – 755 .
- Salthammer , T. , Klipp , H. , Peem , R.-D. and Marutzky , R. 1995 . Formation of Polychlorinated Dibenzo-p-Dioxin (PCDD) and Polychlorinated Dibenzofurans (PCDF) during the Combustion of Impregnated Wood . Chemosphere , 30 : 2051 – 2060 .
- Wan , M.T. 1995 . Treated Wood as a Source of Dioxin/Furan Releases . Organohalogen Compd. , 24 : 109 – 114 .
- Hakk , H. and Larsen , G. 2000 . Levels of Polychlorinated Dioxin and Furan Emission from Pentachlorophenol Treated Wood . Organohalogen Compd. , 45 : 348 – 351 .
- Yasuhara , A. , Katami , T. and Shibamoto , T. 2003 . Formation of PCDDs, PCDFs and Coplanar PCBs from Incineration of Various Woods in the Presence of Chlorides . Environ. Sci. Technol. , 37 : 1563 – 1567 .
- Hansen , C.L. and Hansen , E. 2004 . Survey of Dioxin Emission from PCP-Treated Wood; Environmental Project No. 940 , Copenhagen , , Denmark : Danish Environmental Protection Agency .
- Tame , N.W. , Dlugogorski , B.Z. and Kennedy , E.M. 2003 . Increased PCDD/F Formation in the Bottom Ash from Fires of CCA-Treated Woods . Chemosphere , 50 : 1261 – 1263 .
- Tame , N.W. , Dlugogorski , B.Z. and Kennedy , E.M. 2003 . Assessing Influence of Experimental Parameters on Formation of PCDD/F from Ash Derived from Fires of CAA-Treated Wood . Environ. Sci. Technol. , 37 : 4148 – 4156 .
- Tame , N.W. , Dlugogorski , B.Z. and Kennedy , E.M. 2005 . PCDD/F Formation in Flaming Combustion, Smoldering and Oxidative Pyrolysis of “Eco-Friendly” Treated Wood . Proc. Combust. Inst. , 30 : 1237 – 1243 .
- Tame , N.W. , Dlugogorski , B.Z. and Kennedy , E.M. 2007 . Formation of Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans (PCDD/Fs) in Fires of Arsenic-Free Treated Wood: Role of Organic Preservatives . Environ. Sci. Technol. , 41 : 6425 – 6432 .
- Tame , N.W. , Dlugogorski , B.Z. and Kennedy , E.M. 2007 . Formation of Dioxins and Furans during Combustion of Treated Wood . Prog. Energy Combust. Sci. , 33 : 348 – 408 .
- Wasson , S.J. , Linak , W.P. , Gullett , B.K. , King , C.J. , Touati , A. , Huggins , F.E. , Chen , Y. , Shah , N. and Huffman , G.P. 2005 . Emissions of Chromium, Copper, Arsenic and PCDDs/Fs from Open Burning of CCA-Treated Wood . Environ. Sci. Technol. , 39 : 8865 – 8876 .
- Gass , H.C. , Stein , R. and Suritsch , N. 2007 . Elevated Level of PCDD/F Emission from a Wood Chips Fired Boiler after Small Failure of Operating Conditions . Organohalogen Compd. , 69 : 2474 – 2477 .
- Wielgosiński , G. 2003 . Emissions from Waste Incineration Plants—Primary Methods of Emission Reduction . Energetic Policy , 6 : 131 – 140 .
- Gullett , B. and Seeker , R. Chlorinated Dioxin and Furan Control and Monitoring . Presented at the Industrial Combustion Coordinated Rulemaking Meeting . Research Triangle Park , NC
- McKay , G. 2002 . Dioxin Characterization, Formation and Minimization during Municipal Solid Waste (MSW) Incineration: a Review . Chem. Eng. J. , 86 : 343 – 368 .
- Goldfarb , T.D. 1989 . Evidence for Post-Furnace Formation of PCDDs and PCDFs—Implication for Control . Chemosphere , 18 : 1051 – 1055 .
- Ishikawa , R. , Buekens , A. , Huang , H. and Watanbe , K. 1997 . Influence of Combustion Conditions on Dioxin in Industrial Scale Fluidised Bed Incinerator. Experimental Study and Statistical Modeling . Chemosphere , 35 : 465 – 477 .
- Lenoir , D. , Kaune , A. , Hutzinger , O. , Mutzenich , G. and Horch , K. 1991 . Influence of Operating Parameters and Fuel Type on PCDD/PCDF Emissions from Fluidized Bed Incinerator . Chemosphere , 23 : 1491 – 1500 .
- Seeker, R. Personal communication; 2001.
- Lindbauer , L.R. , Wurst , F. and Prey , T. 1992 . Combustion Dioxin Suppression in Municipal Solid Waste Incineration with Sulphur Additives . Chemosphere , 25 : 1409 – 1414 .
- Gullett , B.K. , Bruce , K.R. and Beach , L.O. 1992 . Effect of Sulfur Dioxide on Formation Mechanism of Polychlorinated Dibenzodioxin and Dibenzofuran in Municipal Waste Combustors . Environ. Sci. Technol. , 26 : 1938 – 1943 .
- Ruokojärvi , P. , Halonen , I. , Tuppurainen , K. , Tarhanen , J. and Ruuskanen , J. 1998 . Effect of Gaseous Inhibitors on PCDD/F Formation . Environ. Sci. Technol. , 32 : 3099 – 3103 .
- Xhrouet , C. and de Pauw , E. 2003 . Prevention of Dioxins De Novo Synthesis by Ethanolamines . Environ. Chem. Lett. , 1 : 51 – 56 .
- Ruokojärvi , P. , Asikainen , A. , Tuppurainen , K. and Ruuskanen , J. 2004 . Chemical Inhibition of PCDD/F Formation in Incineration Processes . Sci. Total Environ. , 325 : 83 – 94 .
- Kazuhara , S. , Sato , H. , Tsubouchi , N. , Ohtsuka , Y. and Kasai , E. 2005 . Effect of Nitrogen-Containing Compounds on Polychlorinated Dibenzo-p-Dioxin/Dibenzofuran Formation through De Novo Synthesis . Environ. Sci. Technol. , 39 : 795 – 799 .
- Samaras , P. , Blumenstock , M. , Lenoir , D. , Schramm , K.W. and Kettrup , A. 2000 . PCDD/F Prevention by Novel Inhibitors: Addition of Inorganic S-and N- Compounds in the Fuel before Combustion . Environ. Sci. Technol. , 34 : 5092 – 5096 .
- Pandelova , M.E. , Lenoir , D. , Kettrup , A. and Schramm , K.W. 2005 . Primary Measures for Reduction of PCDD/F in Co-Combustion of Lignite Coal and Waste: Effect of Various Inhibitors . Environ. Sci. Technol. , 39 : 3345 – 3350 .
- Pandelova , M. , Lenoir , D. and Schramm , K.W. 2006 . Correlation between PCDD/F, PCB and PCBz in Coal/Waste Combustion. Influence of Various Inhibitors . Chemosphere , 62 : 1196 – 1205 .
- Pandelova , M. , Lenoir , D. and Schramm , K.W. 2007 . Inhibition of PCDD/F and PCB Formation in Co-Combustion . J. Hazard. Mater. , 149 : 615 – 618 .
- Ruokojärvi, P. Ph.D. thesis, University of Kuopio, Kuopio, Finland, 2002.
- Cudahy , J.J. and Helsel , R.W. 2000 . Removal of Products of Incomplete Combustion with Carbon . Waste Manage. , 20 : 339 – 345 .
- Fell , H.J. and Tuczek , M. 1998 . Removal of Dioxins and Furans from Flue Gases by Non-Flammable Adsorbents in a Fixed Bed . Chemosphere , 37 : 2327 – 2334 .
- Blumbach , J. and Nethe , L.P. 1994 . PCDD/F/PCB Reduction in Flue Gases and Residual Materials from Waste Incineration by Use of Carbonaceous Adsorbents . Organohalogen Compd. , 19 : 305 – 310 .
- Okajima , S. , Kojima , T. , Ueoka , S. and Ozaki , H. 2001 . Fixed-Bed Adsorption of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans from Flue Gas in a Municipal Solid Waste Incinerator . Organohalogen Compd. , 54 : 193 – 196 .
- Maierhofer , R. and Grochowski , H. 1994 . Removal of Dioxins and Furans with Activated Carbon Countercurrent Flow Process . Organohalogen Compd. , 19 : 361 – 364 .
- Everaert , K. , Baeyens , J. and Creemers , C. 2003 . Adsorption of Dioxins and Furans from Flue Gases in an Entrained Flow or Fixed/Moving Bed Reactor . J. Chem. Technol. Biotechnol. , 78 : 213 – 209 .
- Everaert , K. and Baeyens , J. 2004 . Removal of PCDD/F from Flue Gases in Fixed on Moving Bed Adsorbers . Waste Manage. , 24 : 37 – 42 .
- Mori , K. , Matsui , H. , Yamaguchi , N. and Nakagawa , Y. 2005 . Multi-Component Behavior of Fixed-Bed Adsorption of Dioxins by Activated Carbon Filter . Chemosphere , 61 : 941 – 946 .
- Yates , M. , Blanco , J. , Avila , P. and Martin , M.P. 2000 . Honeycomb Monoliths of Activated Carbons for Effluent Gas Purification . Microporus Mesoporus Mater. , 37 : 201 – 208 .
- Reinmark , M. , van der Kleut , D. and van der Akker , B. 1996 . Norit Powdered Activated Carbon in Flue Gas Clean Up . Organohalogen Compd. , 27 : 177 – 182 .
- Hagenmeier , H. 1989 . Catalytic Oxidation of Halogenated Hydrocarbons with Special Regard to the Dioxin Problem . VDI Berichte , 730 : 239 – 254 .
- Hiraoka , M. , Takeda , N. , Okijama , S. , Kasakura , T. and Imoto , Y. 1989 . Catalytic Destruction of PCDDs in Flue Gas . Chemosphere , 19 : 361 – 366 .
- Hagenmaier , H. and Mittelbach , G. 1990 . Investigation of Catalytic Reduction NOx and Dioxins in the Exhaust Gases from Municipal Solid Waste Incinerator . VGB Kraftwerkstechnik , 70 : 491 – 493 .
- von Fahlenkamp , H. , Mittlebach , G. , Hagenmeier , H. , Brunner , H. and Tichaczek , K.-H. 1991 . Catalytic Oxidation. A Technique for Reducing PCDD/PCDF Emissions from Waste Incinerators to Less Than 0.1 ng TE/m3 . VGB Kraftwerkstechnik , 71 : 671 – 674 .
- Boos , R. , Budin , R. , Hartl , M. , Stock , M. and Wurst , F. 1992 . PCDD and PCDF Destruction by SCR Units in a Municipal Waste Incinerator . Chemo-sphere , 25 : 375 – 382 .
- Ide , Y. , Kashiwabara , K. , Okada , S. , Mori , T. and Hara , M. 1996 . Catalytic Decomposition of Dioxin from MSW Incinerator Flue Gas . Chemo-sphere , 32 : 189 – 198 .
- Andersson , P. , Rappe , C. , Maaskant , O. , Unsworth , J.F. and Marklund , S. 1998 . Low Temperature Catalytic Destruction of PCDD/F in Flue Gas from Waste Incineration . Organohalogen Compd. , 36 : 109 – 248 .
- Schüttenhelm , W. , Wemhöner , R. , Hartenstein , H.U. and Werner , K. 1999 . Reduction of PCDD/F Emission from Iron Ore Sintering Plants—the First Full-Scale SCR Installation . Organohalogen Compd. , 40 : 453 – 456 .
- Weber , R. , Sakurai , T. and Hagenmeier , H. 1999 . Low Temperature Decomposition of PCDD/PCDF, Chlorobenzenes and PAHs by TiO2-Based V2O5-WO3 Catalyst . Appl. Catal. B. , 20 : 249 – 256 .
- Choi , H.C. , Park , C.G. , Seung , H.J. , Oh , T.Y. and Lee , Y.D. 1999 . The Dioxin Removal in Flue Gas of the Waste Incineration Plant by FLR and SCR Units . Organohalogen Compd. , 40 : 493 – 496 .
- Choi , H.C. , Park , C.G. , Seung , H.J. and Oh , T.Y. 2000 . Catalytic Destruction of PCDDs/DFs by the SCR Units . Organohalogen Compd. , 45 : 387 – 391 .
- Bertinchamps, F. Ph.D. thesis, Universite Catholique de Louvain, Louvain, Belgium, 2005.
- Finocchio , E. , Busca , G. and Notaro , M. 2006 . A Review of Catalytic Processes for the Destruction of PCDD and PCDF from Waste Gases . Appl. Catal. B. , 62 : 12 – 20 .
- de Jong , V. , Cieplik , M.K. and Louw , R. 2004 . Formation of Dioxins in the Catalytic Combustion of Chlorobenzene and a Micropollutant-Like Mixture on Pt/γ-Al2O3 . Environ. Sci. Technol. , 38 : 5217 – 5223 .
- van den Brink , R.W. , Louw , R. and Mulder , P. 1998 . Formation of Polychlorinated Benzenes during Catalytic Combustion of Chlorobenzene Using Pt/γ-Al2O3 Catalyst . Appl. Catal. B , 16 : 219 – 226 .
- Hart , J.R. 2008 . Verification of Dioxin Formation in a Catalytic Oxidizer . Chemosphere , 72 : 75 – 78 .
- Plinke , M. , Fritsky , K. , Gantara , C.P. , Wilken , M. , Gass , H. , Weber , R. and Mogami , Y. 2000 . Catalytic Dioxin/Furan Removal from Flue Gas Streams . Organohalogen Compd. , 45 : 452 – 455 .
- Weber , R. , Plinke , M. and Xu , Z. 2000 . Dioxin Destruction Efficiency of Catalytic Filters—Evaluation in Laboratory and Comparison to Field Operation . Organohalogen Compd. , 45 : 427 – 430 .
- Fritsky , K.J. , Kumm , J.H. and Wilken , M. 2000 . Combined PCDD/F Destruction and Particulate Control Using a Catalytic Filter System at a Medical Waste Incineration Plant . Journal of the Air & Waste Management Association , 50 : 1642 – 1649 .
- Pranghofer, G. Destruction of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Fabric Filters: Recent Experiences with a Catalytic Filter System. Presented at the Third International Symposium on Incineration and Flue Gas Treatment Technologies “Incineration’2001” Brussels, Belgium, June, 2001.
- Bonte , J.L. , Plinke , M. , Dandaraw , R. , Brinckman , G. , Waters , M. , van Overberghe , K. and van den Heuvel , H. 1999 . Catalytic Filtration: Dioxin/ Furan Destruction in the Baghouse. Experiences at the IVRO Municipal Waste Incinerator in Roeselare, Belgium . Organohalogen Compd. , 40 : 459 – 464 .
- Bonte , J.L. , Fritsky , K.J. , Plinke , M.A. and Wilken , M. 2002 . Catalytic Destruction of PCDD/F in a Fabric Filter: Experience at Municipal Waste Incinerator in Belgium . Waste Manage. , 22 : 421 – 426 .
- Syc , M. , Pekarek , V. , Fioserova , E. , Puncochar , M. , Karban , J. and Kokes , O. 2006 . Catalytic Filter Application in the Termizo Municipal Waste Incineration Plant in Liberec . Organohalogen Compd. , 68 : 1232 – 1235 .
- Kreisz , S. , Hunsinger , H. and Vogg , H. 1997 . Technical Plastics as PCDD/F Adsorbers . Chemosphere , 34 : 1045 – 1052 .
- Kreisz , S. , Hunsinger , H. and Seifert , H. 2000 . Propylene as Regenerable Absorber for PCDD/F Emission Control . Chemosphere , 40 : 1029 – 1031 .
- Kreisz , S. , Hunsinger , H. and Seifert , H. 2002 . Carbon Doped Propylene as PCDD/F Adsorber . Organohalogen Compd. , 56 : 369 – 372 .
- Andersson , S. , Kreisz , S. and Hunsinger , H. 2002 . PCDD/F Removal from Flue Gases in Wet Scrubbers—a Novel Technique . Organohalogen Compd. , 58 : 157 – 160 .
- Andersson , S. , Kreisz , S. and Hunsinger , H. 2003 . Innovative Material Technology Removes Dioxins from Flue Gases . Filtrat. Separ. , 40 : 22 – 25 .
- Andersson , S. , Kreisz , S. and Hunsinger , H. 2003 . PCDD/F Removal from Flue Gases Using Adiox . Organohalogen Compd. , 63 : 244 – 247 .
- Andersson , S. 2005 . PCDD/F Removal from Gases Using a Dry Adiox Absorber . Organohalogen Compd. , 67 : 2226 – 2229 .
- Andersson , S. , Kreisz , S. and Hunsinger , H. 2005 . Dioxin Removal: ADIOX for Wet Scrubbers and Dry Adsorbers . Filtrat. Separat. , 42 : 22 – 25 .
- Paur , H.R. , Baumann , W. , Mätzing , W. and Jay , K. 1998 . Electron Beam Induced Decomposition of Chlorinated Aromatic Compounds in Waste Incinerator Off Gas . Radiat. Phys. Chem. , 52 : 355 – 359 .
- Gerasimov , G.Y. 2003 . Simulation of the Behavior of Dioxins under the Conditions of Electron-Beam Gas Cleaning of Sulfur and Nitrogen Oxides . High Energy Chem. , 37 : 141 – 145 .
- Nichipor , H. , Dashouk , E. , Yacko , S. , Chmielewski , A.G. , Zimek , Z. and Sun , Y. 2002 . Chlorinated Hydrocarbons and PAHs Decomposition in Dry and Humid Air by Electron Beam Irradiation . Radiat. Phys. Chem. , 65 : 423 – 427 .
- Hirota , K. , Hakoda , T. , Mitsumasa , T. , Takigami , M. , Kim , H. and Kojima , T. 2003 . Application of Electron Beam for the Reduction of PCDD/F Emission from Municipal Solid Waste Incinerators . Environ. Sci. Technol. , 37 : 3164 – 3170 .
- Chmielewski , A.G. , Sun , Y.X. , Bułka , S. and Zimek , Z. 2004 . Chlorinated Aliphatic and Aromatic VOC Decomposition in Air Mixture by Using Electron Beam Irradiation . Radiat. Phys. Chem. , 71 : 435 – 438 .
- Chmielewski , A.G. , Sun , Y. , Bułka , S. and Zimek , Z. 2007 . Review on Gaseous Chlorinated Organics Pollutants Electron Beam Treatment . Radiat. Phys. Chem. , 76 : 1795 – 1801 .
- Hirota , K. and Kojima , T. 2005 . Decomposition Behavior of PCDD/F Isomers in Incinerator Gases under Electron-Beam Irradiation . Bull. Chem. Soc. Jap. , 78 : 1685 – 1690 .
- Jian , H.Y. , Zheng , P. , Sheng , Y.L. , Chang , M.D. , Xiao , D.L. , Ming , J.N. and Ka , F.C. 2006 . Destruction of PCDD/Fs Formed by Pentachlorophenol via Gliding Arc Discharges . Organohalogen Compd. , 68 : 1247 – 1250 .
- Nifuku , M. , Horvath , M. , Bodnar , J. , Zhang , G.Y. , Tanaka , T. , Kiss , E. , Woynarovich , G. and Katoh , H. 1997 . A Study on Decomposition of Volatile Organic Compounds by Pulse Corona . J. Electrostatics , 40 : 687 – 692 .
- Crespo , D. and Yang , R.T. 2006 . Adsorption of Organic Vapors on Single- Walled Carbon Nanotubes . Ind. Eng. Chem. Res. , 45 : 5524 – 5530 .
- Fagan , S.B. , Santos , E.J.G. , Souza-Filho , A.G. , Mendes-Filho , J. and Fazzio , A. 2007 . Ab Initio Study of 2,3,7,8-Tetrachlorinated Dibenzo-p-Dioxin Adsorption on Single Wall Carbon Nanotubes . Chem. Phys. Lett. , 437 : 79 – 82 .
- Long , R.Q. and Yang , R.T. 1999 . Adsorbents for Dioxins: a New Technique for Sorbent Screening for Low-Volatile Organics . Ind. Eng. Chem. Res. , 38 : 2726 – 2731 .
- Long , R.Q. and Yang , R.T. 2001 . Carbon Nanotubes as Superior Sorbent for Dioxin Removal . J. Am. Chem. Soc. , 123 : 2058 – 2059 .
- Shih , Y.H. and Li , M.S. 2008 . Adsorption of Selected Volatile Organic Vapors on Multiwall Carbon Nanotubes . J. Hazard. Mater. , 154 : 21 – 28 .
- Choi , J. , Kim , O. and Kwak , S.Y. 2007 . Suppression of Dioxin Emission in Co-Incineration of Polyvinyl Chloride with TiO2-Encapsulating Polystyrene . Environ. Sci. Technol. , 41 : 5833 – 5838 .
- Graham , J.L. , Almquist , C.B. , Kumar , S. and Sidhu , S. 2003 . An Investigation of Nanostructured Vanadia/Titania Catalysts for the Oxidation of Monochlorobenzene . Catal. Today , 88 : 73 – 82 .
- Jiamg , Y. , Decker , S. , Mohs , C. and Klabunde , K.J. 1998 . Catalytic Solid State Reactions on the Surface of Nanoscale Metal Oxide Particles . J. Catal. , 180 : 24 – 35 .
- Kim , S.H. , Ahn , S.Y. and Kwak , S.Y. 2008 . Suppression of Dioxin Emission in Incineration of Polyvinyl Chloride (PVC) as Hybridized with Titanium Dioxide Nanoparticles . Appl. Catal. B. , 79 : 296 – 305 .
- Kulkarni , P.S. , Branco , L.C. , Crespo , J.G. and Alfonso , C.A.M. 2008 . Capture of Dioxins by Ionic Liquids . Environ. Sci. Technol. , 42 : 2570 – 2574 .
- Yearbook: Environmental Protection—1998–2007; Polish National Statistical Office: Warsaw, Poland.
- Stockholm Convention on Persistent Organic Pollutants; U.N. Environment Programme: Geneva, Switzerland, 2001.